Coronavirus disease 2019 (COVID-19) is the pandemic challenge striking many health systems worldwide. The new coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2), which has a well-known tropism to lung cells, is the cause.
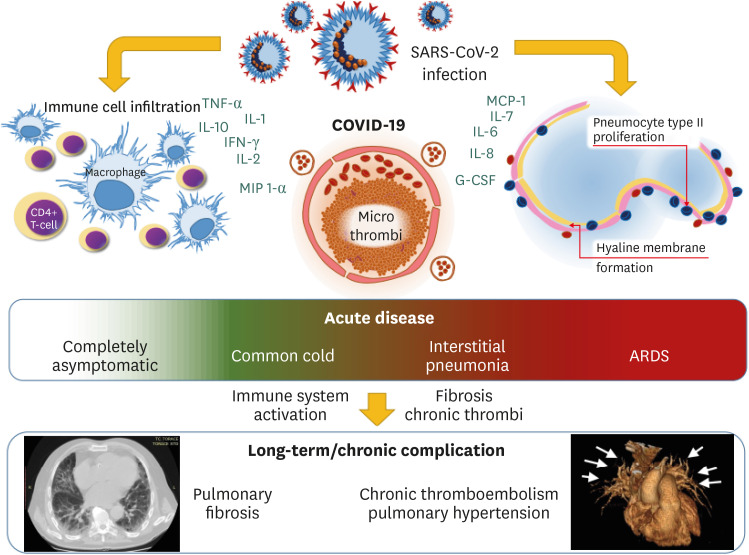
Acute COVID-19 patients present with a wide range of clinical manifestations, ranging from an asymptomatic or mild-symptomatic form (common cold) up to bilateral interstitial pneumonia with moderate-to-severe oxygen desaturation and hypoxia. The consequent respiratory failure may turn into acute respiratory distress syndrome (ARDS) [1] requiring promptly admission in intensive care unit and representing a considerable stressor for healthcare systems worldwide. Differently to usual ARDS, these patients present a normal or slightly increased lung compliance and mostly need high-flow oxygen or continue positive airway pressure ventilation [2]. Acute cardiac involvement of COVID-19 infection [1] was described and it has been hypothesized a direct effect of the virus on the myocardium and heart vessels [3].
According to preliminary observation, inflammatory activation plays a relevant role in the natural course of COVID-19. Immune system dysregulation has been reported to be associated with the disease severity. A cytokine profile in the severe disease is characterised by increased interleukin (IL)-1, IL-2, IL-6, IL-7, IL-8, IL-10, granulocyte-colony stimulating factor, interferon (IFN)-γ inducible protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1-α, and tumour necrosis factor-α [4]. In particular, Chinese data indicate high ferritin and low lymphocyte count as predictor of fatality [1,4]. Immune activation may result in endothelial damage and a consequent activation of coagulation and fibrosis processes in host lungs.
Supporting this hypothesis, reports on COVID-19 patients’ autopsies described alveolar damage including immune cell infiltration, mostly by macrophages and monocytes associated to CD4 positive T-lymphocytes, with hyaline membrane formation, proliferation of type II alveolar epithelia and congested blood vessels associated with hyaline thrombi in microvessels [3]. Authors have also observed a focal pulmonary fibrosis associated with SARS-CoV-2 antigen in lung epithelia and macrophages [3]. Moreover, vascular thrombosis associated to immune infiltration of lung capillary wall has been also described [5].
These findings are at some extent similar to lung biopsies of non-specific interstitial pneumonia (NSIP) or autoimmune interstitial lung disease (ILD) [6]. Moreover, despite both need high-flow oxygen, the COVID-19 patient presents an increased ventilation compliance [2], and ventilation outcome seems to be similar to pneumonia respiratory failure in ILD [7]. According to the previous severe acute respiratory syndrome (SARS), the first coronavirus lung fatal infection known, some further considerations should be add. Clinical observation from patients affected by SARS, reports an increase of lung fibrosis after recovery from the infection [8]. In these patients, the hyperactivation of epidermal growth factor receptor signalling has been indicated in response to the acute lung injury [9]. In SARS infected mice, immune response appears affected by a Th2 bias as a result in deficiency of IFN production due to signal transducer and activator of transcription 1 deficiency. These mice are also prone to fibrosis as a consequence of infection [9]. Moreover, recent data had indicated that patients with ILD other than idiopathic pulmonary fibrosis (IPF) have a subsequent clinical course similar to patients with untreated IPF [6]. In this prospective, an early detection of ILD may be useful for a better treatment and prognosis.
On the other hand, venous thromboembolism (both as deep vein thrombosis and pulmonary embolism) may often complicate COVID-19 [10]. Speculation on an activation of coagulation process directly in pulmonary capillaries may be possible. The micro-thrombi in small lung capillaries might lead to a small vessels vasculopathy through hyperperfusion of non-obstructed lung segments, as in chronic thromboembolic pulmonary hypertension (CTEPH).
The immunological and clinical alterations are red flags for the future. The spread of COVID-19 in general population may be a trigger for an increase in lung rare disease such as NSIP or CTEPH (Fig. 1). It is reasonable to expect chronic complications in COVID-19 survivors and a long-term follow-up of patients may be something to take into consideration. Due to the multiorgan involvement in COVID-19, multi-disciplinary team (including internist, infectious disease specialist, immunologist, pulmonologist, and cardiologist) is mandatory for an adequate follow-up and treatment. Despite all patients might need controls after remission, it could be reasonable to be stricter on patients recovered after a moderate-to-severe form of this disease or those who will present a reduced lung capacity during efforts. The results may be particularly important considering the asymptomatic patients and women, because their follow-up could be the key in better understand and treat COVID-19 and lung rare diseases.
Figure 1. Possible clinical evolution of COVID-19. After the acute phase, the chronic evolution may reveal rare lung diseases.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumour necrosis factor; IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; MCP, monocyte chemoattractant protein; G-CSF, granulocyte-colony stimulating factor; ARDS, acute respiratory distress syndrome.
ACKNOWLEDGMENTS
The authors thanks to dr. Christel Cariddi, Department of Anaesthesia, University of Bari Aldo Moro, for her precious support and fruitful discussions.
Footnotes
Conflict of Interest: No conflict of interest.
- Conceptualization: SC, AMM.
- Data curation: SC.
- Writing - original draft: SC, AMM.
- Writing - review & editing: SC, AV, AC, AMM.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KK, Martinez FJ, Walsh SLF, Thannickal VJ, Prasse A, Schlenker-Herceg R, Goeldner RG, Clerisme-Beaty E, Tetzlaff K, Cottin V, Wells AU. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J. 2020;55:2000085. doi: 10.1183/13993003.00085-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliberti S, Messinesi G, Gamberini S, Maggiolini S, Visca D, Galavotti V, Giuliani F, Cosentini R, Brambilla AM, Blasi F, Scala R, Carone M, Luisi F, Harari S, Voza A, Esquinas A, Pesci A. Non-invasive mechanical ventilation in patients with diffuse interstitial lung diseases. BMC Pulm Med. 2014;14:194. doi: 10.1186/1471-2466-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu HH, Tzao C, Wu CP, Chang WC, Tsai CL, Tung HJ, Chen CY. Correlation of high-resolution CT, symptoms, and pulmonary function in patients during recovery from severe acute respiratory syndrome. Chest. 2004;126:149–158. doi: 10.1378/chest.126.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res. 2017;143:142–150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macías M, Toledo-Samaniego N, García-García A, García-Fernández-Bravo I, Ji Z, de-Miguel-Diez J, Álvarez-Sala-Walther LA, Del-Toro-Cervera J, Galeano-Valle F. Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res. 2020;192:23–26. doi: 10.1016/j.thromres.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]