Abstract
Background
A novel antiviral agent, remdesivir (RDV), is a promising candidate treatment for coronavirus disease 2019 (COVID-19) in the absence of any proven therapy.
Materials and Methods
This retrospective case series included 10 patients with a clinically and laboratory confirmed diagnosis of severe COVID-19 pneumonia who had received RDV for 5 days (n = 5) or 10 days (n = 5) in the Phase III clinical trial of RDV (GS-US-540-5773) conducted by Gilead Sciences. The clinical and laboratory data for these patients were extracted.
Results
One patient in the 10-day group received RDV for only 5 days because of nausea and elevated liver transaminases. No patient had respiratory comorbidity. Seven patients had bilateral lesions and three had unilateral lesions on imaging. All patients had received other medications for COVID-19, including lopinavir/ritonavir and hydroxychloroquine, before administration of RDV. Five patients required supplemental oxygen and one required mechanical ventilation. All patients showed clinical and laboratory evidence of improvement. Half of the patients developed elevated liver transaminases and three had nausea. There were no adverse events exceeding grade 2.
Conclusion
Our experience indicates that RDV could be a therapeutic option for COVID-19. A well-designed randomized controlled clinical trial is now needed to confirm the efficacy of RDV in patients with COVID-19.
Keywords: Coronavirus disease 2019, COVID-19, Severe acute respiratory syndrome coronavirus 2, Remdesivir, Pneumonia
Introduction
Coronavirus disease 2019 (COVID-19) was declared a pandemic just 4 months after the first case was reported in December 2019 in Wuhan, China. At least 3,000,000 confirmed COVID-19 cases and 210,000 deaths due to COVID-19 had been reported by April 30, 2020 [1]. Initially, COVID-19 spread from China to adjacent Asian countries but soon spread worldwide because of extensive movement of populations between countries.
The first case of COVID-19 in Korea was in a Chinese traveler and reported on January 20, 2020. By April 30, 2020, there were 10,765 confirmed cases of COVID-19 and 247 COVID-19-related deaths in Korea [2]. The daily incidence of COVID-19 in Korea is now below 10. Nevertheless, the situation is still being monitored carefully in view of the small outbreaks that have occurred in health care institutions and religious facilities and the potential for a second wave.
There is still no known effective treatment for COVID-19. However, numerous candidate agents have been identified. Remdesivir (RDV), a monophosphoramidate prodrug of a C-adenosine nucleoside analogue, is expected to be a particularly effective treatment for COVID-19 and is currently under investigation in large-scale clinical trials worldwide [3]. RDV was confirmed to be effective in the treatment of severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) at in vitro setting in previous studies [4,5]. Seoul Medical Center, a dedicated hospital for COVID-19 in Korea, is the one of the sites participating in one such clinical trial and has experience using RDV in patients with clinically severe COVID-19. The purpose of this report is to share our experience of treating COVID-19 with RDV.
Material and Methods
1. Patient selection and study protocol
This retrospective case series included 10 patients confirmed to have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Korea and treated with RDV. In Korea, individuals with fever or respiratory symptoms and a history of overseas travel and those with atypical pneumonia of unknown cause are screened for SARS-CoV-2 infection. If infection is confirmed, the patient is transferred to a hospital with an airborne infection isolation room. Treatment of all patients with COVID-19 at Seoul Medical Center, one of the hospitals treating the disease, is managed by infectious diseases specialists according to their symptoms, disease severity, and comorbidities. Patients who have a still positive SARS-CoV-2 PCR test but in whom symptoms improve after treatment in hospital may be transferred to an isolation facility for patients with COVID-19.
The patients included in this study were selected according to the RDV clinical trial performed by Gilead Sciences. This randomized open-label Phase III trial (GS-US-540-5773) included patients with a confirmed diagnosis of SARS-CoV-2-related pneumonia who were aged ≥18 years (or 12 – 18 years if they weighed ≥40 kg) and had an oxygen saturation of ≤94% in room air. COVID-19 was diagnosed by SARS-CoV-2 PCR and a chest X-ray or chest computed tomography (CT) scan. The following exclusion criteria were applied: (1) evidence of multi-organ failure; (2) mechanical ventilation for longer than 5 days; (3) a serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level more than 5 times the upper limit of normal (ULN); (4) creatinine clearance <50 mL/min (by the Cockcroft-Gault formula in patients aged ≥18 years and by the Schwartz formula in those aged younger than 18 years); (5) pregnancy or breastfeeding; (6) known hypersensitivity to RDV or its metabolites; and (7) participation in another clinical trial. The patients were randomized to receive RDV for 5 or 10 days. The 5-day group received RDV 200 mg intravenously on day 1 followed by RDV 100 mg/day on days 2 – 5. The patients in the 10-day group received RDV 200 mg intravenously on day 1 followed by RDV 100 mg/day on days 2 – 10. All study participants were hospitalized in an airborne infection isolation room. RDV was discontinued in patients who (1) experienced any severe adverse event above grade 3, (2) had an increase in ALT to above 5 times the ULN, in AST to above 3 times the ULN, or in total bilirubin to above twice the ULN, or (3) had a decrease in creatinine clearance to below 30 mL/min. Concomitant use of other antiviral agents, such as lopinavir/ritonavir, hydroxychloroquine (HCQ)/chloroquine, and interferon, was prohibited during administration of RDV.
2. Data collection
Data were collected on patient characteristics at baseline, including age, sex, smoking history, onset of symptoms, and pre-existing pulmonary and other comorbidities, including chronic obstructive lung diseases, pulmonary tuberculosis, asthma, hypertension, chronic cardiac disease, chronic liver disease, chronic neurological disorder, solid organ cancer, chronic hematologic disease (including malignancy), peptic ulcer disease, diabetes, and immune disease. Information on medications including antiviral agents and antibiotics, treatment modalities, and interventions such as supplemental oxygen, vasopressor therapy, and hemodialysis were collected.
Clinical, laboratory, and imaging data were also collected. Blood pressure, heart rate, respiratory rate, temperature, and symptoms such as fever, cough, production of sputum, sore throat, rhinorrhea, ear pain, myalgia, fatigue, malaise, dyspnea, headache, abdominal pain, vomiting/nausea, and diarrhea were included in the clinical information.
Laboratory data, including a complete blood count, C-reactive protein (CRP), procalcitonin, blood urea nitrogen, creatinine, AST, ALT, total bilirubin, lactate dehydrogenase (LDH), and ferritin levels, prothrombin time (international normalized ratio), arterial blood gas analysis, and electrolyte levels, were also obtained. The interpretation reports for simple chest radiographs and CT scans were collected. Viral load was determined by real-time RT-PCR for the SARS-CoV-2 Envelope gene and RNA-dependent RNA polymerase gene in specimens from the nasopharynx/oropharynx and sputum using a PowerChek 2019-nCoV Real-time PCR Kit (Kogene Biotech, Seoul, Korea).
3. Statistical analysis
Continuous variables are expressed as the median (interquartile range [IQR]). The study population was too small to allow a statistical analysis. However, a frequency analysis was performed using SPSS version 18.0 (IBM Corp., Armonk, NY, USA).
4. Ethical statement
The study protocol was approved by the institutional review board of Seoul Medical Center (2020-03-004). Investigators in this study provided the information about the study protocol to all patients enrolled in this study and obtained the informed consent.
Results
1. Patient characteristics
The 10 patients enrolled in this study received RDV during their hospital stay. The median patient age was 52 years (Interquartile range [IQR], 37.5 – 55.5). Five patients (50%) were men. One patient was a current smoker and two were ex-smokers. The most common symptom was fever (n = 8, 80%) followed by cough (n = 5, 50%). Patient 2 reported the first symptom after admission to hospital (Fig. 1). The median duration of illness at the time of admission was 4.00 days (IQR, 3.75 – 9.50). No patient had an underlying respiratory disease. Three patients (30%) had hypertension; one also had dyslipidemia and alcoholic fatty liver disease (Table 1).
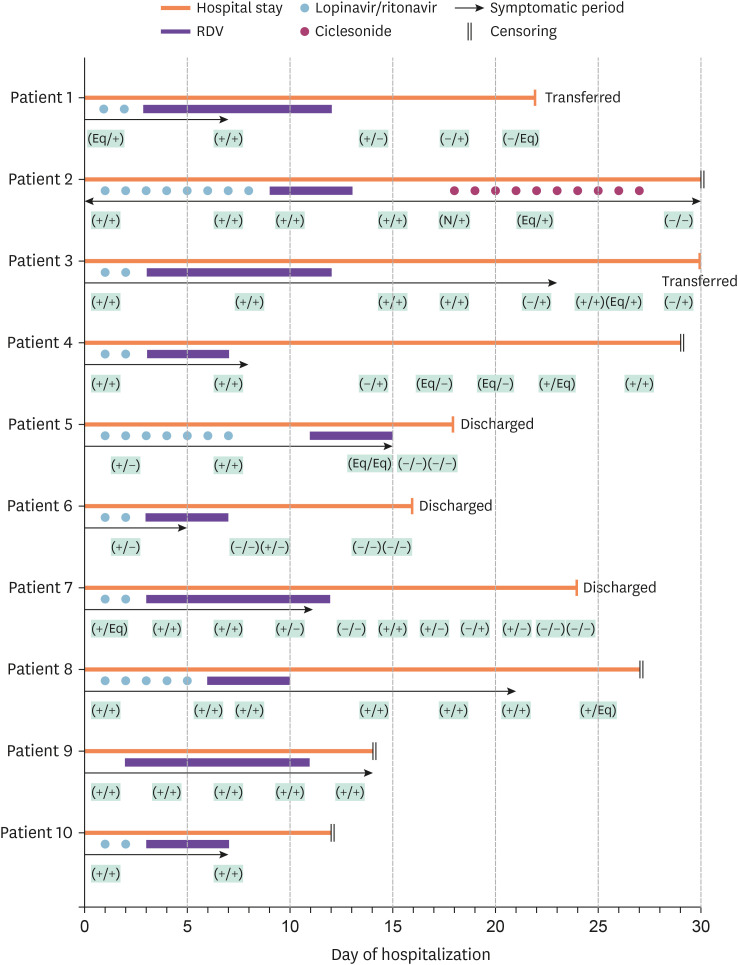
Figure 1. Schematic representation of 10 cases treated with RDV and other treatments for COVID-19 during hospitalization. Patient data were censored when the data were blocked on April 15, 2020. The black arrows indicate the 'symptomatic period', i.e., from the day the patient reported respiratory symptoms. The results of the SARS-CoV-2 PCR analysis of a sputum sample and a nasopharyngeal/pharyngeal swab are shown in parentheses. (+) Positive result. (-) Negative result. (Eq) Obvious result. (N) Patient was not examined.
RDV, remdesivir; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction.
Table 1. Clinical characteristics of patients with COVID-19 and laboratory results prior to administration of remdesivir.
| Characteristic | Patients with COVID-19 (n = 10) | ||
|---|---|---|---|
| Age, years | 52.0 (37.5 – 55.5) | ||
| Male sex (%) | 5 (50.0) | ||
| Smoking history, yes (%) | 3 (30.0) | ||
| Symptom, yes (%) | |||
| Cough | 5 (50.0) | ||
| Sputum | 2 (20.0) | ||
| Rhinorrhea | 1 (10.0) | ||
| Dyspnea | 2 (20.0) | ||
| Fever | 8 (80.0) | ||
| Fatigue | 1 (10.0) | ||
| Malaise | 0 (0.0) | ||
| Headache | 0 (0.0) | ||
| Gastrointestinal symptomsa | 1 (10.0) | ||
| Duration of illness, days | 4.00 (3.75 – 9.50) | ||
| Underlying respiratory disease, yes (%) | |||
| Chronic obstructive pulmonary disease | 0 (0.0) | ||
| Pulmonary tuberculosis, both active and inactive | 0 (0.0) | ||
| Asthma | 0 (0.0) | ||
| Other lung disease | 0 (0.0) | ||
| Other comorbidities, yes (%) | |||
| Hypertension | 3 (30.0) | ||
| Diabetes | 0 (0.0) | ||
| Cardiovascular disease (non-hypertensive) | 0 (0.0) | ||
| Cerebrovascular disease | 0 (0.0) | ||
| Autoimmune disease | 0 (0.0) | ||
| Peptic ulcer disease | 0 (0.0) | ||
| Chronic kidney disease | 0 (0.0) | ||
| Chronic liver disease | 1 (10.0) | ||
| Solid organ cancer | 0 (0.0) | ||
| Hematological malignancy | 0 (0.0) | ||
| Laboratory test | |||
| White blood cells (WBC), /mm3 | 4,750 (4,250 – 5,275) | ||
| Leukocytosisb, yes (%) | 0 (0.0) | ||
| Leukopeniac, yes (%) | 2 (20.0) | ||
| Platelets, × 103 cells/mm3 | 203 (172 – 219) | ||
| Platelets <150 × 103 cells/mm3, yes (%) | 0 (0.0) | ||
| Severe thrombocytopeniad, yes (%) | 0 (0.0) | ||
| AST, IU/L | 35 (22 – 43) | ||
| ALT, IU/L | 23 (15 – 36) | ||
| Total bilirubin, mg/dL | 0.43 (0.33 – 0.91) | ||
| Blood urea nitrogen, mg/dL | 10.5 (8.5 – 13.0) | ||
| Creatinine, mg/dL | 0.76 (0.61 – 0.93) | ||
| C-reactive protein, mg/L | 9.75 (2.73 – 32.80) | ||
| Lactate dehydrogenase, U/L | 281 (229 – 329) | ||
| Procalcitonin, ng/mL | 0.06 (0.05 – 0.13) | ||
Data are presented as the median (interquartile range) or as the number (percentage) unless otherwise indicated.
aGastrointestinal symptoms included nausea, vomiting, diarrhea, and abdominal pain.
bLeukocytosis was defined as a WBC count ≥10,000/mm3.
cLeukopenia was defined as a WBC count <4,000/mm3.
dSevere thrombocytopenia was defined as a platelet count <50,000 cells/mm3.
COVID-19, coronavirus disease 2019;AST, aspartate aminotransferase; ALT, alanine aminotransferase.
At admission, the median white blood cell count was 4,750/mm3 (IQR, 4,250 – 5,275) and the median platelet count was 203 × 103/mm3 (IQR, 177 – 219). Leukopenia was observed in two patients (20%); no patient had leukocytosis or thrombocytopenia. The median serum AST/ALT levels were 35/23 IU/L (IQR, 22 – 43/15 – 36). The AST level was higher than 40 IU/L in four patients (40%) and ALT was higher than 40 IU/L in only one patient (10%). The median serum creatinine level was 0.76 mg/dL (IQR, 0.61 – 0.93), the median serum CRP level was 9.75 mg/L (IQR, 2.73 – 32.80), the median serum LDH level was 281 U/L (IQR, 229 – 329), and the median serum procalcitonin level was 0.06 ng/mL (IQR, 0.05 – 0.13). The serum ferritin levels were checked in four patients (40%) at admission (Table 1).
Plain chest radiographs and CT scans were obtained in all patients at admission. Five (50%) had bilateral opacities, one (10%) had unilateral opacities, and four (40%) had normal radiographic findings. The CT scans showed ground-glass opacities that were bilateral in seven patients (70%) and unilateral in three (30%). Bilateral consolidations were observed in patients 7 and 9 (Table 2).
Table 2. Progress of each patient during hospital admission.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age, years | F/28 | M/54 | M/33 | F/57 | F/52 | M/39 | M/52 | M/60 | F/55 | F/50 |
| Country of infection | Korea | Korea | Korea | Korea | Korea | Philippines | Korea | Korea | UK | Korea |
| Underlying respiratory disease | None | None | None | None | None | None | None | None | None | None |
| Other comorbidity | None | Hypertension, dyslipidemia, GERD, alcoholic fatty liver | Hypertension | None | None | None | None | Hypertension | Hydatidiform mole | Rt. Renal cyst |
| Smoking history | None | Ex-smoker | None | None | None | Current smoker | None | Ex-smoker | None | None |
| Symptoms | Fever, cough | Cough, sore throat | Fever | Fever, chills, sputum sore throat, myalgia | Fever, chills, myalgia | Fever, chills, myalgia | Fever, cough, shortness of breath | Cough, rhinorrhea | Fever, shortness of breath, sore throat, nausea | Fever, cough, sputum, sore throat, myalgia, fatigue |
| Duration of illnessa, days | 4 | 1 | 3 | 9 | 4 | 4 | 11 | 8 | 27 | 4 |
| Chest CT findings | Bilateral GGO | Unilateral GGO | Bilateral GGO | Bilateral GGO | Bilateral GGO | Unilateral GGO | Bilateral GGO, bilateral consolidations | Bilateral GGO | Bilateral GGOs | Unilateral GGO |
| Bilateral consolidations | ||||||||||
| Other antiviral agents used for COVID-19, days | Lopinavir/ritonavir (2) | Lopinavir/ritonavir (8) | Lopinavir/ritonavir (2) | Lopinavir/ritonavir (2) | Lopinavir/ritonavir (7) | Lopinavir/ritonavir (2) | Lopinavir/ritonavir (3) | Lopinavir/ritonavir (5) | Lopinavir/Ritonavir (10) | Lopinavir/Ritonavir (2) |
| Ciclesonide (10) | HCQ (2) | |||||||||
| Interval between symptom onset and starting RDV, days | 6 | 9 | 5 | 11 | 14 | 6 | 13 | 13 | 28 | 6 |
| Duration of RDV, days | 10 | 5 | 10 | 5 | 5 | 5 | 10 | 5 | 10 | 5 |
| AE during RDV | None | None | Diarrhea (G1), AST, ALT elevation (G1) | Nausea (G2), chest discomfort (G2) | None | AST, ALT elevation (G1), insomnia (G1) | AST, ALT elevation (G2) | None | AST, ALT elevation (G1), nausea (G1) | AST, ALT elevation (G2), nausea (G2) |
| ICU care | No | Yes | No | No | No | No | Yes | No | Yes | No |
| Respiratory support modalityb | None | MV | None | Nasal O2 | None | None | Nasal O2 | Nasal O2 | HFNC | None |
| Vasopressor | No | Norepinephrine | No | No | No | No | No | No | No | No |
| ECMO | No | No | No | No | No | No | No | No | No | No |
| Hemodialysis | No | No | No | No | No | No | No | No | No | No |
| Antibiotics | No | Moxifloxacin, piperacillin/tazobactam, teicoplanin, meropenem, ampicillin/sulbactam, minocycline | No | No | No | No | Ceftriaxone, azithromycin, moxifloxacin | No | Moxifloxacin | No |
| Use of steroids | No | Yes | No | No | No | No | No | No | No | No |
| Time to negative PCR, daysc | N/A | 29 (21) | N/A | N/A | 20 (7) | 17 (12) | 23 (11) | N/A | N/A | N/A |
| Prognosis | Transferred | On admission | Transferred | On admission | Discharged | Discharged | Discharged | On admission | On admission | On admission |
aTime from onset of COVID-19-related symptoms and hospital admission.
bHighest level of respiratory support during hospital admission.
cTime from onset of COVID-19-related symptoms to confirmation of a first negative SARS-CoV-2 PCR result. Time from start of RDV to confirmation of a negative SARS-CoV-2 PCR result is given in parentheses.
F, female; M, male; GERD, gastroesophageal reflux disease; CT, computed tomography; GGO, ground glass opacity; COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; RDV, remdesivir; AE, adverse event; G, grade; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ICU, intensive care unit; MV, mechanical ventilation; HFNC, high flow nasal cannula; ECMO, extracorporeal membrane oxygenation; PCR, polymerase chain reaction; N/A, not applicable.
2. Clinical course and adverse events during treatment with RDV
Six patients received RDV for 5 days and the remaining four received it for 10 days. The interval between symptom onset and the first dose of RDV ranged from 5 to 28 days. Figure 1 provides a schematic description of the major events that occurred in each case during up to 30 days of follow-up. The clinical course was variable in this cohort. After completion of 5 or 10 days of treatment with RDV, negative SARS-CoV-2 PCR results were obtained from both nasopharyngeal/oropharyngeal swabs and sputum samples in four patients (40%; Fig. 1).
Several adverse reactions were reported during treatment with RDV, the most common of which was elevated liver transaminase levels (50%). Other adverse events included nausea (30%), diarrhea (10%), chest discomfort (10%), and insomnia (10%). There were no adverse events exceeding grade 2 (Table 2). Patient 10 was included in the 10-day group initially but received only 5 doses of RDV because of elevated transaminase levels and nausea; although these events were not severe enough to warrant cessation of RDV, the investigators decided to withdraw the drug because the patient’s nausea was worsening in the opinion of the attending physician. These adverse events started after 3 days of RDV (Table 2Fig. 1).
3. Treatment outcomes
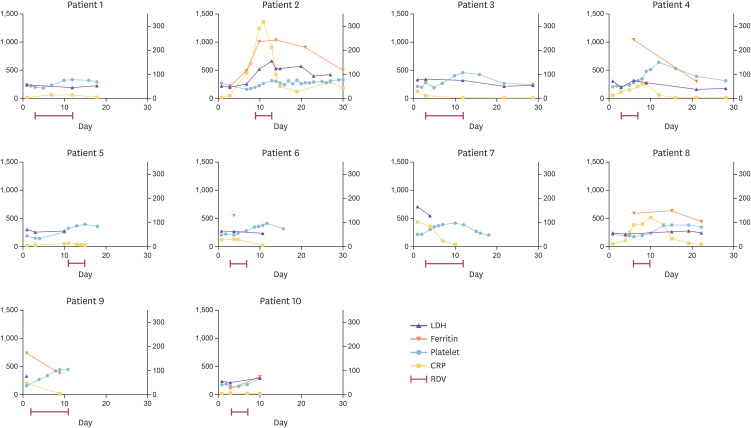
Five patients (50%) required supplemental oxygen therapy; two of these patients were hospitalized in a general ward and three in the intensive care unit (ICU). The two patients on the general ward and one in the ICU required nasal low-flow oxygen (30%) and another patient in the ICU required supplemental oxygen via a high-flow nasal cannula. Patient 2, who was the most severely ill, subsequently developed acute respiratory distress syndrome and received invasive mechanical ventilation, a vasopressor, and systemic glucocorticoids in the ICU. No patient required extracorporeal membrane oxygenation or hemodialysis (Table 2). Figure 2 shows the changes in serum LDH, ferritin, and CRP levels and the platelet count in each patient during admission.
Figure 2. Changes in laboratory test results in 10 patients treated with RDV for COVID-19. Changes in serum LDH (U/L), ferritin (ng/mL), platelet count (× 103 cells/mm3), and CRP (mg/L) levels in each patient. The LDH level, ferritin level, and platelet count are represented on the left y axis and the CRP level is represented on the right y axis.
RDV, remdesivir; COVID-19, coronavirus disease 2019; LDH, lactate dehydrogenase; CRP, C-reactive protein.
Three patients fully recovered from their symptoms, were confirmed to have negative SARS-CoV-2 PCR results in two consecutive examination, and were discharged from hospital. Five patients, who have partially improved symptoms and a positive SARS-CoV-2 PCR result, remained in hospital. The remaining two patients fully recovered from their symptoms but still had a positive SARS-CoV-2 PCR result and were transferred to an isolation facility (Fig. 1).
4. Other therapeutic agents administered
All patients had been treated with lopinavir/ritonavir for more than 2 days before they were started on RDV. Patient 9 had received other anti-COVID-19 agents (lopinavir/ritonavir for 10 days and HCQ for 2 days) at another hospital before admission. Patient 2 was treated with ciclesonide (an inhaled glucocorticoid) and intravenous methylprednisolone for 10 days followed by prednisolone orally for 5 days (Fig. 1).
Three patients (30%) received a variety of antibacterial agents for possible bacterial coinfection (Table 2). Staphylococcus hominis was isolated from a blood culture in patient 2. There was no bacterial growth in any specimen from the other patients.
Discussion
All patients enrolled in this study were considered recovered from COVID-19 or improved based on their clinical symptoms and laboratory and radiologic results. Markers of disease severity, such as CRP, ferritin, and LDH, improved after administration of RDV (Fig. 2). However, this study did not provide sufficient evidence to confirm the efficacy of RDV because the patients had previously received lopinavir/ritonavir or HCQ. Furthermore, more than half of the patients developed elevated transaminases, albeit no worse than grade 2.
In a recently reported study from Europe, one of three patients with COVID-19 who received RDV developed elevated ALT and a maculopapular rash [6]. Furthermore, Grein et al. reported that 12 of 53 patients with COVID-19 from nine countries developed elevated hepatic enzymes after treatment with RDV [7]. It was unclear if the hepatic enzyme elevation was caused by RDV, the viral infection itself, the medications used for symptom control, or underlying comorbidities. However, RDV cannot be excluded as a cause of elevated hepatic enzymes, so close monitoring of liver enzymes is necessary in patients receiving RDV.
A number of drugs are under investigation for treatment of COVID-19. In the early days of the outbreak, lopinavir/ritonavir, two protease inhibitors that are used in combination to treat human immunodeficiency virus infection, were investigated for their anti-SARS-CoV-2 activity. It was reported that lopinavir/ritonavir effectively inhibited replication of coronavirus in an animal model and in vitro [8,9,10]. Many physicians have used lopinavir/ritonavir in patients with COVID-19. However, Cao et al. found that lopinavir/ritonavir had no clinical benefit in COVID-19 and did not improve mortality beyond that achieved by standard care [11]. A large-scale, well-designed investigation is required in the future to determine the activity of lopinavir/ritonavir against SARS-CoV-2 in vivo.
HCQ is another potential candidate for treatment of COVID-19. Chloroquine, an agent that is similar to HCQ, has proven activity against SARS-CoV-2 in vitro [5]. HCQ was found to be more effective than chloroquine as an inhibitor of SARS-CoV-2 in a recent in vitro study [12]. However, HCQ cannot be used as a first-line antiviral agent in patients with COVID-19 because of the lack of clinical data. Moreover, HCQ did not show the improvement of disease progression and mortality in recent two observational studies [13,14]. Well-designed randomized clinical trials are also needed to confirm the clinical benefits of HCQ. However, HCQ is thought to have already been eliminated in candidates for the treatment of COVID-19.
In Japan, three patients with severe COVID-19 showed an improvement in symptoms after being treated with ciclesonide, which is now also widely recognized as a candidate treatment. An inhaled steroid, ciclesonide is thought to inhibit replication of human coronavirus with lower cytotoxicity and more potent suppression of viral growth than other steroids, including prednisolone, dexamethasone, and fluticasone. A report on the findings using ciclesonide in the above-mentioned patients is presently under peer review (Matsuyama et al, manuscript in preparation). The investigators in our study prescribed ciclesonide for patients in whom a steroid would typically be indicated. Ciclesonide also needs to be investigated in a well-designed clinical trial to determine its efficacy as a treatment for SARS-CoV-2.
RDV was originally developed as a treatment for Ebola virus [15]. After administration, RDV is metabolized to GS-441524, which inhibits viral replication by interrupting the function of RNA polymerase and proofreading exoribonuclease [4]. This novel agent was also shown to be an effective inhibitor of filovirus, paramyxovirus, and pneumovirus in the in vitro setting [16]. Moreover, several in vitro studies have reported that RDV can inhibit coronaviruses, including SARS-CoV and MERS-CoV [5,17]. Given that SARS-CoV-2 shares 90.18% of its RNA-dependent RNA polymerase with SARS-CoV [18], RDV can be considered a potential treatment for COVID-19. However, RDV did not show clinical benefits in a recent randomized, double-blind, placebo-controlled study to 237 patients with severe COVID-19 [19]. Nevertheless, RDV is the most potent of the therapeutic candidates for COVID-19 at this time considering findings of recent studies [4,7,19,20,21,22]. Although it was conducted in preliminary setting, RDV contributed to decrease of recovery time in patients with COVID-19 pneumonia in ACTT-1 trial [23]. Korea Centers for Disease Control and Prevention decided to import RDV as therapeutic agent for severe COVID-19 based on the result of this study. However, there are concerns that RDV was not effective in Asian patients in the results of the ACTT-1 trial.
The clinical data for RDV are presently limited, and our experience with RDV is insufficient to confirm its efficacy as a treatment for patients with COVID-19. Our data thus far are based on a case series, albeit obtained from a randomized clinical trial. This preliminary study has some limitations in that (1) the sample size was too small to determine the statistical significance of the findings and (2) some patients had received lopinavir/ritonavir or HCQ before administration of RDV. However, the value of this report is that it summarizes our experience of using RDV in Asian patients with severe COVID-19.
In conclusion, our experience indicates that RDV is a potential therapeutic option for COVID-19 but that a well-designed randomized controlled clinical trial is needed to confirm its efficacy.
Acknowledgments
The authors are grateful to all the medical staff, paramedics, and other staff members of Seoul Medical Center for their work in caring for patients with COVID-19.
Footnotes
Funding: This work was performed using data from the GS-US-540-5773 study that was supported by Gilead Sciences. However, the funder had no role in the study design, data collection, and data analysis in this study.
Conflict of Interest: DHO, MYA, KB, JC, CH, HK, SK, THK, and JO received research grants from Gilead Sciences.
- Conceptualization: DHO.
- Data curation: CL, MYA, KB, JC, CH, HK, SK, THK, JO, DHO.
- Formal analysis: CL, CH, SK, DHO.
- Funding acquisition: MYA.
- Investigation: MYA, DHO.
- Methodology: CH, SK.
- Project administration: DHO.
- Resources: MYA, JC, HK, THK, JO, DHO.
- Software: KB, JO.
- Supervision: DHO.
- Validation: KB, CH, HK, SK, THK, JO, DHO.
- Visualization: CL.
- Writing - original draft: CL, DHO.
- Writing - review & editing: MYA, JC, DHO.
References
- 1.World Health Organization (WHO) Coronavirus disease 2019 (COVID-19) situation report - 101. [Accessed 30 April 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200430-sitrep-101-covid-19.pdf?sfvrsn=2ba4e093_2.
- 2.Korea Centers for Disease Control and Prevention (KCDC) Coronavirus disease 2019 (COVID-19) domestic outbreak status. [Accessed 30 April 2020]. Available at: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=354313&contSeq=354313&board_id=&gubun=ALL.
- 3.Gilead. Company statements: Gilead Sciences statement on the company's ongoing response to the 2019 Novel Coronavirus (2019-nCoV) [Accessed 30 April 2020]. Available at: https://www.gilead.com/news-and-press/company-statements/gilead-sciences-statement-on-the-company-ongoing-response-to-the-2019-new-coronavirus.
- 4.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9:e0221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, Gaymard A, Bouscambert-Duchamp M, Donati F, Le Hingrat Q, Enouf V, Houhou-Fidouh N, Valette M, Mailles A, Lucet JC, Mentre F, Duval X, Descamps D, Malvy D, Timsit JF, Lina B, van-der-Werf S, Yazdanpanah Y. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg P, Kirkwood J, Muse A, DeHovitz J, Blog DS, Hutton B, Holtgrave DR, Zucker HA. Association of treatment with hydroxychloroquine or azithromycin with in-hospital Mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO, Mackman RL, Hui HC, Perron M, Ray AS, Cihlar T, Nichol ST, Spiropoulou CF. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augustin M, Hallek M, Nitschmann S. Remdesivir for patients with severe COVID-19. Internist (Berl) 2020;61:644–645. doi: 10.1007/s00108-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: An evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon A, Le NT, Selisko B, Eydoux C, Alvarez K, Guillemot JC, Decroly E, Peersen O, Ferron F, Canard B. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res. 2020;178:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]