Abstract
Coronavirus disease 2019 (COVID-19) outbreak is spreading rapidly all over the world, being a major threat to public health. Since clinical feature of COVID-19 has not been fully evaluated yet, empirical antibacterial agents are frequently combined for the treatment of COVID-19 in addition to antiviral agents, concerning co-existing bacterial pathogens. We experienced a case of severe thrombocytopenia with epistaxis and petechiae, while treating a COVID-19 patient with ceftriaxone, levofloxacin, and lopinavir/ritonavir. The platelet count decreased to 2,000/mm3 and recovered after discontinuation of the three suspected drugs. In treating a potentially fatal emerging infectious disease, empirical and/or experimental approach would be unavoidable. However, the present case suggests that the possibility of adverse effects caused by polypharmacy should also be carefully considered.
Keywords: COVID-19, Lopinavir/ritonavir, Ceftriaxone, Levofloxacin, Thrombocytopenia
Introduction
Coronavirus disease 2019 (COVID-19) outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is spreading rapidly all over the world, including Korea [1]. As clinical feature of COVID-19 has not been fully evaluated yet, clinicians frequently combine empirical antibacterial agents for the treatment of COVID-19 in addition to antiviral agents, concerning co-existing bacterial pathogens [2]. However, empirical combinations of antibiotic agents may also increase risk of severe adverse effects. We report a case of severe thrombocytopenia with epistaxis and petechiae, while treating a patient with COVID-19 with ceftriaxone, levofloxacin, and lopinavir/ritonavir. Informed consents for publication of clinical data were obtained from the patient.
Case Report
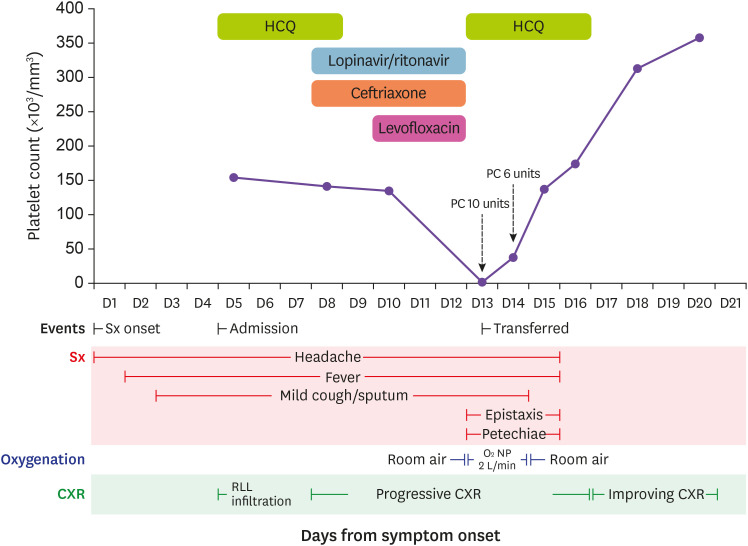
This is a 54-year-old Korean man who was diagnosed with hypertension and diabetes mellitus at the most recent health checkup two months ago. He was not on regular medication yet. He experienced headache on February 19, 2020 (symptom onset day, D1; Fig 1). On the next day he became febrile and productive coughs and myalgia followed. He visited a screening center to be tested for COVID-19, and the diagnosis of COVID-19 was confirmed on February 22 (D4). He was admitted at a public medical center for the treatment of COVID-19 on February 23 (D5). His exposure history to SARS-CoV-2 was unclear.
Figure 1. Clinical course, drug administration, and platelet count of a patient with COVID-19.
A 54-year-old Korean male patient with COVID-19 experienced severe thrombocytopenia with epistaxis and petechiae. Platelet count dropped to 2,000/mm3 while receiving ceftriaxone, levofloxacin, and Lopinavir/ritonavir, while it recovered soon after discontinuation of all the suspected drugs.
COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; PC, platelet concentrate; Sx, symptom; NP, nasal prong; CXR, Chest X-ray; RLL, right lower lobe.
At the time of admission, his chest X-ray (CXR) showed mild bilateral infiltration, but predominantly on the right lower lobe. Routine blood tests including complete blood counts (CBC) were unremarkable; white blood cell (WBC) count 5,490/mm3, hemoglobin 15.1 g/dL, platelet count 154,000/mm3, erythrocyte sedimentation rate (ESR) 24 mm/hr, and C-reactant protein (CRP) 0.12 mg/dL. Hydroxychloroquine (HCQ) 400 mg PO qd was prescribed as an antiviral agent for COVID-19. On February 26 (D8), the antiviral agent was changed to lopinavir/ritonavir 400/100 mg PO twice daily and ceftriaxone 2 g IV q24hr was started as fever and headache persisted with worsening infiltration on CXR. Fever and infiltrates on CXR did not improve by February 28 (D10), levofloxacin 750 mg IV q24hr was added consequently. CBC on this day was as follows: WBC count 7,510 /mm3, hemoglobin 14.9 g/dL, and platelet count 135,000 /mm3.
On March 2 (D13), he had epistaxis on the right nostril, gum bleeding, and petechiae on both arms and legs. CBC showed severe thrombocytopenia (WBC count 5,770 /mm3, hemoglobin 13.0 g/dL, platelet count 2,000 /mm3), while coagulation times were not much prolonged (prothrombin time 14.1 seconds (INR 1.21, 69.1%) and activated partial thromboplastin time 35.0 seconds). A repeat test confirmed the findings were not by an error. Due to high risk of critical bleeding, he was transferred to a tertiary care center after 10 units of platelet concentrates transfused. On arrival at the tertiary care center, his vital signs were stable, but complained of headache and nausea. Intra-cranial hemorrhage was concerned, but close monitoring without immediate imaging was decided since he did not show any neurologic deficit and the headache persisted since the diagnosis of COVID-19 without significant changes in intensity. Follow-up CBC showed platelet count of 38,000 /mm3, and pseudo-thrombocytopenia could be excluded by a peripheral blood smear. After additional 6 units of platelet concentrates were given, his platelet count rose to 113,000/mm3 next morning. After reviewing his clinical course, laboratory tests, and prescriptions, drug-induced thrombocytopenia was strongly suspected considering rapidly progressed thrombocytopenia despite relatively stable course of COVID-19. Three antibiotic agents, ceftriaxone, levofloxacin, and lopinavir/ritonavir, were suspected to be the culprit and all of these were discontinued. HCQ 400 mg PO qd was re-started as the antiviral agent, since he was still febrile and had oxygen requirement of 2 L/minute via nasal prong. His platelet count kept rising without additional transfusion afterward (137,000/mm3 on March 4 (D15), 174,000/mm3 on March 5 (D16), and 313,000/mm3 on March 7 (D18)). His symptoms also improved, and HCQ was discontinues on March 6 (D17). Cycle threshold of polymerase chain reaction for SARS-CoV-12 consequently increased, and the virus became undetected from upper respiratory tract specimen on March 9 (D20).
Discussion
We presented a case of severe thrombocytopenia that occurred in a course of COVID-19 treatment. In this case, the culprit could have either been viral infection itself or the drugs used empirically to treat pneumonia. Thrombocytopenia is commonly found in viral infections [3]. This may be also true in COVID-19, as several recent publications reported thrombocytopenia in this patient group, although its severity was not always described [2,4,5,6]. In a randomized clinical trial which examined the efficacy of lopinavir/ritonavir in COVID-19 [4], researchers found thrombocytopenia occurred in 16 out of 194 participants, and 3 out of 16 who developed thrombocytopenia had platelet counts below 50,000 /mm3.
Lopinavir/ritonavir, a protease inhibitor used for human immunodeficiency virus (HIV) infection, is used experimentally for the treatment of COVID-19, based on previous in vitro and/or in vivo data for severe acute respiratory syndrome coronavirus (SARS-CoV) [7] and Middle East respiratory syndrome coronavirus (MERS-CoV) [8,9]. Although thrombocytopenia with platelet count <50,000 /mm3 had been reported as an adverse effect of lopinavir/ritonavir [10], only one case report of drug-induced thrombocytopenia in a HIV-infected patient could be found in the literature [11]. Considering over 10 years’ experience of clinical use since 2010, incidence of drug-induced thrombocytopenia caused by lopinavir/ritonavir would be low. Reports of drug-induced thrombocytopenia (DITP) by levofloxacin is also limited [12,13,14,15]. Some reported shorter time to reach nadir in platelet count in patients with prior exposure to levofloxacin [13] or even ciprofloxacin [14]. On the contrary, DITP related to ceftriaxone and associated antibody have been reported, although number of cases are also limited [16,17,18,19]. In the most recent case report, abrupt-onset severe thrombocytopenia with platelet count of 1,000 /mm3 occurred after six doses of ceftriaxone [16]. Although exposure history to antibiotics of the present case is not certain, previous sensitization to third-generation cephalosporin and/or fluoroquinolones would be possible, since they are commonly prescribed in primary care settings. The cause of the acute thrombocytopenia in the present case would be likely to be ceftriaxone or levofloxacin, although the possibility of lopinavir/ritonavir cannot be excluded.
Data released by present suggest that bacterial co-infection is not common in COVID-19 [2]. However, clinicians often prescribe empirical antibiotics for the patients with COVID-19 even when the possibility of combined bacterial pneumonia would be low. It would be because we are not familiar with the disease yet, and a possibility of combined bacterial pneumonia cannot be excluded completely as we experience post-influenza bacterial pneumonia [20]. In addition to antibiotic agents, various treatment modalities including corticosteroids and convalescent plasma can be considered, and medications for underlying diseases such as diabetes and hypertension are administered together. In treating a potentially fatal emerging infectious disease, empirical and/or experimental approach is unavoidable. However, the present case suggests that the possibility of adverse effects caused by polypharmacy should also be carefully considered.
In conclusion, we experienced a case of severe thrombocytopenia with epistaxis and petechiae, while treating a COVID-19 patient with ceftriaxone, levofloxacin, and lopinavir/ritonavir. Although severe thrombocytopenia has not been commonly encountered with this widely used empiric antibiotics, they should not be ruled out as a potential culprit.
ACKNOWLEDGMENTS
We express our sincere condolence for the patients and their families who had COVID-19 in the Republic of Korea. We greatly appreciate the efforts of all the hospital employees and their families who are working tirelessly during this outbreak.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: EN, JHK, BHJ, KH, SYC, CIK, DRC, KRP.
- Writing - original draft: EN, JHK, KRP.
- Writing - review & editing: EN, JHK, BHJ, KH, SYC, CIK, DRC, KRP.
References
- 1.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases. Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Kao RYT, Poon LLM, Wong CLP, Guan Y, Peiris JSM, Yuen KY HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, van den Hoogen BG, Neyts J, Snijder EJ. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JFW, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. National Library of Medicine. Drug. Lopinavir/Ritonavir. [Accessed March 13, 2020]. Available at: https://aidsinfo.nih.gov/drugs/316/lopinavir-ritonavir/0/professional.
- 11.Colebunders R, De Schacht C, Vanwolleghem T, Callens S. Lopinavir/ritonavir- and indinavir-induced thrombocytopenia in a patient with HIV infection. Int J Infect Dis. 2004;8:315–316. doi: 10.1016/j.ijid.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Salloum R, Liu CY, Weise AM. Possible case of levofloxacin-induced thrombocytopenia. Am J Health Syst Pharm. 2011;68:43–46. doi: 10.2146/ajhp090564. [DOI] [PubMed] [Google Scholar]
- 13.Shih AW, Lam AS, Warkentin TE. Levofloxacin-induced acute immune-mediated thrombocytopenia of rapid-onset. J Pharm Pract. 2018;31:234–237. doi: 10.1177/0897190017702306. [DOI] [PubMed] [Google Scholar]
- 14.Landi AJ, Burkes R. Probable levofloxacin-induced thrombocytopenia in a patient previously on ciprofloxacin: a case report and literature review. Case Rep Med. 2016;2016:2860645. doi: 10.1155/2016/2860645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polprasert C, Prayongratana K. Levofloxacin-induced severe thrombocytopenia. J Med Assoc Thai. 2009;92(Suppl 3):S69–S71. [PubMed] [Google Scholar]
- 16.Piedra Abusharar S, Shah N, Patel R, Jain R, Polimera HV. A case of confirmed ceftriaxone-induced immune thrombocytopenia. Cureus. 2019;11:e4688. doi: 10.7759/cureus.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquot C, Moayeri M, Kim B, Shugarts S, Lynch KL, Leavitt AD. Prolonged ceftriaxone-induced immune thrombocytopenia due to impaired drug clearance: a case report. Transfusion. 2013;53:2715–2721. doi: 10.1111/trf.12138. [DOI] [PubMed] [Google Scholar]
- 18.George JN. Drug-induced thrombocytopenia. Available at: https://www.ouhsc.edu/platelets/ditp.html.
- 19.Kam T, Alexander M. Drug-induced immune thrombocytopenia. J Pharm Pract. 2014;27:430–439. doi: 10.1177/0897190014546099. [DOI] [PubMed] [Google Scholar]
- 20.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]