Abstract
Vivax malaria which had been highly prevalent in Korea disappeared rapidly from the 1960s to 1984 when domestic occurrence of cases stopped. However, malaria reemerged in 1993 near the demilitarized zone (DMZ) bordering with North Korea. The number of patients thereafter increased exponentially year after year totaling 35,526 cases by the end of 2015. A small number of cases (1 - 53 patients annually) also occurred among the United States military personnel camping in Korea. However, after the 2010s the number of annual malaria cases has been decreasing slowly in Korea. Several reports on malaria situation in North Korea described high malaria prevalence after 1997 which peaked during 1999 - 2002 and has been decreasing thereafter. At the beginning of the reemergence, the majority of cases (60 - 90%) were soldiers aged 20 - 25 years camping around the northern parts of Gyeonggi-do and Gangwon-do (Province), Korea just facing the DMZ. However, as the outbreak continued more civilians were infected. The course of illness was relatively mild, and chemotherapy with chloroquine in combination with primaquine was successful in most of the patients. Mass chemoprophylaxis combined with mosquito control activities greatly contributed to the decline of malaria situation among Korean military soldiers.
Keywords: Vivax malaria, Plasmodium vivax, History, Current status, Epidemiology
Domestic outbreak and endemic of malaria
1. From Independence (1945) to the 1980s
The malaria that is naturally prevalent in Korea is vivax malaria, and the parasite responsible is Plasmodium vivax. The disease has been widespread in Korea for many years; however, it was first recorded in the modern literature in 1913 [1]. In 1927, there were reports that the infection rate was 1.3 - 16.1% among elementary and middle school students in Chuncheon and Cheorwon [2] areas, and even with a slight lull, the trend seems to have persisted after 1945. For instance, in 1949, 14.7% of the 3,983 students in three middle schools in Seoul answered that they had contracted malaria recently (in 1948) [3].
Malaria briefly disappeared for a short period subsequently, nonetheless after the Korean war in 1950, it recurred and continued until 1953, which was a typical form of so-called “war malaria” [4]. In a small village with a population of approximately 10,000 in Yangyang-gun, Gangwon-do, 1,032 patients (10.3%) were diagnosed with malaria between April and September of 1952. This was a total of more than 2,000 patients per year (20.6%) [4]. Another example was the onset of malaria in soldiers (Canadians) who returned to Canada after serving as part of the United Nations (UN) forces during the Korean War. Among 1,350 Canadian soldiers, 152 in 1952 had malaria between January and December [5]. This indicates an incidence rate for malaria of 11.3% (most of them likely to be patients with a long incubation period). Although “war malaria” somewhat improved due to the settling of residents, the supply of antimalarial drugs, and control of mosquitos, the outbreak of patients continued until 1960.
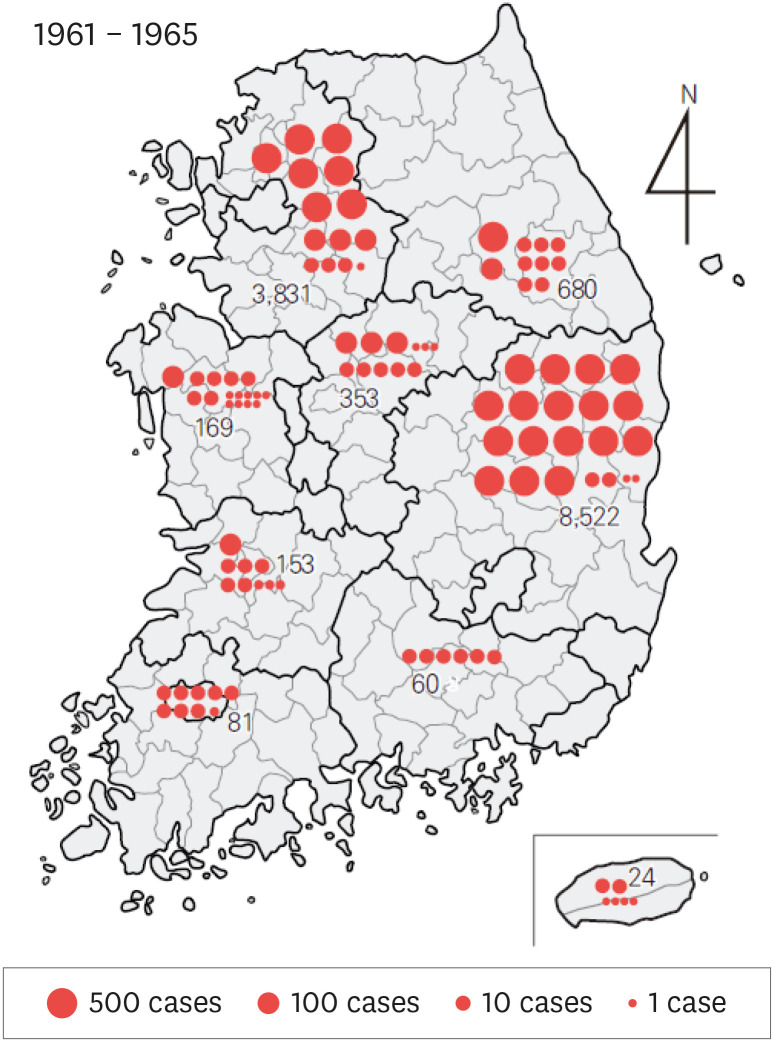
Recognizing this situation, the World Health Organization (WHO) and the Korean government (the Ministry of Health and Social Affairs at the time) jointly established the National Malaria Eradication Service (NMES) and initiated several programs for the treatment and management of malaria. The first project was a blood smear test collected from 18,697 residents in 278 villages (a total population of 166,241) among 41 cities and counties across 9 provinces in 1960. As a result, 212 people tested positive for malaria, resulting in a 1.1% positive rate. During this time, the region with the highest positive rate was Gyeongsangbuk-do (183 people, 2.5%), followed by Jeollabuk-do (9 people, 0.7%), Gyeongsangnam-do (8 people, 0.5%), Gyeonggi-do (8 people, 0.2%), and Chungcheongbuk-do (1 person, 0.2%). Another major project implemented by the NMES was the completion of passive case detection (PCD) between 1961 and 1965. This was a system to check the rate of malaria among patients with fever by blood collection and testing for the parasite. As a result, it was confirmed that within these five years, fever occurred in 13,929 (30.7%) of the 45,395 patients nationwide was caused by malaria [4]. The region with the highest positive rate was Gyeongsangbuk-do (41.5%), followed by Gangwon-do (32.7%), Gyeonggi-do (30.6%), Chungcheongbuk-do (20.6%), and Jeju-do (10.9%) (Fig. 1).
Figure 1. Geographical distribution (1961 - 1965) of Plasmodium vivax positive patients diagnosed via passive case detection (PCD) by the National Malaria Eradication Service. A total of 13,929 out of 45,395 subjects tested positive (30.7%).
Source: Paik et al. [4] (1966) Statistics from Ministry of Health and Social Affairs.
Due to the success of the NMES, transmission of malaria virtually ceased in most regions except for the northern and eastern part of Gyeonggi-do and the north of Gyeongsangbuk-do [4]. Afterwards, the papers that reviewed the trend of malaria [6,7] reported that the trend continued to decrease between the mid-1960s and the early 1970s and almost disappeared since the late 1970s. Even in the northern part of Gyeongsangbuk-do, where the infection continued the longest, the outbreak completely stopped. After 2 cases in 1984 [7]. No local cases of malaria were reported for 10 years until 1993. However, the distribution of the malaria vector mosquitos (Anopheles spp.) did not decrease significantly, and they existed in the previous high epidemic areas as well as rural areas of the country. Therefore, if P. vivax emerged again, the possibility of recurrence could not be ruled out. In particular, the possibility of malaria reemergence in Korea seemed to exist according to the malaria situation in North Korea.
2. From the 1990s to 2015 (The resurgence of malaria)
1) Beginning of the resurgence
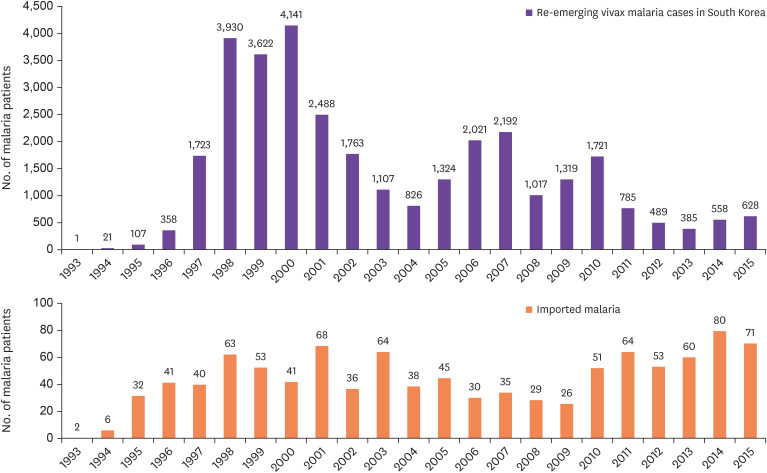
In Korea, indigenous vivax malaria was completely eradicated for approximately 10 years from 1984 to June 1993, and during this period only cases of imported malaria were at times diagnosed [8]. However, in July 1993, one patient with no foreign travel history was diagnosed with malaria near the demilitarized zone (DMZ) in northern Gyeonggi-do [9]. This was not transient, and thereafter, a total of 32,526 malaria cases (an average of 1,414 patients per year) occurred for 23 years until 2015 (Fig. 2). The first patient was a soldier in a military unit in northern Gyeonggi-do [9]. Fever and splenomegaly were observed, and several developmental stages of P. vivax were observed in blood smear test. Consequently, the patient was diagnosed with vivax malaria [9]. The patient was definitely contracted by malaria in Korea; however, it had been 10 years since the eradication of malaria in Korea; thus, there was no way to identify the source of infection. In May and June 1994, 10 - 11 months after the first patient was identified, two malaria patients [10], each from different military units nearby, were identified, strongly suggesting that this was not a transient phenomenon.
Figure 2. Re-emerging vivax malaria and imported malaria in Korea (1993 - 2015).
Source: Korea Centers for Disease Control and Prevention (KCDC) website.
2) Continuation of the resurgence and its' characteristics
The number of patients with resurgent vivax malaria continued to increase with 21 patients in 1994, 107 patients in 1995, and 358 patients in 1996, with steep increase to 1,723 patients in 1997, and for three years, between 1998 and 2000, the number of patients peaked with 3,930 patients in 1998; 3,622 patients in 1999; and 4,141 patients in 2000 (Fig. 2, 3).
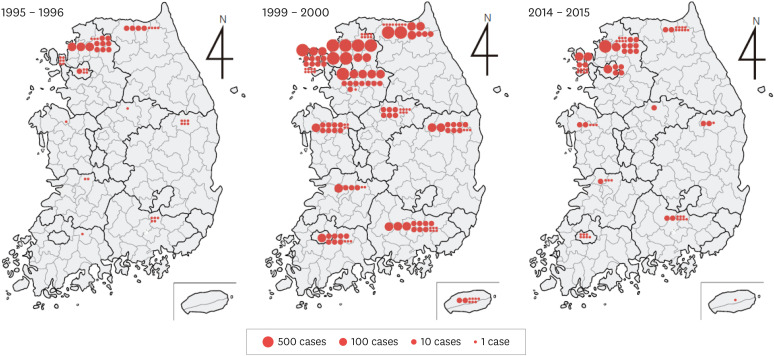
Figure 3. Local frequency of the malaria resurgence (1995 - 1996, 1999 - 2000 and 2014 - 2015). There were 465 cases in the early stages of the resurgence (1995 - 1996), 7,763 cases during the peak of the epidemic (1999 - 2000), and 1,186 cases in the lull period (2014 - 2015). Northern and western Gyeonggi-do as well as northern Gangwon-do were the main areas of epidemic. Cases that occurred in other regions were primarily from patients with a long incubation period and were either discharged soldiers or travelers from northern Gyeonggi-do and northern Gangwon-do.
Fortunately, the number of patients, after a slight decrease and increase, gradually decreased with 1,107 patients in 2003, 2,021 patients in 2006, 1,319 patients in 2009, 489 patients in 2012, and 628 patients in 2015 (Fig. 2) [8,11]. The malaria resurgence had very unusual characteristics in terms of the patients' occupations and ages. That is, the majority of the patients with resurgent malaria between 1993 and 1996 (100% in 1993, 90.5% in 1994, 93.5% in 1995, and 87.2% in 1996) were acting or discharged soldiers [8]. However, as the resurgence progressed, the number of civilian patients increased, exceeding 50% of the total number of patients (percentage of civilian patients: 38.2% in 2000, 50.6% in 2003, 63.2% in 2006, 60.8% in 2010, and 59.0% in 2013) [11]. The age distribution was wide among the civilian patients, ranging from 4 - 5 years children to adults over 60 years. This phenomenon showed that the resurgent malaria was initially prevalent in small areas, mainly around military units located near the DMZ; nonetheless, it gradually spread to a wider area.
3) Distribution and early infection sources of resurgent malaria
The main area of outbreak in the first four years of malaria resurgence was distributed from the northern Gyeonggi-do to the east and west along the DMZ, and most patients resided within 10 km of the DMZ [12]. Most of the cases (80.0%) occurred in northern Gyeonggi-do, especially in Yeoncheon-gun and Paju-gun, followed by Cheorwon-gun (12.3%), with some cases in Seoul, Gimpo, Pocheon, and Dongducheon [12]. A small number of cases occurred in Daegu/Gyeongsangbuk-do, Busan/Gyeongsangnam-do, Gwangju/Jeollanam-do, and Chungcheongbuk-do (Fig. 2). These cases were confirmed to be discharged soldiers who had served in military units near the DMZ within the past year [12]. Therefore, including these cases, most of the initial patients seem to have been infected during military service in the units near the DMZ. In particular, 20 cases from the first outbreak occurred near the DMZ in the north of Gyeonggi-do and northwest of Gangwon-do without exception [12]. Considering this, it is highly likely that a mosquito with blood from a malaria patient in North Korea near the DMZ, flew to the south and infected a Korean soldier. A normal travel distance for mosquitoes is known to be within a radius of 1 km. However, this distance may increase slightly if a mild wind blows from the north, and if the temperature and humidity are appropriate (such as when traveling along the river).
4) Trends of the resurgence
The malaria resurgence caused many changes in the affected areas over the course of 10 years. Initially, the main area of the epidemic was Paju-gun and Yeoncheon-gun in Gyeonggi-do and Cheorwon-gun in Gangwon-do. However, after 10 years of resurgence, additional regions such as Gimpo-si, Donducheon-si, Ganghwa-gun, and Ongjin-gun in Gyeonggi-do were involved, showing the trend of gradual spread towards the south and the west (Fig. 3). A large number of civilian patients outside the primary area of the epidemics were among those who were discharged from military units near the epidemic areas within the past year (cases after a long-term incubation period). Yet, the remainder were purely civilians with no history of travelling to countries affected by malaria. This suggested the possibility that the area of epidemics was gradually spreading. Cases of infection from blood transfusion were also reported [13,14].
However, the malaria resurgence appeared to be entering a lull period in the 2010s, with occurrence of less than 1,000 patients per year (Fig. 2). This could also mean that it was in the process of settling in some local epidemic areas in Korea (mainly the north of Gyeonggi-do, such as Paju-si and Ganghwa-gun). Although it is impossible to completely rule out the possibility of continued mutual spread involving South and North Korea, such possibility has greatly decreased. Rather, it is suspected that the secondary and tertiary infections continue to occur in the main endemic areas in Korea.
5) Malaria resurgence among the US soldiers
Recurrent vivax malaria continuously appeared among US military personnel stationed in Korea. The number of patients began with 1 in 1993 and rapidly increased to 11 in 1996 and 53 in 1999. Then, the number of cases gradually decreased to 45 in 2002, 18 in 2005, and 32 in 2007 [15]. Of the total 363 US military cases, 166 occurred (febrile paroxysm began) in Korea, and 197 after return to the US or being dispatched to another country [15]. Many of these are estimated to be cases with a long incubation period. Most of the patients with disease onset in Korea were soldiers stationed in the northern part of Gyeonggi-do, with the majority of cases in the military unit stationed near the DMZ in Paju [16].
6) Malaria epidemic in North Korea
It can be speculated in various circumstances that the malaria resurgence in Korea may have been caused by mosquitos infected in North Korea. However, in the early stages of resurgence, no data were available on the malaria situation in North Korea, thus it was difficult to infer the source of infection. The first official data on the malaria situation in North Korea was published on the WHO website, UN Pyeongyang office website, and the UN website between 2002 and 2005 [17]. Shortly thereafter, the WHO Pyeongyang office officially reported on the malaria situation in North Korea in conjunction with Mahidol University in Thailand [18]. According to this report, malaria in North Korea was extinguished in the 1970s and began to reemerge in 1998. However, we cannot rule out the possibility that malaria, which had been lull for some time, revived beginning in the early 1990s due to frequent flooding and deteriorated living standards.
The summary of data from the WHO website, previous studies [18], and the data presented by North Korean scholars at a conference in Shanghai, China is as follows. Malaria resurgence became a serious problem in 1997, yet no specific number of patients was recorded, and, in 1998, 2,100 malaria patients were first identified in Gangwon-do, Hwanghaenam-do, and Kaesong city [18]. The following year, in 1999, the number of patients rapidly increased to 95,960, followed by peaking with 204,428 patients in 2000, 295,570 in 2001, and 240,339 in 2002 [17]. Subsequently, the number significantly decreased with 46,251 patients in 2003 and 33,677 in 2004 [17], continuing with 11,507 in 2005, 9,353 in 2006, and 7,436 in 2007 (unofficial data). The main areas of the epidemic were Hwanghaebuk-do, Hwanghaenam-do, Kaesong city, and Gangwon-do in 2006 and 2007. The situation in Hwanghaenam-do and Gangwon-do improved while Pyeonganbuk-do was added as a new area of the epidemic, resulting in Hwanghaebuk-do, Kaesong city, and Pyeonganbuk-do as the primary areas to be managed (unofficial data).
7) Imported malaria
While the malaria resurgence began in 1993 and lasted for 23 years until 2015, the number of cases due to imported malaria from overseas travel gradually increased (Fig. 2). According to a report which collected data from 18 - 26 general hospitals across the country [7], 80 malaria cases were recorded from 1970 to 1985, with 35 falciparum malaria cases, 11 vivax malaria cases, 1 ovale malaria case, and 37 unknown malaria cases (4 cases of mixed infections). Between 1993 and 2015, when the Korea Centers for Disease Control and Prevention (KCDC) began receiving reports of imported malaria cases, a total of 1,082 cases were reported, and the majority of them were falciparum and vivax malaria cases (yearly average of 44.7 patients) [8,11].
Disease patterns of malaria in Korea
1. Clinical characteristics
1) Incubation period
In general, the incubation period of vivax malaria is known to be approximately 10 - 15 days, although in temperate regions, there are rare cases with long incubation periods of more than 10 months [19]. Vivax malaria in Korea is also a type of temperate zone malaria, and shows a very unique pattern in terms of its' incubation period [8,20]. In other words, the rate of patients with short incubation periods (within 1 month) and long incubation periods (7 - 12 months) are 1:3, showing that many patients have long incubation periods [8]. The mechanism of long-term incubation is unknown. A long incubation period may be necessary in the human body for P. vivax to survive. In the body of mosquitoes, P. vivax cannot survive for long periods during winter season, November to April, because mosquitoes are not active during winter season (some mosquitoes hibernate) and malaria may have difficulty to survive. Among mammals, only humans can be the host for human malaria. Thus, the symptom onset of vivax malaria (febrile attack) can begin in areas remote from endemic localities of malaria [17]. For instance, it is possible for a soldier bitten by an infected mosquito during military service to have febrile attack from malaria a year after being discharged and returning to his hometown. There were 152 cases who had late febrile attack due to long incubation periods among 1,350 UN troops who returned to Canada after the Korean war [5].
2) Clinical symptoms
Resurgent vivax malaria patients showed various clinical symptoms (Table 1). Approximately two-thirds of patients have the typical fever cycle of P. vivax (48 hours). For example, 21 out of 26 soldiers (80.8%) revealed typical 48-hour febrile attack (coldness, fever, and sweating), and 4 had initial irregular fever, which gradually changed to a 48-hour cycle of febrile attack [21]. In comparison, only 69 out of the 101 (68.3%) civilian patients (including discharged former soldiers) showed a typical 48-hour fever cycle [22]. Other clinical symptoms included 43 cases of splenomegaly (42.0%), 16 cases of hepatomegaly (15.8%), 84 cases of headache (83.2%), 43 cases of muscle pain (42.6%), 24 cases of nausea (23.8%), 24 cases of diarrhea (23.8%), 17 cases of vomiting (16.8%), 15 cases of cough (14.9%), and 9 cases of epigastric pain (8.9%) [22]. Conversely, symptoms that appeared in 341 patients at a university hospital located in Gyeonggi-do included 337 cases of fever (98.8%), 213 cases of chills (62.5%), 115 cases of headache (33.7%), 85 cases of muscle pain (24.9%), 61 cases of nausea (17.9%), 36 cases of vomiting (10.6%), 31 cases of abdominal pain (9.1%), and 14 cases of diarrhea (4.1%) [23].
Table 1. Major clinical symptoms and hematological findings of the reemerging vivax malaria in Korea.
| Major clinical symptoms (frequency, %) | Major hematological findings (frequency, %) | ||
|---|---|---|---|
| Fever | 98.8 - 100 | Thrombocytopenia | 61.5 - 99.7 |
| Chills | 62.5 - 100 | Hemoglobin reduction (Anemia) | 28.5 - 61.5 |
| Headache | 33.7 - 83.2 | Leukopenia | 19.9 - 50.0 |
| Muscle pain | 24.9 - 88.5 | Leukocytosis | 2.9 - 19.2 |
| Splenomegaly | 23.1 - 69.2 | Increased aminotransferases | 14.9 - 54.8 |
| Hepatomegaly | 7.7 - 15.8 | Increased bilirubin | 10.9 - 53.7 |
| Nausea | 17.9 - 23.8 | ||
| Vomiting | 10.6 - 16.8 | ||
| Cough | 14.9 | ||
| Diarrhea | 4.1 - 23.8 | ||
| Stomach pain | 8.9 - 9.1 | ||
3) Hematological properties
Anemia is less frequent in patients with resurgent vivax malaria (Table 1). A paper that analyzed 101 patients reported that 52 patients (51.5%) had anemia (hemoglobin <12.0 g/dL), where about a half had a hemoglobin level of 10.1 - 12.0 g/dL with mild anemia [22]. Another hematological finding with high frequency was thrombocytopenia, which was observed in 61.5 - 100% of the patients (different by each institution) [8]. In addition, other laboratory findings included leukopenia, increased levels of aminotransferases, and increased levels of bilirubin [22,23].
4) Complications and sequelae
One of the most serious complications of vivax malaria was spontaneous rupture of the spleen [22,23]. In addition, hypotension, mental disorders, azotemia, and spleen infarction occurred [23]. Other sequelae included relapse after a treatment. With regular chloroquine treatment, febrile paroxysm soon disappears; however, if primaquine is not properly administered, which can kill cryptomerozoite in hepatocytes, the parasite may proliferate again. This may lead to a relapse of febrile attack. Even after receiving both chloroquine and primaquine treatment, relapse may occur in 1 in 101 patients due to a treatment failure [23]. Moreover, in Gyeonggi-do, 11 (3.2%) of 341 patients who received both chloroquine and primaquine treatment relapsed [23]. However, one point to note is that the average total dose of primaquine in these cases was 3.01 mg/kg, which was slightly lower than the dose that did not relapse, which was 3.39 mg/kg [23].
5) Drug resistance
In a paper that analyzed the drug resistance of vivax malaria, two out of 484 patients did not respond to chloroquine treatment and were judged to be drug resistant [24]. Fortunately, however, there were no further reports of drug resistance. In most foreign countries, there have been no drug resistance problems, except in Indonesia, Myanmar, Vietnam, Papua New Guinea, and India that reported some resistant cases [25].
2. Epidemiological characteristics
1) Vector mosquitoes
Eight species of Anopheles spp. mosquitoes have been identified in Korea [26]. They included A. sinensis (sensu stricto), A. pullus (syn. A. yatsushiroensis), A. lesteri, A. kleini, A. belenrae, A. sineroides, A. lindesayi japonicus, and A. koreicus. However, only four among them can be vector mosquitoes for malaria: A. sinensis [27,28], A. pullus [28,29], A. lesteri [30,31], and A. kleini [28] (Table 2). Of these, A. sinensis is the primary vector mosquito that is widely distributed and mainly active at night time (between 8 pm and 5 am). This species has strong zoophilism, but if there are no animals nearby, it has high tendency to attack humans [27].
Table 2. Major vector mosquitoes of the reemerging vivax malaria in Korea.
| Anopheles sinensis |
| Anopheles pullus (syn. Anopheles yatsushiroensis) |
| Anopheles lesteri |
| Anopheles kleini |
2) Seasonal tendency
Because there were a mixed number of cases with short and long incubation periods, the timing of fever onset varied widely. Overall, however, the occurrence of the resurgent vivax malaria (onset of febrile attack) tended to be between May and September (from late spring to early autumn) [8,25]. These seasonal characteristics are almost identical to that of the old Korean vivax malaria recorded in 1913 [8]. Between May and September is the time when adult mosquitoes are predominantly active. This means that by causing malaria in humans during this time, the parasite can be passed to next mosquitoes and can survive a long time.
3) Potential indigenous occurrence of falciparum malaria and quartan malaria
The only malaria that can occur indigenously in Korea is vivax malaria. There are four vector mosquitoes known to transmit vivax malaria [28,31]. but there are no vector mosquitoes that can transmit faciparum malaria or quartan malaria in Korea. In the 1930s, there were a few reports of quartan malaria in Seosan-gun, Chungcheongnam-do [32]; nevertheless, no further cases were recorded. Falciparum malaria had also been reported during the 1950s among drug addicts who shared contaminated syringes [33], yet it soon disappeared. One patient with no overseas travel history died of falciparum malaria recently [34]. However, this case was confirmed to have been due to a nosocomial (in-hospital) infection from another malaria patient who became infected in Africa [34].
Prevention and management
1. Basic strategy for malaria management
The four basic strategies for malaria management are as follows [35]: (1) early diagnosis and treatment of patients, (2) establishment and implementation of preventive measures and mosquito control programs, (3) early detection of epidemics and implementation of suppression and preventive actions, and (4) regular inspection of ecological and socio-economic conditions related to the epidemic.
2. Early diagnosis and treatment of patients
In the mid- to late-1990s with the widespread malaria resurgence, the government and military (including the US troops in Korea) implemented several measures for early patient detection, including passive case detection (PCD) and active case detection (ACD). As a result, medical workers at local health centers and hospitals rapidly gained experience with malaria, and the average time from patient occurrence to diagnosis was reduced from 23.6 days in 1995 to 9.5 days in 1997 and to 8.0 days in 2000 [36]. Thereafter (between 2000 and 2002), 61.7 - 73.6% of patients were diagnosed within 6 days of symptom onset [17]. In addition, since 2008, rapid diagnostic tests (RDT) were distributed to rapidly detect malaria antibodies, and the time required to diagnose malaria patients has been further reduced [37].
3. Establishment and implementation of continuous preventive measures and mosquito control programs
1) Personal protection
The government provided health education for residents and soldiers in the main endemic areas to raise awareness of malaria and to prevent mosquito bites. To control malaria mosquitoes, the military also provided measures for personal protection, such as supplying mosquito nets, treating combat uniforms with pesticides, and supplying mosquito repellents [37].
2) Mass chemoprophylaxis
One of the more aggressive methods compared to personal defense is mass chemoprophylaxis. In the case of vivax malaria, a single treatment of 300 mg of chloroquine once a week or 15 mg of primaquine daily for 14 days or combination therapy using both chloroquine and primaquine can be used. Chloroquine is used to kill the parasite and has a half-life (late half-life) of up to 1 - 2 months [19]. Accordingly, it is administered only once a week, and there are no known significant side effects even when using a cumulative dose of 100 g for 6 years. Primaquine is the drug that kills tissue-stage parasites that are lurking in hepatocytes and it should be administered for at least 14 days. Therefore, administering primaquine for 14 days in winter season can kill Plasmodium parasites that have been in the liver for a long period of time, preventing the occurrence of malaria the following year.
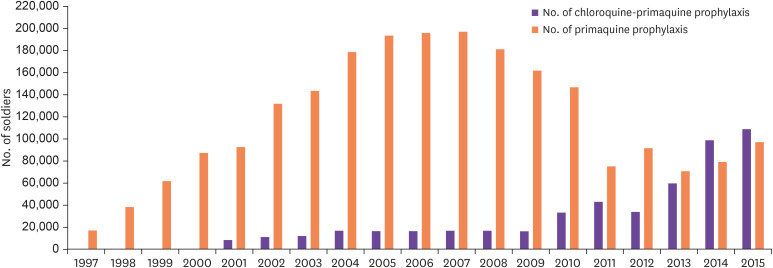
This mass chemoprophylaxis was primarily used in military units. In 1997, at the beginning of the resurgence, combination chemoprophylaxis using chloroquine and primaquine was first used on 15,981 people [17,37]. Thereafter, the number of subjects increased to 37,529 in 1998 and 61,722 in 1999. In 2007, up to 190,000 people received mass chemoprophylaxis for malaria, and the method continued until 2015 (Fig. 4) [37]. In addition, since 2001, single treatment with primaquine began separately for discharged soldiers, and this has continued for approximately 10,000 people each year (Fig. 4). Thus, in 2015, 90,000 people received combination therapy with chloroquine and primaquine, and 100,000 received a single therapy with primaquine [37]. This chemoprophylaxis in the military is estimated to have had a great effect on the management of malaria resurgence.
Figure 4. Malaria chemoprophylaxis conducted by the Korean military. Between 1997 and 2015, a combination therapy of chloroquine and primaquine was primarily administered. Since 2001, a single therapy of primaquine was prescribed for discharged soldiers. The use of primaquine monotherapy significantly expanded since 2014 - 2015.
※ Source: Jung J [37].
3) Mosquito control
As malaria resurgence persisted for several years after the first case in 1993, the government and the military (including US troops in Korea) also made great efforts to control mosquitoes. In 2001, both the Korean and North Korean government jointly agreed on the need for malaria management and began activities such as mosquito control [17]. In Korea, Gyeonggi-do, Gangwon-do, and Incheon were designated as centralized management areas, and the KCDC and local health authorities allocated budgets to control mosquitoes. North Korea received budget support from the WHO, the International Red Cross Foundation (IFRC), and the Korean government for mosquito control as well as to purchase anti-malarial drugs [17]. The military also conducted various activities to control mosquitoes, including filling puddles around the garrison, spraying of mosquito repellent, and fumigation.
Footnotes
This secondary publication is based on ‘Korean Society of Infectious Diseases (KSID). Korean History of Infectious Diseases II. Seoul: Koonja; 2018. (ISBN 979-11-5955-379-0)’.
Conflict of Interest: No conflicts of interest.
References
- 1.Hasegawa Y. Malaria in Korea. Chosen Igakkai Zasshi. 1913;4:53–69. [Google Scholar]
- 2.Tanabe M. Distribution of malaria in Korea: on the endemicity of malaria in Chunchon and Cholwon, Kangwon-do. J Chosun Med Soc. 1927;82:882–885. [Google Scholar]
- 3.Rim HJ. Malaria. Comp Med. 1968;13:18–22. [Google Scholar]
- 4.Paik YH, Ree HI, Shim JC. Malaria in Korea. Jpn J Exp Med. 1988;58:55–66. [PubMed] [Google Scholar]
- 5.Hale TR, Halpenny GW. Malaria in Korean veterans. Can Med Assoc J. 1953;68:444–448. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DC. Status of malaria infection in the Republic of Korea. Yonsei Rep Trop Med. 1982;13:59–62. [Google Scholar]
- 7.Soh CT, Lee KT, Im KI, Min DY, Ahn MH, Kim JJ, Yong TS. Current status of malaria in Korea. Yonsei Rep Trop Med. 1985;16:11–18. [Google Scholar]
- 8.Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–143. doi: 10.3347/kjp.1999.37.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasitol. 1994;32:195–200. doi: 10.3347/kjp.1994.32.3.195. [DOI] [PubMed] [Google Scholar]
- 10.Cho SY, Kong Y, Park SM, Lee JS, Lim YA, Chae SL, Kho WG, Lee JS, Shim JC, Shin HK. Two vivax malaria cases detected in Korea. Korean J Parasitol. 1994;32:281–284. doi: 10.3347/kjp.1994.32.4.281. [DOI] [PubMed] [Google Scholar]
- 11.Korean Centers for Disease Control and Prevention (KCDC) Malaria management guidelines (2015~2017) Osong: KCDC; 2017. [Google Scholar]
- 12.Chai JY. Re-emerging malaria. J Korean Med Assoc. 1997;40:728–733. [Google Scholar]
- 13.Jeong IK, Oh MD, Chai JY, Lee HW, Lee WJ, Lee JS, Soe DH, Choe KW. A case of transfusion-induced malaria presenting as fever of unknown origin. Korean J Infect Dis. 1999;31:41–45. [Google Scholar]
- 14.Lee YH, Lee HK, Choi KH, Hah JO, Lim SY. Transfusion-induced malaria in a child after open heart surgery in Korea. J Korean Med Sci. 2001;16:789–791. doi: 10.3346/jkms.2001.16.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein TA, Pacha LA, Lee HC, Kim HC, Lee WJ, Lee JK, Jeung GG, Sames WJ, Gaydos JC. Plasmodium vivax malaria among U.S. forces Korea in the Republic of Korea, 1993-2007. Mil Med. 2009;174:412–418. doi: 10.7205/milmed-d-01-4608. [DOI] [PubMed] [Google Scholar]
- 16.Kim HC, Pacha LA, Lee WJ, Lee JK, Gaydos JC, Sames WJ, Lee HC, Bradley K, Jeung GG, Tobler SK, Klein TA. Malaria in the Republic of Korea, 1993-2007. Variables related to re-emergence and persistence of Plasmodium vivax among Korean populations and U.S. forces in Korea. Mil Med. 2009;174:762–769. doi: 10.7205/milmed-d-01-6208. [DOI] [PubMed] [Google Scholar]
- 17.Han ET, Lee DH, Park KD, Seok WS, Kim YS, Tsuboi T, Shin EH, Chai JY. Reemerging vivax malaria: changing patterns of annual incidence and control programs in the Republic of Korea. Korean J Parasitol. 2006;44:285–294. doi: 10.3347/kjp.2006.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chol PT, Suwannapong N, Howteerakul N. Evaluation of a malaria control project in DPR Korea, 2001-2003. Southeast Asian J Trop Med Public Health. 2005;36:565–571. [PubMed] [Google Scholar]
- 19.Chai JY, Hong ST, Choi MH, Shin EH, Bae YM, Hong SJ, Sohn WM, Yu JR, Koh WG, Seo M, Park YK, Han ET. Clinical parasitology. Seoul: Seoul National University Press; 2011. [Google Scholar]
- 20.Tiburskaja NA, Vrublevskaja OS. The course of infection caused by the North Korean strain of Plasmodium vivax. WHO; 1977. pp. 1–19. [Google Scholar]
- 21.Kim KH, Lim CS. Clinical observation in 26 cases of indigenous malaria in 1995. Korean J Med. 1997;52:577–583. [Google Scholar]
- 22.Oh MD, Shin H, Shin D, Kim U, Lee S, Kim N, Choi MH, Chai JY, Choe K. Clinical features of vivax malaria. Am J Trop Med Hyg. 2001;65:143–146. doi: 10.4269/ajtmh.2001.65.143. [DOI] [PubMed] [Google Scholar]
- 23.Kwak YG, Lee HK, Kim M, Um TH, Cho CR. Clinical characteristics of vivax malaria and analysis of recurred patients. Infect Chemother. 2013;45:69–75. doi: 10.3947/ic.2013.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KS, Kim TH, Kim ES, Lim HS, Yeom JS, Jun G, Park JW. Chloroquine-resistant Plasmodium vivax in the Republic of Korea. Am J Trop Med Hyg. 2009;80:215–217. [PubMed] [Google Scholar]
- 25.Yeom JS. Malaria. Korean J Med. 2014;86:265–270. [Google Scholar]
- 26.Jeong KY, Un S, Lee J, Lee IY, Yong TS, Ree HI. Population dynamics of five Anopheles species of the hyrcanus group in northern Gyeonggi-do, Korea. Korean J Parasitol. 2010;48:351–353. doi: 10.3347/kjp.2010.48.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ree HI. Studies on Anopheles sinensis, the vector species of vivax malaria in Korea. Korean J Parasitol. 2005;43:75–92. doi: 10.3347/kjp.2005.43.3.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee WJ, Klein T, Kim HC, Choi YM, Yoon SH, Chang KS, Chong ST, Lee IY, Jones JW, Jacobs JS, Sattabongkot J, Park JS. Anopheles kleini, Anopheles pullus, and Anopheles sinensis: potential vectors of Plasmodium vivax in the Republic of Korea. J Med Entomol. 2007;44:1086–1090. doi: 10.1603/0022-2585(2007)44[1086:akapaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Hong HK. Ecological studies of important mosquitoes in Korea. PhD thesis. Dongguk University Graduate School; 1978. Print. [Google Scholar]
- 30.Shin EH, Kim TS, Lee HW, Lee JS, Lee WJ. Vector competence of Anopheles lesteri Baisas and Hu (Diptera: Culicidae) to Plasmodium vivax in Korea. Korean J Parasitol. 2002;40:41–44. doi: 10.3347/kjp.2002.40.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi D, Choochote W, Park MH, Kim JY, Kim TS, Suwonkerd W, Min GS. The susceptibility of Anopheles lesteri to infection with Korean strain of Plasmodium vivax . Malar J. 2009;8:42. doi: 10.1186/1475-2875-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh SN. On quartan malaria in Korea. Mansen No Ikai. 1930;112:18–19. [Google Scholar]
- 33.Seo BS, Rim HJ. A survey of malaria amongst narcotic addicts in Seoul. Seoul Nat Univ J (Med & Pharm Series) 1959;8:213–220. [Google Scholar]
- 34.Kim JY, Kim JS, Park MH, Kang YA, Kwon JW, Cho SH, Lee BC, Kim TS, Lee JK. A locally acquired falciparum malaria via nosocomial transmission in Korea. Korean J Parasitol. 2009;47:269–273. doi: 10.3347/kjp.2009.47.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization (WHO) Turning malaria around. Geneva: WHO; 1998. [Google Scholar]
- 36.Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003;69:159–167. [PubMed] [Google Scholar]
- 37.Jung J. Effectiveness of malaria chemoprophylaxis in military units (seminar presentation) 2017. [Google Scholar]