Abstract
Paralytic ileus is the condition where the motor activity of the bowel is impaired, usually not associated with a mechanical cause. Although the condition may be self‐limiting, it is serious and if prolonged and untreated will result in death in much the same way as in acute mechanical obstruction. Management of paralytic ileus depends on the knowledge of the most likely cause and the perceived chance of resolution without operation. Postoperative ileus is the single largest factor influencing length of hospital stay after bowel resection, and has great implications for patients and resource utilization. Early diagnosis and correct management is essential in reducing complications. This article briefly outlined the plausible pathophysiological mechanisms and clinical implications of paralytic ileus.
Keywords: ileus, intestine, obstruction, paralytic
Paralytic ileus is the condition where the motor activity of the bowel is impaired, usually without the presence of a physical obstruction. Although the condition may be self‐limiting, it is serious and if prolonged and untreated will result in death in much the same way as in acute mechanical obstruction. Management of paralytic ileus depends on the knowledge of the most likely cause and the perceived chance of resolution without operation.
Introduction
Paralytic ileus (or adynamic ileus) is a condition in which there is a functional motor paralysis of the digestive tract secondary to neuromuscular failure involving the myenteric (Auerbach’s) and submucous (Meissner’s) plexus. The intestine fails to transmit peristaltic waves, resulting in a functional obstruction, and allowing fluid and gas to collect in the intestine. It is the small intestine that is predominantly affected, but the colon and stomach could also be involved. The resultant stasis leads to accumulation of fluid and gas within the bowel with associated distension, vomiting, decrease of bowel sounds, and absolute constipation. Paralytic ileus is a neurogenic condition, in which the normal electrical slow wave is present in the smooth muscle, but does not excite any action potentials. The mechanism is thought to be adrenergic stimulation, possibly involving dopamine release, but adrenoceptor blockade or dopamine inhibition have not been shown to be therapeutically effective. 1 , 2 In postoperative ileus, it is the inhibitory alpha2‐adrenergic reflexes with peptidergic afferents that contribute. 2 The motor inhibition is triggered by a variety of stimuli, the commonest being tactile stimulation during surgery and peritonitis. Paralytic ileus is commonly seen in postoperative ileus which resolves after 24–48 h or from hypokalemia with diuretic use. 1 The whole intestine could be affected and could become grossly distended. Small bowel motility returns within a few hours and gastric and colonic motility after a few days, depending on the degree of trauma. 1 , 3 It might be prolonged if there is hypokalemia, hypoproteinemia, or renal failure or if the bowel is allowed to get grossly distended. 4 Uremia could also produce ileus, often with distention, vomiting, and hiccups. 1 Peritonitis (infection) causes ileus. Ileus is also sometimes associated with retroperitoneal trauma and hemorrhage, spine or rib fractures, severe trauma outside the abdomen, and the application of a plaster jacket. This occurs through a reflex sympathetic overstimulation. Paralytic ileus could also be classified on the basis of the site of hypomotility.
Acute gastric dilatation, which probably represents extreme gastric stasis from atonia following any abdominal surgical procedure, carries a high risk of aspiration and mortality. It can occur in patients with anorexia and other psychiatric conditions when they have been prescribed large doses of psychotropic drugs and with the gastric autonomic neuropathy of diabetes mellitus. Whilst remedied by insertion of a wide nasogastric tube, contributory electrolyte imbalances, particularly hypokalemia, must be corrected. 1 Acute colonic pseudo‐obstruction (Ogilvie syndrome) results from colonic hypomotility and produces a massive but reversible dilatation of the colon. It occurs in critically ill or postoperative patients. It could carry a mortality rate as high as 45% if not recognized or colonoscopically or operatively decompressed following failure of conservative treatment, or laparotomy not carried out when clinical signs indicate cecal ischemia or perforation. 5
Manifestations of paralytic ileus
Sequelae of acute mechanical intestinal obstruction
Intestinal obstruction could be mechanical, which presents with colicky pain from the increased peristalsis against the obstructive lesion, or paralytic, which is painless being aperistaltic. There is a degree of overlap as mechanical obstruction can progress to a paralytic obstruction (ileus). Obstruction of the bowel leads to bowel distension above the block, with increased secretion of fluid into the distended bowel. Bacterial contamination occurs in the distended stagnant bowel. Prolonged increase in intraluminal pressure impairs the viability of the bowel wall with subsequent diffusion of toxic bacterial products into the peritoneal cavity where they are absorbed and produce toxemia and death. Thus, there is a role for i.v. prophylactic antibiotics in ileus. A further complicating factor is potassium depletion, which leads to weakness and smooth muscle paresis, and could superimpose a state of adynamic ileus on the existing obstructive lesion. The consequences of intestinal obstruction are therefore a combination of progressive dehydration, electrolyte imbalance, and systemic toxicity due to migration of toxins and bacterial translocation either through the intact but ischemic bowel or through a perforation.
Localized ileus
A localized ileus consists of a localized loop (sentinel loop) of dilated small bowel. The appearance is not diagnostic of intra‐abdominal infection but rather an associated feature of a local irritation such as acute pancreatitis, with the ileus being in the same anatomical region as the pathology. Radiologically, there are one or two loops of small or large bowel with often air‐fluid levels in the sentinel loops. In localized or generalized ileus there is air in the rectum or sigmoid colon unlike in mechanical small or large bowel obstruction. 4
Generalized peritonitis
Generalized peritonitis is a serious condition resulting from irritation of the peritoneum due to infection, such as perforated appendix, or from chemical irritation due to leakage of intestinal contents, such as perforated duodenal ulcer. In the latter case, superadded infection gradually occurs and Escherichia coli and Bacteroides are the commonest organisms. The peritoneal cavity becomes acutely inflamed with production of an inflammatory exudate that spreads throughout the peritoneum, leading to intestinal dilatation and paralytic ileus. Peritonitis causes ileus initially from the inflammation, second from the bacterial toxins, and finally the fibrinous adhesions produced could delay the return of bowel function. Bacterial lipopolysaccharide (LPS) causes ileus by initiating an inflammatory response within the intestinal smooth muscle layers and a subsequent decrease in in vitro and in vivo smooth muscle contractility. 1 , 2 The ileus in generalized peritonitis is characterized by multiple distended loops of bowel. 1 , 2 , 3 , 4
Postoperative ileus
Although definitions vary considerably in the literature, postoperative ileus (POI) can be characterized by a temporary inhibition of gastrointestinal motility after surgical intervention due to non‐mechanical causes that promote deficient oral intake. The paralytic state usually lasts from a few hours to 24 h in the small bowel, from 24 to 48 h in the stomach, and from 48 to 72 h in the colon. 1 , 2 , 3 , 4 The duration of POI correlates with the degree of surgical trauma and is most extensive (10–30%) following colon and rectal surgery. 3 However, POI can develop after all types of surgery, including extraperitoneal surgery. The clinical consequences of postoperative paralytic ileus are profound, contributing to discomfort, increased catabolism because of hindered oral nutrition, immobilization, increased risk of pulmonary complications, and increased length of hospital stay. 1 , 2 , 3 , 4 Preliminary clinical studies on laparoscopic colon surgery have shown normalization of gastrointestinal function within 24–48 h, thereby shortening recovery and length of hospital stay. 3 The pathophysiology of POI is multifactorial and complex involving pharmacological (opioids, anesthetics), with possible direct inhibition of enteric or spinal nerves, neural, and immune‐mediated mechanisms. 1 , 2 , 3 , 4 The electrolyte imbalance following major surgery could contribute to paralytic ileus by interfering with the normal ionic shifts that occur during smooth muscle contraction. 1 , 3 The early neural phase, triggered by activation of afferent nerves during the surgical procedure, is short‐term compared to the later inflammatory phase. The latter starts after 3–6 h and lasts several days, making it a more interesting target for treatment. 6 In addition, there is need for the study of the potential alteration of neurotransmitter receptor activity within the enteric nervous plexus after manipulation of the bowel. The two insults, the manipulation of the small intestine and bacterial translocation, act synergistically to promote paralytic ileus. 7 Gentle manipulation of the intestine leads to postoperative ileus through the promotion of a postoperative cellular inflammatory response within the manipulated intestinal smooth muscle layer. 8 This is evident by visualizing the escape of fluorescent microspheres from the gut lumen in response to intestinal manipulation. 3 Within a short time the endogenous luminal particles and bacterial products will activate or prime circulating leucocytes that eventually extravasate into the manipulated muscularis. This is in response to the upregulation of the intercellular adhesion molecule‐1 on the endothelium of the muscularis vasculature and potentiates ileus. 3 , 9 , 10 Postoperative ileus is known to favor intestinal bacterial overgrowth, a major potential morbidity factor in ileus. 1 , 9 Risk factors for development of postoperative paralytic ileus include increasing age, American Society of Anesthesiologists score of 3–4, open approach, operative difficulty, operation duration more than 3 h, bowel handling, drop in hematocrit, or need for a transfusion, increasing crystalloid administration, and delayed postoperative mobilization. 1 , 2 , 3 , 4 , 11 While treatment is expectant and supportive, significant investigations into strategies to mitigate development of POI or shorten the duration have been undertaken, with mixed results. However, there is significant evidence that a minimally invasive surgical approach and multimodal pain regimens, including epidural anesthesia/analgesia and opioid‐free or opioid‐reduced analgesia, reduce the development of POI. 12 , 13 , 14 , 15 , 16 , 17 , 18 Chewing gum and early oral feeding postoperatively could stimulate gut motility but the data are not all conclusive. 17 , 19 , 20 , 21 , 22 In addition, early enteral nutrition reduces the infectious complications of ileus. 3 , 20 Early enteral nutrition is a component of the enhanced recovery after surgery protocol that decreases the development of POI in patients who undergo elective major gastrointestinal surgery, such as colorectal surgery. 3 , 20 , 21
Prolonged postoperative ileus
Short‐lasting local or generalized ileus occurs with any abdominal operation. However, paralytic ileus could be prolonged if there is hypokalemia, hypoproteinemia, renal failure, a localized peritoneal sepsis, leakage of visceral contents such as pancreatic juice, intraperitoneal or retroperitoneal hematoma, or if the bowel is allowed to get grossly distended. 3 , 4 , 22 Mechanical obstruction must be differentiated from paralytic ileus during the first 4–5 days, as the former might require surgical intervention. 3 , 4 Mechanical obstruction could be caused by pre‐existing adhesions, new fibrinous adhesions, sepsis perhaps due to an intestinal leak or a hematoma, a technically inadequate anastomosis or stoma causing undue narrowing of the lumen, and an internal hernia or volvulus. 23 , 24 , 25 After 5 days, the infective causes of obstruction, such as anastomotic leakage or other intra‐abdominal collection, become more important in the differential diagnosis. If a limited disruption of a large bowel anastomosis is suspected, a careful gastrografin enema could confirm a leak. 12 , 26 Acute gastric dilatation is a complication of major upper abdominal surgery, commonly seen post‐splenectomy. The presentation is subtle with persistent left shoulder tip pain, and hiccups as early warning signs. Unrecognized and untreated cases can have a fatal outcome due to vomiting and aspiration. Correction of biochemical abnormalities, such as potassium, is essential and some cases might require prokinetic drugs to help improve the motility. 27
Clinical features of paralytic ileus
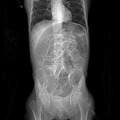
The signs of ileus are a cessation of any motor activity. There is usually no abdominal pain, certainly no colic, but possibly some tenderness from distension. There could be tachypnea because the diaphragms are pushed up and tachycardia from hypovolemia. Bowel sounds are absent, flatus is not passed, and there is consequent gastric stasis that could lead to hiccups, discomfort, and effortless vomiting, unless gastric aspiration has been carried out. The abdomen is distended and tympanic. The abdomen is usually silent on auscultation or quiet “tinkling” sounds due to gut distension are heard as the abdomen is moved. 1 , 2 , 3 , 4 In pseudo‐obstruction, the motility disorder commonly affects the whole gastrointestinal tract, although it is colonic distension that is often most prominent. 5 Dilatation of the colon with signs of systemic upset (“toxic dilatation”) is becoming less common as acute attacks of ulcerative colitis are recognized and appropriately treated. Clinical appearance can be deceptive due to steroids; the danger if surgery is inappropriately delayed is colonic perforation, which still carries a high mortality. Colitis can also occur in Crohn’s and rarely in ischemic or infective colitis (e.g., Yersinia enterocolitica , Campylobacter sp., and pseudomembranous colitis due to Clostridium difficile). 28 The radiological appearance of paralytic ileus is diagnostic. A plain X‐ray of the abdomen in the upright posture shows dilated loops of bowel with multiple fluid levels, indicating distension with fluid and air in all of the small bowel and often much or all of the large bowel. 3 , 6 An abdominal and pelvic computed tomography (CT) scan is used to confirm the diagnosis of postoperative ileus in cases when X‐ray is not diagnostic. Findings on CT scan diagnostic of POI include multiple air‐fluid levels throughout the abdomen, elevated diaphragm, dilatation of both large and small intestine, and no evidence of mechanical obstruction. Computed tomography scan with i.v. contrast and oral water soluble contrast can also distinguish early postoperative ileus from mechanical obstruction. 29
Treatment of paralytic ileus
Preventative measures include avoiding unnecessary exposure and excessive handling of the bowel or traction on its mesentery. 4 Treatment is conservative, as the condition is mostly self‐limiting. Recovery is hastened by the correction of any fluid or electrolyte deficits by giving fluid and nutrients parenterally, and by measures to “defunction” the small bowel by nasogastric decompression. Hypokalemia especially needs attention. Fluids and food by mouth are withheld until bowel sounds return or flatus passed. The cause of the ileus should be found and dealt with. Conservative treatment often succeeds in postoperative ileus and contractility could return, particularly if electrolyte and fluid balance can be restored. Recently, several studies on nasogastric intubation questioned its efficacy in upper gastrointestinal surgery. 30 , 31 , 32 The insertion of a nasogastric tube could postpone the return of bowel sounds and increase the incidence of nausea and patient discomfort, but it does not affect the incidence of postoperative ileus. 30 The fluid lost by intubation is predominantly alkaline and this leads to acidosis. Parenteral infusion of normal saline should thus be supplemented by lactate or carbonate or by a balanced salt solution (Ringer’s or Hartmann’s solution). It is important to remember while calculating the daily fluid balance that these patients will need fluid to replace not only the ongoing losses (e.g., nasogastric aspirates) and baseline daily requirements but also the long‐standing fluid deficits, or third‐space loss. The patients with long‐standing fluid losses could be deficient of 5 L or more of extracellular fluid, which should be replaced slowly over the first 48 h to prevent overexpansion of the circulating plasma volume. Drugs have little place, but prokinetic drugs such as cisapride have been used parenterally to stimulate peristalsis. Trials of adrenergic blockade combined with cholinergic stimulation have met with little success. 2 , 3 , 33 The return of function is heralded by decreasing aspirates and abdominal girth, and the passage of flatus. If ileus is associated with a mechanical cause such as intra‐abdominal sepsis, strangulated obstruction, or peritonitis, the relief of distension by intubation and rapid resuscitation are critically important prior to immediate surgical intervention. 3 , 6 , 7 , 33 It is physiologically important that a patient should not remain obstructed for more than 48 h as both the ensuing local bowel and systemic complications would worsen the prognosis. 3
Conclusions
Paralytic ileus has important clinical implications in the perioperative care of the surgical patient. For its prevention, it is important to recognize the synergistic interaction between the traumatized intestine and exogenous endotoxin. Minimally invasive surgery and the enhanced recovery after surgery protocol would ameliorate the prevalence of this pathophysiological condition.
Disclosure
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding Information
No funding information provided.
References
- 1. Schwarz NT, Beer‐Stotz D, Bauer AJ. Pathogenesis of paralytic ileus. Ann. Surg. 2002; 235(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vather R, O'Grady G, Bissett IP, Dinning PG. Postoperative ileus: mechanisms and future directions for research. Clin. Exp. Pharmacol. Physiol. 2014; 41(05): 358–70. [DOI] [PubMed] [Google Scholar]
- 3. Harnsberger CR, Markel JA, Avavi K. Postoperative ileus. Clin. Colon Rectal Surg. 2019; 32(3): 166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lubawski J, Saclarides T. Postoperative ileus: strategies for reduction. Ther. Clin. Risk Manag. 2008; 4(5): 913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vanek VW, Al‐Salti M. Acute pseudo‐obstruction of the colon (Ogilvie’s syndrome). An analysis of 400 cases. Dis. Colon Rectum 1986; 29(3): 203–10. [DOI] [PubMed] [Google Scholar]
- 6. Stakenborg N, Gomez‐Pinilla J, Boeckastaas GE. Postoperative ileus: Pathophysiology. Current therapeutic approaches. Hands Exp. Pharmacol. 2017; 239: 39–57. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz NT, Simmons RL, Bauer AJ. Minor intraabdominal injury followed by low dose LPS administration act synergistically to induce ileus. Neurogastroenterol. Motil. 2000; 11(2): 288. [Google Scholar]
- 8. Kalff JC, Schrant WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in paralytic ileus. Ann. Surg. 1998; 228: 625–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deitch EA, Specian RD, Berg RD. Endotoxin‐induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit. Care Med. 1991; 19: 785–91. [DOI] [PubMed] [Google Scholar]
- 10. Frank PG, Lisanti MP. ICAM‐1: role in inflammation and in the regulation of vascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2008; 295(3): H926–H927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bragg D, El‐Sharkawy AM, Psaltis E, Maxwell‐Armstrong CA, Lobo DN. Postoperative ileus: recent developments in pathophysiology and management. Clin. Nutr. 2015; 34(03): 367–76. [DOI] [PubMed] [Google Scholar]
- 12. Grass F, Slieker J, Jurt J, et al Postoperative ileus in an enhanced recovery pathway‐a retrospective cohort study. Int. J. Colorectal. Dis. 2017; 32(05): 675–81. [DOI] [PubMed] [Google Scholar]
- 13. Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta‐analysis. JAMA Surg. 2014; 149(12): 1224–30. [DOI] [PubMed] [Google Scholar]
- 14. Halabi WJ, Kang CY, Nguyen VQ, et al Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg. 2014; 149(02): 130–6. [DOI] [PubMed] [Google Scholar]
- 15. daSilva M, Lomelin D, Tsui J, Klinginsmith M, Tadaki C, Langenfeld S. Pain control for laparoscopic colectomy: an analysis of the incidence and utility of epidural analgesia compared to conventional analgesia. Tech. Coloproctol. 2015; 19(09): 515–20. [DOI] [PubMed] [Google Scholar]
- 16. Liu H, Hu X, Duan X, Wu J. Thoracic epidural analgesia (TEA) vs. patient controlled analgesia (PCA) in laparoscopic colectomy: a meta‐analysis. Hepatogastroenterology 2014; 61(133): 1213–9. [PubMed] [Google Scholar]
- 17. Shum NF, Choi HK, Mak JC, Foo DC, Li WC, Law WL. Randomized clinical trial of chewing gum after laparoscopic colorectal resection. Br. J. Surg. 2016; 103(11): 1447–52. [DOI] [PubMed] [Google Scholar]
- 18. Schwenk W, Böhm B, Haase O, Junghans T, Müller JM. Laparoscopic versus conventional colorectal resection: a prospective randomised study of postoperative ileus and early postoperative feeding. Langenbecks Arch. Surg. 1998; 383(01): 49–55. [DOI] [PubMed] [Google Scholar]
- 19. Fujii S, Ishibe A, Ota M, et al Short‐term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg. Endosc. 2014; 28(02): 466–76. [DOI] [PubMed] [Google Scholar]
- 20. Ng WQ, Neill J. Evidence for early oral feeding of patients after elective open colorectal surgery: a literature review. J. Clin. Nurs. 2006; 15(06): 696–709. [DOI] [PubMed] [Google Scholar]
- 21. Carmichael JC, Keller DS, Baldini G, et al Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon Rectum 2017; 60(08): 761–84. [DOI] [PubMed] [Google Scholar]
- 22. Wolthuis AM, Bislenghi G, Fieuws S, de Buck van Overstraeten A, Boeckxstaens G, D'Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta‐analysis. Colorectal Dis. 2016; 18(01): O1–O9. [DOI] [PubMed] [Google Scholar]
- 23. Northover JMA. Intestinal surgery. In: Postoperative complications in surgery. Eds Alan V Pollock, Mary Evans, Blackwell scientific publications 1991 London.
- 24. Artinyan A, Nunoo‐Mensah JW, Balasubramaniam S, et al Prolonged postoperative ileus‐definition, risk factors, and predictors after surgery. World J. Surg. 2008; 32(07): 1495–500. [DOI] [PubMed] [Google Scholar]
- 25. Vather R, Josephson R, Jaung R, Robertson J, Bissett I. Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 2015; 157(04): 764–73. [DOI] [PubMed] [Google Scholar]
- 26. Vather R, Josephson R, Jaung R, Kahokehr A, Sammour T, Bissett I. Gastrografin in prolonged postoperative ileus: a double‐blinded randomized controlled trial. Ann. Surg. 2015; 262(01): 23–30. [DOI] [PubMed] [Google Scholar]
- 27. Todd SR, Marshall GT, Tyroch AH. Acute gastric dilatation revisited. The American Surgeon 2000; 65(8): 709–10. [PubMed] [Google Scholar]
- 28. Magee C, Blackwell V, Travis S. Toxic dilatation of the colon. Medicine 2015; 43(3): 171–3. [Google Scholar]
- 29. Frager DH, Baer JW, Rothpearl A, Bossart PA. "Distinction between postoperative ileus and mechanical small‐bowel obstruction: value of CT compared with clinical and other radiographic findings". AJR Am. J. Roentgenol. 1995; 164(4): 891–4. [DOI] [PubMed] [Google Scholar]
- 30. Jangjoo A, Mohammadipoor F, Fazel A, Mehrabi Bahar M, Aliakbarian M, Jabbari Nooghabi M. The role of nasogastric intubation on postoperative gastrointestinal function in patients with obstructive jaundice. Indian J. Surg. 2012; 74(5): 376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koukouras D, Mastronikolis NS, Tzoracoleftherakis E, Angelopoulou E, Kalfarentzos F, Androulakis J. The role of nasogastric tube after elective abdominal surgery. Clin. Ter. 2001; 152: 241–4. [PubMed] [Google Scholar]
- 32. Cutillo G, Maneschi F, Franchi M, Giannice R, Scambia G, Benedetti‐Panici P. Early feeding compared with nasogastric decompression after major oncologic gynecologic surgery: a randomized study. Obstet. Gynecol. 1999; 93: 41–5. 10.1016/S0029-7844(98)00401-3.]. [DOI] [PubMed] [Google Scholar]
- 33. Wittbrodt E. The impact of postoperative ileus and emerging therapies. Pharm. Treatment 2006; 31: 39–59. [Google Scholar]


