Abstract
Sarcopenia, which is the loss of muscle mass and strength that occurs with aging, involves imbalanced muscle protein turnover (i.e., protein breakdown exceeding synthesis), which in turn exacerbates other clinical conditions such as type 2 diabetes mellitus, obesity, osteoporosis, and cancer, thereby worsening the quality of life in older adults. This imbalance is attributed in part to the resistance of aged muscle to anabolic stimuli such as dietary protein/amino acids and resistance exercise known as anabolic resistance. Despite research efforts, no practical therapeutics have been successfully discovered possibly because of a lack of understanding of the dynamic nature of muscle protein, and the use of indirect assessments of muscle mass. Herein, we briefly discuss the regulation of protein turnover in response to the abovementioned anabolic stimuli with respect to anabolic resistance and optimal protein intake, followed by methodological considerations for advancing sarcopenia research, including assessments of muscle mass and dynamics.
Keywords: Sarcopenia, Metabolism, Essential amino acids, Stable isotope labeling
INTRODUCTION
Sarcopenia, the age-related degenerative loss of muscle mass and function, is considered a muscle disease (i.e., muscle failure)1) and is central to functional and metabolic alterations in various clinical conditions such as critical illness (e.g., burn injury and cancer), chronic diseases (e.g., heart failure), insulin resistance, obesity, and osteoporosis.2) Therefore, discovering effective therapeutic means to counteract sarcopenia progression is of utmost importance to improve the quality of life in older adults and is a major target for drug development; however, efforts have not yet led to clinically meaningful success.3) The etiology of sarcopenia is multifactorial, including alterations in hormones and sex steroids, physical inactivity, and comorbidities.4) It is, therefore, difficult to understand the underlying mechanism(s) at molecular and cellular levels, which may explain the lack of meaningful success in the development of effective drugs to treat sarcopenia. Regardless of the complexity of etiology, sarcopenia is the direct result of dysregulation in muscle proteostasis that is maintained through orchestrated changes in the rates of protein synthesis and breakdown in response to various physiological challenges.5) While it is important to appreciate the dynamic nature of the proteome, most previous studies largely depend on “snap-shot” information obtained through molecular and cellular biological tools or -omics data that lack information on the actual rates of muscle protein kinetics.6,7) Furthermore, the invasiveness of muscle biopsy required to assess muscle protein dynamics is a burden that impedes subjects participating in sarcopenia research. In this regard, stable isotope tracer techniques are helpful as they provide information on protein dynamics in vitro and in vivo in both animal and human studies.8,9) Furthermore, it is important to accurately determine muscle mass to evaluate the efficacy of therapeutic candidates (e.g., nutrition, exercise, and/or drugs) using minimally invasive techniques; however, no commonly used methods (such as dual-energy X-ray absorptiometry [DEXA]) directly assess muscle mass.10,11) Therefore, this short review discusses the following topics: (1) regulation of muscle protein dynamics in response to anabolic stimuli such as resistance exercise and amino acid (AA)/protein nutrition, (2) anabolic resistance to those anabolic stimuli in older adults, (3) importance of dietary protein or balanced essential AAs (EAA) for effective muscle protein synthesis (MPS), and (4) methodological considerations for advancing medical research in sarcopenia, including the critical role of stable isotope tracer methodologies to assess muscle protein dynamics and muscle mass.
REGULATION OF MUSCLE PROTEIN KINETICS: EFFECTS OF RESISTANCE EXERCISE AND DIETARY PROTEINS/AMINO ACIDS
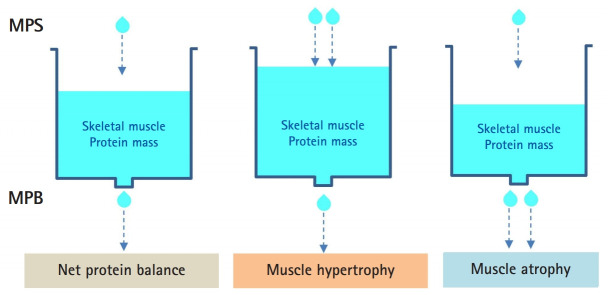
The muscle protein pool is in a constant state of turnover (i.e., protein synthesis and protein breakdown). Thus, the muscle protein pool (mass) is determined by the balance between rates of protein synthesis and breakdown. As long as the two rates are constant, muscle mass will not change, regardless of the actual rates. If the MPS rate exceeds that of breakdown, muscle mass will hypertrophy over time. In contrast, muscle atrophy will occur in the opposite state (i.e., protein breakdown > synthesis) (Fig. 1).
Fig. 1.
Muscle (protein) mass is regulated by the balance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB). The pool size of the muscle protein (i.e., mass) is represented as the volume in the water tank. Muscle hypertrophy is defined as MPS > MPB and muscle atrophy as MPS < MPB.
Resistance exercise and dietary EAA/protein are the two most prominent anabolic stimuli. In the resting fasted state, muscle protein breakdown (MPB) typically exceeds MPS, implying a net negative protein balance.12,13) Resistance exercise stimulates muscle protein turnover, with MPS increasing to a greater extent than MPB, leading to an improvement in the net protein balance. However, the net protein balance remains negative (MPB > MPS) because MPB remains greater than MPS.14) Therefore, resistance exercise alone does not lead to a net positive protein balance (i.e., muscle hypertrophy) until the provision of nutrients, particularly high-quality dietary protein or balanced EAAs.15-18) In contrast, dietary protein alone can induce a net positive protein balance (i.e., anabolic response: MPS > MPB) even without resistance exercise,15) although the anabolic effect is relatively transient (approximately 2–3 hours post-feeding).19) The anabolic response to dietary protein or AAs is achieved by stimulating MPS with no apparent change in MPB20-22) after the intake of AAs/proteins. This may not be the case following the intake of mixed meals containing proteins; however, no definite evidence with respect to MPB is currently available.23-25) The stimulatory effect of dietary protein consumption on MPS is attributable to the EAA component because the consumption of non-essential AAs (NEAAs) alone or with EAAs failed to further stimulate MPS.26) Resistance exercise can amplify15) and prolong anabolic responses to dietary protein/EAA.14)
Anabolic Resistance to Dietary Protein/EAA and Resistance Exercise in Older Adults with Sarcopenia
It was once believed that age-related sarcopenia resulted from alterations in protein kinetics in the basal fasted state.27,28) However, using state-of-the-art stable isotope tracer methodologies with muscle biopsy and arteriovenous balance techniques, it was demonstrated that no difference in basal fasted protein kinetics between older adults with sarcopenia and normal healthy young adults exists.28) Instead, the efficiency of stimulation of net muscle protein synthetic response to a small EAA intake was attenuated in older adults compared with that in healthy young adults (intake of 6.7 g EAA), a phenomenon termed anabolic resistance.20) However, the attenuated response to dietary protein/EAA was rescued when the amount of EAA intake (contained in beef steak) was doubled.29) Similarly, anabolic resistance occurs in response to resistance exercise in older adults with sarcopenia. For example, Kumar et al.30) determined MPS after resistance exercise at a wide range of intensities while holding the volume of exercise constant in young and older subjects. They found that MPS increased linearly as a function of intensity until 60% repetition maximum (a maximal weight to be lifted once) in both age groups, but to a greater extent in young subjects than in older subjects at all intensities. Anabolic resistance could be partly because of the attenuated activation of mammalian target of rapamycin complex 1 (mTORC1) in response to anabolic stimuli such as dietary protein/EAA27) or resistance exercise.30) Consistent with this notion, increasing the proportion of leucine in AA mixtures overcomes the blunted anabolic response in rest and post-exercise conditions in older adults.31,32) Although leucine may have a therapeutic potential to counteract muscle wasting owing to its potency in activating mTORC1, the activation of mTORC1 is only one of several important components required for the complete synthesis of new proteins, including the availability of precursor AAs.
All AAs Work Together to Make New Proteins
Appreciation of the process of protein synthesis makes clear the requirement that all AA precursors must be present in adequate quantities to produce a protein. The requirement for all AAs can be envisioned with the following analogy. If you bought a sports car, you can drive at 100 mph as long as you have gasoline in the tank. However, you cannot go without gasoline (i.e., no AAs) despite having a powerful engine (i.e., a fully activated mTORC1). In short, to make new proteins, all AAs required for protein synthesis need to be available. As discussed above, because the consumption of NEAAs does not lead to the stimulation of MPS26) and because they are sufficiently produced endogenously, the consumption of balanced EAA should be mainly considered. Consistent with this notion, no clinical studies have shown the positive effects of leucine supplementation on lean body mass and strength, particularly in older adults.33,34) In contrast, EAA or protein supplementation improved lean body mass in older adults, with35) or without exercise training.36) Moreover, at the whole-body level, the consumption of balanced EAA was more effective in inducing a greater anabolic response than that of protein in young37) and older adults.38) However, leucine alone could have nutraceutical potential in counteracting sarcopenia if the following two criteria are met: (1) acceleration of protein breakdown drives loss of muscle mass and (2) leucine effectively inhibits protein breakdown. First, the underlying kinetic mechanism may vary depending on muscle-wasting conditions (e.g., sarcopenia vs. cachexia). Cachexia is a much stronger driver of accelerated MPB than sarcopenia. Thus, it is important to determine protein kinetics to understand if the underlying mechanisms reside in the alteration of protein breakdown or synthesis. Second, an anti-proteolytic potential of leucine that involves mTORC1 activation, which in turn suppresses autophagy through the phosphorylation of an important autophagy initiating kinase, Unc-51-like autophagy-activating kinase 1 (ULK1), has been reported.39,40) However, its quantitative contribution to muscle wasting remains to be determined.
Optimal Dietary Protein Intake for Maximal Anabolic Response
Protein dose-response studies in older adults have largely been conducted with protein or AA alone.41,42) However, most of these studies only quantified the synthesis side of the protein balance equation. In this respect, the optimal amount of protein, i.e., the minimum amount of protein that induces a maximal anabolic response, ranges from 20 to 35 g per meal or more specifically 0.24 g protein/kg body weight per meal for healthy young adults.43) The corresponding amount for healthy older adults is 0.40 g protein/kg body weight per meal, reflecting 70% anabolic resistance.41) Based on these data, distributing the total amount of protein evenly throughout the day rather than the more conventional approach of consuming one large meal containing most of the dietary protein (typically dinner) may provide a near-maximal anabolic response per meal.44) For example, if an older adult weighing 70 kg consumes 1.2 g protein/kg/day (84 g/day), corresponding to 1.5 times the recommended dietary allowance (0.8 g/kg/day),45) a typical distribution pattern might be 20% (0.24 g/kg or 16.8 g per meal) at breakfast, 30% (0.36 g/kg or 25.2 g per meal) at lunch, and 50% (0.6 g/kg or 42 g per meal) at dinner. With this uneven pattern of protein intake, a near-maximal anabolic response can occur only at dinner (above 0.4 g/kg per meal).41) However, if consumed evenly throughout the day (one-third per meal), the same person can achieve maximal anabolic response by consuming the optimal 0.4 g/kg at every meal. While this theory appears to be logical, the optimal dose (0.4 g/kg per meal) may be underestimated in the real world for several reasons. First, the optimal protein dose was based on the assessment of the anabolic response to increasing doses of high-quality (animal source) protein,41) whereas a normal diet contains proteins with varying degrees of quality. Second, for most (>95%) older adults to achieve maximal anabolic response, they may need to consume approximately 0.6 g protein/kg/meal (3 meals × 0.6 g/kg/meal = approximately 1.8 g/kg/day).23) Third, and most importantly, people consume most protein in the context of mixed meals and not in isolation, which induces different physiological responses such as higher insulin and lower EAA responses in the blood for a given amount of protein or AA consumed.23-27,46) Consistent with this notion, we showed an increased whole-body anabolic response following the consumption of protein above the amount considered optimal.27,28) Furthermore, the response was dose dependent (dose range: approximately 6.4–91.7 g per meal), with an increasing protein intake in the context of mixed meals largely improving the net protein balance by suppressing protein breakdown via insulin-dependent and insulin-independent pathways.23,24) The results of a 12-week chronic study support our findings by showing a close correlation between lean body mass and the amount of protein intake within a wide range of protein intake.47) However, the role of the suppression of protein breakdown in inducing an anabolic response in muscle remains to be confirmed because the rate of MPB with increasing amounts of protein or EAA intake has not been directly measured. Furthermore, despite its major role in inducing an anabolic response, it is still unclear if inducing an anabolic response by slowing protein turnover, as shown in our previous studies,23-27,46) is optimal for muscle health and the quality of life in older adults. A high rate of protein turnover presumably replaces older proteins with new functional proteins. Thus, further investigations of the optimal total amount of dietary protein intake and the pattern of consumption are warranted.
METHODOLOGICAL CONSIDERATIONS FOR ADVANCING MEDICAL RESEARCH IN SARCOPENIA: A ROLE FOR STABLE ISOTOPE TRACER METHODOLOGIES
To better understand the pathological alterations in sarcopenic skeletal muscle and test the efficacy of potential therapeutics developed to counteract sarcopenia, two important variables need to be accurately assessed, namely (1) muscle protein kinetics and (2) muscle mass. However, quantifying these parameters presents several challenges. First, the assessment of muscle protein turnover requires a minimum of one, and often several, muscle biopsies, which is invasive and may limit participant recruitment. Second, despite the critical importance of an accurate assessment of changes in muscle mass owing to therapeutic interventions (nutrition, exercise, and/or drugs), the approaches commonly utilized in clinical research such as DEXA are indirect measurements of muscle mass, which are highly susceptible to errors.8,48) However, these challenges can be overcome by recent technological advancements with minimal invasiveness, including (1) a deuterium-labeling method combined with “virtual” biopsy (no muscle biopsy) to determine muscle protein dynamics and (2) a D3-creatine dilution method to directly determine muscle mass. In the following section, we will briefly discuss (1) the basic principles of determining muscle protein turnover, (2) the deuterium oxide labeling method combined with virtual biopsy, and (3) the D3-creatine dilution method.
Exploration of Protein Turnover Dynamics
To maintain proteostasis in the body, all proteins are in varying rates of turnover, resulting in a dynamic balance between protein synthesis and breakdown (Fig. 1). In normal conditions, muscle mass is maintained through a close match between the rates of protein synthesis and breakdown in a daily basis. That is, the portion of the day in which protein balance is positive as the result of anabolic stimuli, such as exercise and nutritional intake, is closely balanced with the portion of the day in which protein balance is negative as the result of catabolic stimuli such as overnight fasting and/or post-absorptive periods. However, in muscle-wasting conditions such as sarcopenia, negative protein balance (i.e., loss of muscle mass) predominates owing to protein breakdown exceeding protein synthesis over time. Hence, an understanding of the dynamic nature of protein turnover in the body is of critical importance for elucidating in vivo proteostasis, which can be assessed by stable isotope tracers.
The MPS rate is generally assessed using stable isotope tracers and is reported as a fractional term, namely the fractional synthetic rate (FSR, %/unit time). Briefly, following the administration of a tracer AA (i.e., phenylalanine tracer) that monitors the fate of the trace AA into the body (typically intravenously), muscle protein FSR is estimated by determining the rate of tracer phenylalanine incorporation into muscle protein over time. To obtain the absolute rate of MPS, the muscle protein FSR is multiplied by the pool size (i.e., muscle protein mass), which underpins another reason to correctly determine muscle (protein) mass. In contrast, the rate of MPB is determined similarly in principle, except that the precursor of free AAs is muscle protein. More comprehensive information regarding the principles of stable isotope tracer methodology is available elsewhere.8,9)
Assessment of Muscle Dynamics: Deuterium Oxide and Virtual Biopsy Method
The assessment of muscle protein FSR using the stable isotope tracer methodology typically requires muscle tissue obtained by needle biopsy. However, muscle biopsy is invasive, thus limiting subjects’ willingness to participate. Furthermore, obtaining muscle tissue by biopsy may be difficult in older adults with sarcopenia owing to their greatly reduced muscle mass. The heavy water labeling method combined with “virtual biopsy” allows researchers to avoid problems of obtaining muscle samples.49,50) The method comprises two parts (Fig. 2), namely (1) heavy water labeling of muscle proteins and (2) measurement of labeling of a circulating protein almost exclusively released from skeletal muscle into the blood (e.g., muscle creatine kinase, mCK). To assess the MPS rate using this method, individuals consume a small amount (approximately 100 mL) of heavy water (deuterium oxide, 2H2O) daily from a few days to months. Deuterium from heavy water rapidly equilibrates with the existing body water pool, both of which are then rapidly exchanged with free AAs via transamination and deamination reactions51) and then incorporated into muscle proteins, including mCK. The advantages of measuring mCK include (1) consistent detection in the blood, (2) exclusive derivation from muscle (>90%), and (3) a shorter half-life than that in muscle (approximately 2 months) (Fig. 1).49,52) Therefore, circulating mCK levels reflect the levels in muscle with respect to deuterium labeling and thus the protein FSR of mCK.49) Furthermore, mCK FSR is well equated with the muscle contractile protein FSR.49)
Fig. 2.
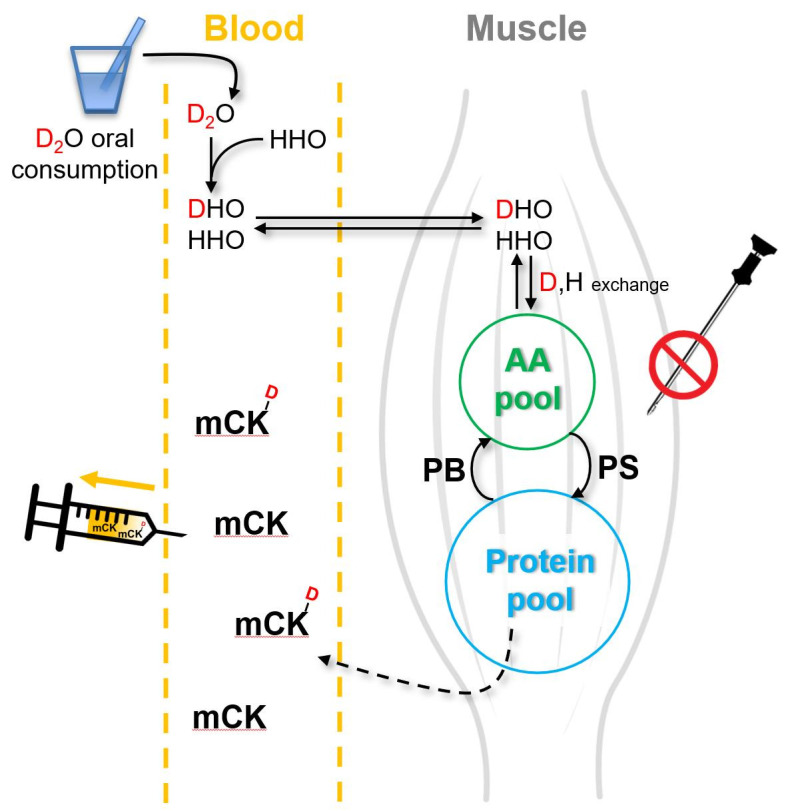
Heavy water labeling combined with virtual biopsy method. Heavy water (2H2O, D2O, or DDO) that is consumed rapidly equilibrates with water in the body (1H2O or 1H1HO) for hydrogen, resulting in deuterium-labeled AAs (the number of deuterium labels vary depending on AAs and thus protein) that are incorporated into muscle proteins, including the muscle isoform of creatine kinase (mCK), which is released into the blood. Circulating mCK is extracted for measuring the amount of deuterium-labeled AA incorporation over time using mass spectrometry. The muscle fractional synthesis rate of mCK is a direct reflection of the total muscle protein fractional synthesis rate. AA, amino acid; PB, protein breakdown; PS, protein synthesis.
Direct Assessment of Skeletal Muscle Mass: The D3-Creatine Dilution Method
The accurate measurement of changes in muscle mass is crucial for assessing the efficacy of potential therapeutics for sarcopenia. DEXA, electrical impedance, computed tomography, and magnetic resonance imaging have been commonly utilized10) to measure muscle mass. However, none of these techniques directly measures skeletal muscle mass because muscle mass is not distinguished from bone and tendon-like connective tissues and the results are significantly affected by hydration status.10) The recently described D3-creatine dilution method more directly estimates muscle mass via the dilution principle of deuterium-labeled creatine (i.e., D3-creatine). The basic principle of the method is based on the calculation of the magnitude of the dilution of D3-creatine in all skeletal muscles in the body (reflected in urine creatinine enrichment) following the oral consumption of a known small amount of D3-creatine.53,54) Briefly, orally consumed creatine mostly enters skeletal muscle via a creatine transporter against a concentration gradient. In muscles, creatine is converted to creatinine by irreversible, non-enzymatic dehydration, whereupon the creatinine is released into the blood and is excreted by the urinary system (Fig. 3). The advantage of using the D3-creatine dilution method to measure muscle mass is that approximately 98% of the total creatine pool is found in skeletal muscle and that creatine is exclusively turned over in muscle and converted to creatinine. Thus, urine enrichment of creatinine reflects creatine enrichment in muscle, enabling the calculation of the total creatine pool size, which is directly related to muscle mass.50)
Fig. 3.
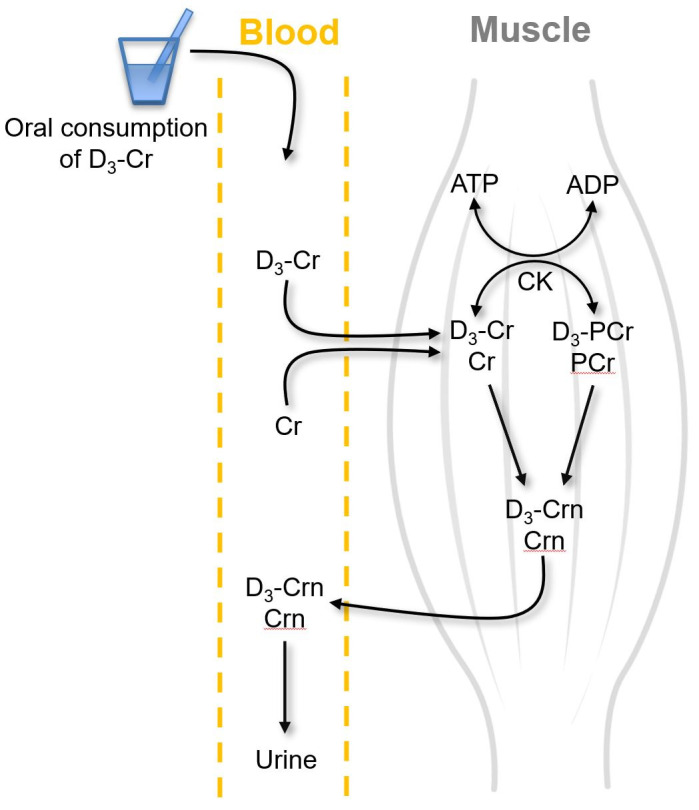
D3-creatine dilution method. Orally consumed D3-creatine (D3-Cr) is absorbed into the circulation and transported into muscles, where it equilibrates with existing unlabeled Cr (i.e., dilution of D3-Cr in unlabeled Cr). Both labeled and unlabeled Cr are phosphorylated to form phosphocreatine (PCr) via the enzymatic action of creatine kinase (CK). Both Cr and PCr are spontaneously converted to creatinine (both labeled and unlabeled Crn) at a constant rate. Crn (both labeled and unlabeled) is then released to the blood and ultimately excreted in the urine. The urine Crn enrichment (ratio of labeled to unlabeled Crn) is equal to the D3-Cr enrichment in muscle. Information on the doses of orally consumed D3-creatine, urine Crn enrichment, and Cr mass per kg muscle mass is used to calculate the muscle mass.
In summary, the heavy water labeling method combined with virtual biopsy and D3-creatine dilution method provided precise, accurate, and convenient tools to analyze protein turnover and muscle mass, respectively, with minimal invasiveness. The application of these techniques can facilitate and advance clinical muscle research, particularly in the field of sarcopenia.
SUMMARY AND CONCLUSIONS
Sarcopenia, which is the progressive loss of muscle mass and strength with aging, is a public health problem affecting the quality of life of older adults. While research efforts to reverse sarcopenia progression have heavily focused on “static” molecular and cellular mechanisms, improved understanding of “kinetic” mechanisms is needed as the muscle protein pool is in a dynamic state of constant turnover. The determination of muscle protein kinetics traditionally requires muscle biopsy, which slows sarcopenia research. In this regard, heavy water labeling combined with “virtual” biopsy is an important tool for determining muscle protein kinetics in free-living conditions without requiring an actual muscle biopsy. Furthermore, the accurate assessment of muscle mass is required to determine the efficacy of potential therapeutics. To date, muscle mass has been indirectly estimated using methods such as DEXA. These indirect methods can be replaced by a direct muscle mass assessment using the D3-creatine dilution method. The virtual biopsy and D3-creatine methods can use stable isotope tracers to quantify the dynamic nature of the muscle proteome and accurately measure muscle mass. These new methods will allow the development of therapeutics based on a quantitative understanding of the physiological basis of sarcopenia.
Footnotes
CONFLICT OF INTEREST
Dr. Wolfe is a shareholder in Essential Blends LLC and The Amino Co. Inc.
FUNDING
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2018R1D1A1B07051053) and a Korea Research Fellowship (KRF) funded by the Ministry of Sciences and ICT and National Research Foundation of Korea (No. 2019H1D3A1A01071043).
AUTHOR CONTRIBUTIONS
Conceptualization, IYK; Funding acquisition, IYK; Writing-original draft, IYK, SP, JWJ; Writing-review & editing, SP, JWJ, IYK, RRW.
REFERENCES
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 3.Saitoh M, Ishida J, Ebner N, Anker SD, Springer J, von Haehling S. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. JCSM Clin Rep. 2017;2:e00037 [Google Scholar]
- 4.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 5.Kim IY, Deutz NE, Wolfe RR. Update on maximal anabolic response to dietary protein. Clin Nutr. 2018;37:411–8. doi: 10.1016/j.clnu.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab. 2015;308:E1056–65. doi: 10.1152/ajpendo.00481.2014. [DOI] [PubMed] [Google Scholar]
- 7.Hellerstein MK. New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng. 2004;6:85–100. doi: 10.1016/j.ymben.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Kim IY, Suh SH, Lee IK, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med. 2016;48:e203. doi: 10.1038/emm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2005. [Google Scholar]
- 10.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–83. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 11.Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74:355–66. doi: 10.1017/S0029665115000129. [DOI] [PubMed] [Google Scholar]
- 12.Loenneke JP, Loprinzi PD, Murphy CH, Phillips SM. Per meal dose and frequency of protein consumption is associated with lean mass and muscle performance. Clin Nutr. 2016;35:1506–11. doi: 10.1016/j.clnu.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Simmons E, Fluckey JD, Riechman SE. Cumulative muscle protein synthesis and protein intake requirements. Annu Rev Nutr. 2016;36:17–43. doi: 10.1146/annurev-nutr-071813-105549. [DOI] [PubMed] [Google Scholar]
- 14.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 15.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–9. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 16.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 17.Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 18.Tipton KD, Elliott TA, Cree MG, Aarsland AA, Sanford AP, Wolfe RR. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab. 2007;292:E71–6. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 19.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532(Pt 2):575–9. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 21.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003;284:E76–89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 22.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308:E21–8. doi: 10.1152/ajpendo.00382.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016;310:E73–80. doi: 10.1152/ajpendo.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IY, Schutzler S, Schrader AM, Spencer HJ, Azhar G, Wolfe RR, et al. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: a randomized-controlled trial. Clin Nutr. 2018;37:488–93. doi: 10.1016/j.clnu.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 28.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–6. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–7. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 32.Murphy CH, Saddler NI, Devries MC, McGlory C, Baker SK, Phillips SM. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lower- and higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104:1594–606. doi: 10.3945/ajcn.116.136424. [DOI] [PubMed] [Google Scholar]
- 33.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, et al. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009;89:1468–75. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 34.Leenders M, van Loon LJ. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr Rev. 2011;69:675–89. doi: 10.1111/j.1753-4887.2011.00443.x. [DOI] [PubMed] [Google Scholar]
- 35.Ten Haaf DSM, Eijsvogels TMH, Bongers CCWG, Horstman AMH, Timmers S, de Groot LCPGM, et al. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10:298–310. doi: 10.1002/jcsm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–7. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S, Church DD, Azhar G, Schutzler SE, Ferrando AA, Wolfe RR. Anabolic response to essential amino acid plus whey protein composition is greater than whey protein alone in young healthy adults. J Int Soc Sports Nutr. 2020;17:9. doi: 10.1186/s12970-020-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim IY, Park S, Smeets ETHC, Schutzler S, Azhar G, Wei JY, et al. Consumption of a specially-formulated mixture of essential amino acids promotes gain in whole-body protein to a greater extent than a complete meal replacement in older women with heart failure. Nutrients. 2019;11:1360. doi: 10.3390/nu11061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanchi NE, Nicastro H, Lancha AH., Jr Potential antiproteolytic effects of L-leucine: observations of in vitro and in vivo studies. Nutr Metab (Lond) 2008;5:20. doi: 10.1186/1743-7075-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 42.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–8. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 44.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung HW, Kim SW, Kim IY, Lim JY, Park HS, Song W, et al. Protein intake recommendation for Korean older adults to prevent sarcopenia: expert consensus by the Korean Geriatric Society and the Korean Nutrition Society. Ann Geriatr Med Res. 2018;22:167–75. doi: 10.4235/agmr.18.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim IY, Shin YA, Schutzler SE, Azhar G, Wolfe RR, Ferrando AA. Quality of meal protein determines anabolic response in older adults. Clin Nutr. 2018;37(6 Pt A):2076–83. doi: 10.1016/j.clnu.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307:47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duchowny KA, Peters KE, Cummings SR, Orwoll ES, Hoffman AR, Ensrud KE, et al. Association of change in muscle mass assessed by D3-creatine dilution with changes in grip strength and walking speed. J Cachexia Sarcopenia Muscle. 2020;11:55–61. doi: 10.1002/jcsm.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankaran M, King CL, Angel TE, Holmes WE, Li KW, Colangelo M, et al. Circulating protein synthesis rates reveal skeletal muscle proteome dynamics. J Clin Invest. 2016;126:288–302. doi: 10.1172/JCI79639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellerstein M, Evans W. Recent advances for measurement of protein synthesis rates, use of the 'Virtual Biopsy' approach, and measurement of muscle mass. Curr Opin Clin Nutr Metab Care. 2017;20:191–200. doi: 10.1097/MCO.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 51.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006;1760:730–44. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 52.Apple FS, Rogers MA, Ivy JL. Creatine kinase isoenzyme MM variants in skeletal muscle and plasma from marathon runners. Clin Chem. 1986;32(1 Pt 1):41–4. [PubMed] [Google Scholar]
- 53.Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark RV, Walker AC, O'Connor-Semmes RL, Leonard MS, Miller RR, Stimpson SA, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985) 2014;116:1605–13. doi: 10.1152/japplphysiol.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]