Abstract
There are no proven therapeutics for Coronavirus disease 2019 (COVID-19) pneumonia outbreak. We observed and analyzed the clinical efficacy of the most used hydroxychloroquine (HCQ) for 30 days. In this study, administration of HCQ <5 days from diagnosis (odds ratio: 0.111, 95% confidence interval: 0.034 - 0.367, P = 0.001) was the only protective factor for prolonging of viral shedding in COVID-19 patients. Early administration of HCQ significantly ameliorates inflammatory cytokine secretion by eradicating COVID-19, at discharge. Our findings suggest that patients confirmed of COVID-19 infection should be administrated HCQ as soon as possible.
Keywords: Early, Hydroxychloroquine, Coronavirus disease 2019, Eradication
Coronavirus disease 2019 (COVID-19) pneumonia outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection emerged in Wuhan, China in December 2019, rapidly spread to produce a worldwide pandemic [1]. There are no proven therapeutics for COVID-19, and current toll estimate more than 2.7 million infected people and almost 200 thousand deaths worldwide as of April 24 2020, according to data compiled by Johns Hopkins University. Recent studies have reported factors in association with prolonged duration of viral RNA shedding [2,3]. But so far, no study has demonstrated the effects of antiviral drug administration on the duration of viral shedding and clinical improvement in COVID-19 patients. We investigated the time of antiviral drug administration in COVID-19 patients with factors and inhibitors associated with prolonged viral shedding, clinical improvement and cytokine storm, respectively.
We enrolled 99 patients confirmed with SARS-CoV-2 infection at Yeungnam University Medical Center in Daegu, Korea. This study was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital (YUH IRB 2020-03-057, 2020-05-031-001). The requirement for informed consent was waived because of the retrospective study design. The primary outcome of this study was the viral clearance rates in the first 30 days after the diagnosis. The secondary outcome was time to clinical improvement. Clinical improvement was analyzed in two variables: (1) temperature recovery, defined as a patient with body temperature below 37.5°C and does not rise again afterwards; (2) radiologic recovery, defined as an improvement compared to initial imaging findings.
Patients were enrolled if they met one of the following criteria: (1) cured and discharged within 30 days without viral shedding; (2) remained in the hospital without viral shedding; (3) viral shedding at 30 days. Nine patients who died less than 30 days after the diagnosis with viral shedding were excluded. One patient was excluded as the patient were less than 30 days after the diagnosis with viral shedding. And 1 patient was excluded as the patient transferred to therapeutic living center. The final date of follow-up was April 17, 2020.
The patients were confirmed positive with real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay (AllplexTM 2019-nCoV Assay targeting E gene, RdRp gene, and N gene by Seegene Technologies Inc, Seoul, Korea) [4]. After the patient's symptoms improved, specimen sampling of the sputum, nasopharyngeal swab were conducted daily. We conducted specimen collection according to the manufacturer's protocol. For each gene, a cycle threshold value (Ct-value) less than 40 was defined as a positive results, while a Ct-value of more than 40 was defined as negative. Specimen with a Ct-value of more than 40 in both E gene, RdRp gene, and N gene was defined as negative results.
All of the following criteria were required for quarantine release: (1) no fever with improved clinical symptoms for at least 3 days, (2) radiologic improvement assessed by chest X-ray or chest computed tomography, (3) two consecutive negative RT-PCR results obtained during a 24-hour interval sampling of nasopharyngeal swab and sputum.
The National Early Warning Score (NEWS) is an early warning score facilitating prompt detection in response to patient deterioration [5]. Acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [6]. Septic shock was defined according to the third international consensus definitions for sepsis and septic shock (Sepsis-3) [7].
Serum levels of inflammatory cytokines IL-1β, IL-6, TNF-α, and IFN-γ were determined in SARS-CoV-2-infected patients and discharged patients using Human IL-1β Quantikine enzyme-linked immunosorbent assay (ELISA) Kit (DLB50, R&D Systems, Minneapolis, MN, USA), Human IL-6 Quantikine ELISA Kit (D6050, R&D Systems, Minneapolis, MN, USA), Human TNF-α Quantikine ELISA Kit (DTA00D, R&D Systems, Minneapolis, MN, USA), and Human IFN-γ Quantikine ELISA Kit (DIF50, R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The results were expressed as pg/mL.
Forty-three patients were determined as men (43.4%) and the median age was 59 years (interquartile range [IQR], 45 - 68 years). The median duration of viral shedding was 33 days (IQR, 27 - 40 days) from the diagnosis. The time between the diagnosis and clinical improvement was significantly longer in prolonged viral shedding groups (temperature recovery, 3 days (IQR, 1 - 5 days) vs. 6 days (IQR, 2 - 9 days), P = 0.002; radiologic recovery, 12 days (IQR, 10 - 16 days) vs. 21.5 days (IQR, 16 - 28 days), P <0.001). There were no significant differences between prolonged viral shedding with age, sex, NEWS, comorbidities, complications, and treatments. However, the time between the point of diagnosis and antiviral medication was significantly longer in prolonged viral shedding groups (lopinavir/ritonavir, 3 days (IQR, 1 - 4 days) vs. 4 days (IQR, 2.5 - 6.5 days), P = 0.020; hydroxychloroquine (HCQ), 3 days (IQR, 1 - 5 days) vs. 7 days (IQR, 3 - 9 days), P <0.001). When continuous variables were converted to categorical variables with a cut off of 5 days, early administration of lopinavir/ritonavir, and hyxroxychroloquine was associated with decreased rates of prolonged viral shedding. Glucocorticoid usage was not associated with prolonged viral shedding (Table 1). From multivariable logistic regression analysis, it was observed that HCQ <5 days from diagnosis (odds ratio [OR]: 0.111, 95% confidence interval [CI]: 0.034 - 0.367, P = 0.001) was the only protective factor for prolonging of viral shedding in COVID-19.
Table 1. Baseline characteristics according to the duration of viral shedding.
| All patients (n = 99) | Viral shedding duration after diagnosis ≤30 days (n = 39) | Viral shedding duration after diagnosis >30 days (n = 60) | P value | |||
|---|---|---|---|---|---|---|
| Characteristics | ||||||
| Age | 59 (45, 68) | 58 (39, 67) | 58.5 (47, 66.8) | 0.636 | ||
| Sex, male | 43 (43.4) | 18 (46.2) | 25 (41.7) | 0.683 | ||
| Duration from diagnosis to admission | 3 (2, 6) | 3 (1, 4) | 4 (2, 7) | 0.060 | ||
| Duration of viral RNA shedding | 33 (27, 40) | 24 (21, 27) | 37 (33, 44.8) | <0.001 | ||
| Duration from diagnosis to temperature recovery | 4 (2, 8) | 3 (1, 5) | 6 (2, 9) | 0.002 | ||
| Duration from diagnosis to radiologic recovery | 18 (12, 23) | 12 (10, 16) | 21.5 (16, 28) | <0.001 | ||
| NEWS | 1 (0, 3) | 2 (1, 3) | 1 (0, 3) | 0.070 | ||
| Any comorbidity | 43 (43.3) | 17 (43.6) | 26 (43.3) | 1.000 | ||
| Cardiovascular disease | 9 (9.1) | 3 (7.7) | 6 (10.0) | 1.000 | ||
| Cerebrovascular disease | 3 (3.0) | 2 (5.1) | 1 (1.7) | 0.560 | ||
| Chronic lung disease | 3 (3.0) | 1 (2.6) | 2 (3.3) | 1.000 | ||
| Dementia | 3 (3.0) | 1 (2.6) | 2 (3.3) | 1.000 | ||
| Diabetes mellitus | 14 (14.1) | 5 (12.8) | 9 (15.0) | 0.780 | ||
| Hypertension | 32 (32.3) | 11 (28.2) | 21 (35.0) | 0.517 | ||
| Complications | ||||||
| ARDS | 11 (11.1) | 5 (12.8) | 6 (10.0) | 0.748 | ||
| Septic shock | 4 (4.0) | 2 (5.1) | 2 (3.3) | 0.645 | ||
| Acute cardiac injury | 5 (5.1) | 3 (7.7) | 2 (3.3) | 0.380 | ||
| Acute kidney injury | 4 (4.0) | 2 (5.1) | 2 (3.3) | 0.645 | ||
| Medication | ||||||
| Lopinavir/ritonavir | 92 (92.9) | 34 (87.2) | 58 (96.7) | 0.109 | ||
| Diagnosis to first lopinavir/ritonavir days | 3 (2, 6) | 3 (1, 4) | 4 (2.5, 6.5) | 0.020 | ||
| Lopinavir/ritonavir < 5 days of diagnosis | 66 (71.7) | 29 (85.3) | 37 (63.8) | 0.032 | ||
| Lopinavir/ritonavir ≥5 days of diagnosis | 26 (28.3) | 5 (14.7) | 21 (36.2) | |||
| Hydroxychloroquine | 90 (90.9) | 35 (89.7) | 55 (91.7) | 0.736 | ||
| Diagnosis to first hydroxychloroquine, days | 5 (3, 8) | 3 (1, 5) | 7 (3, 9) | <0.001 | ||
| Hydroxychloroquine <5 days of diagnosis | 42 (46.7) | 24 (68.6) | 18 (32.7) | 0.001 | ||
| Hydroxychloroquine ≥5 days of diagnosis | 48 (53.3) | 11 (31.4) | 37 (67.3) | |||
| Glucocorticoid | 22 (22.2) | 10 (25.6) | 12 (20.0) | 0.622 | ||
| Glucocorticoid <5 days of diagnosis | 7 (68.2) | 8 (80.0) | 7 (58.3) | 0.381 | ||
| Glucocorticoid ≥5 days of diagnosis | 15 (31.8) | 2 (20.0) | 5 (41.7) | |||
| Treatment | ||||||
| Oxygen | 28 (28.3) | 10 (25.6) | 18 (30.0) | 0.657 | ||
| HFNC | 7 (7.1) | 3 (7.7) | 4 (6.7) | 1.000 | ||
| IMV | 5 (5.1) | 3 (7.7) | 2 (3.3) | 0.380 | ||
| CRRT | 1 (1.0) | 1 (2.6) | 0 (0.0) | 0.394 | ||
| ECMO | 3 (3.0) | 2 (5.1) | 1 (1.7) | 0.560 | ||
Continuous variables are expressed as median (IQR) and were compared using a Student's t-test. Categorical variables were compared using the chi-squared test or Fisher's exact test. Multivariable logistic regression analysis was performed to identify risk factors for prolonged viral shedding using variables with P values <0.1 in univariable analyses.
RNA, ribonucleic acid; NEWS, national early warning score; ARDS, acute respiratory distress syndrome; HFNC, high flow nasal cannula; IMV, invasive mechanical ventilation; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
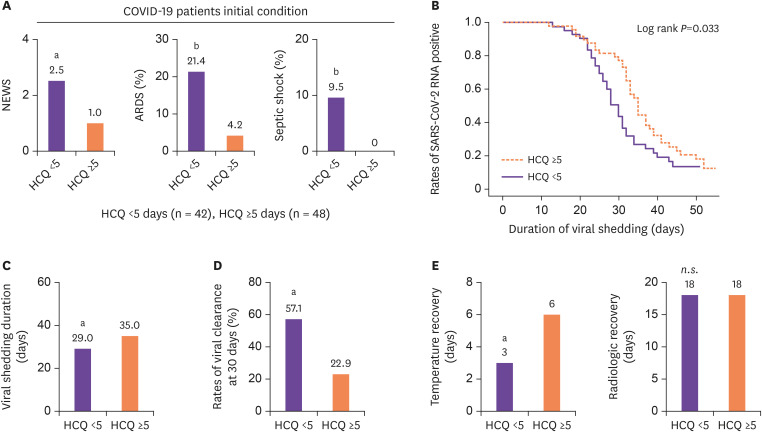
Patients were divided into two groups; one group of patients who took HCQ <5 days from diagnosis (n = 42), and another group of patients who took HCQ ≥5 days from diagnosis (n = 48). Early HCQ administration group had a higher initial NEWS (2.5 (IQR 0.75 - 5.0) vs. 1.0 (IQR 0 - 2.0), P = 0.002), and many had progressed toward ARDS (21.4% vs. 4.2%, P = 0.021) and septic shock (9.5% vs. 0.0%, P = 0.044) (Fig. 1A). Despite the severity of the disease, early administration group showed a significantly shorter viral shedding duration (29 days (IQR 24 - 34.8 days) vs. 35 days (IQR 31 - 41.8 days), P = 0.006), and a higher rate of viral clearance at 30 days (57.1% vs. 22.9%, P = 0.001) (Fig. 1B-1D) than later administration group. When it comes to clinical improvement indicators, early administration group showed a significantly shorter temperature recovery duration (3 days (IQR 0.75 - 5 days) vs. 6 days (IQR 1.25 - 9.75 days), P = 0.002). However, there were no significant difference in radiologic recovery duration between HCQ <5 days and HCQ ≥5 days patients (18 days (IQR 11.75 - 28 days) vs. 18 days (IQR 12 - 23 days), P = 0.563) (Fig. 1E).
Figure 1. Anti-infectious effects of early HCQ administration against COVID-19.
(A) Comparisons of initial NEWS, rates of ARDS, and septic shock early in hospitalization. (B) Cumulative proportion of patients with detectable viral RNA by day after diagnosis confirmed between HCQ <5 days and HCQ ≥5 days patients. (C, D) Comparisons of viral shedding duration, and rates of viral clearance at 30 days between HCQ <5 days and HCQ ≥5 days patients. (E) Comparisons of temperature recovery, and radiologic recovery between HCQ <5 days and HCQ ≥5days patients. Statistical analysis was performed using a two-tailed unpaired t-test. In all analyses, a two-tailed P-value <0.05 was considered to indicate statistical significance. Data are presented as median.
aP <0.01.
bP <0.05.
HCQ, hydroxychloroquine; COVID-19, coronavirus disease 2019; NEWS, national early warning score; ARDS, acute respiratory distress syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RNA, ribonucleic acid; n.s., not significant.
All statistical analyses were performed using SPSS software (ver. 24.0; SPSS Inc., Chicago, IL, USA) and Graphpad Prism.
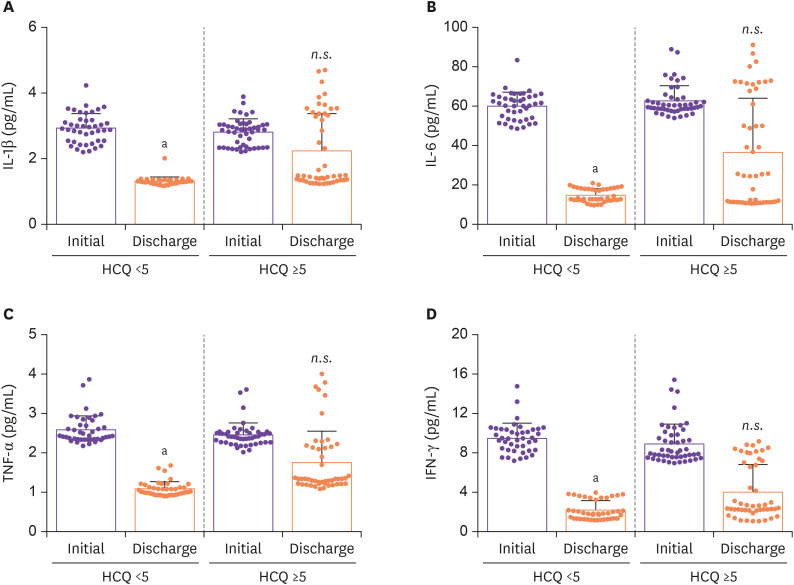
A recent study has reported that the cytokine storm is associated with progression towards ARDS and septic shock [8]. We investigated the level of secreted inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-γ) in the plasma of COVID-19 patients according to the time of HCQ administered (Fig. 2). Based on the ELISA data, it was confirmed that early administration of HCQ significantly ameliorates inflammatory cytokine secretion by eradicating COVID-19, at discharge.
Figure 2. Series of comparisons of plasma cytokines levels in patients at initial and at discharge after HCQ administration. Statistical analysis was performed using a two-tailed unpaired t-test. In all analyses, a two-tailed P-value <0.05 was considered to indicate statistical significance. Data are presented as mean ± SEM.
aP <0.01.
bP <0.05.
HCQ, hydroxychloroquine; SEM, standard error of the mean; n.s., not significant.
All statistical analyses were performed using SPSS software (ver. 24.0; SPSS Inc., Chicago, IL, USA) and Graphpad Prism.
Recent observational study involving 1,446 COVID-19 patients revealed that there were no significant association between HCQ administration and intubation or death. HCQ also revealed no benefit as postexposure prophylaxis for COVID-19 in a randomized trial [9]. Food and Drug Administration (FDA) raises concerns about using HCQ with other medications known to prolong the QT interval. In this study, we only focused on the association between the time of HCQ administration and duration of viral shedding, and proinflammatory cytokine secretion at discharge. Our research alone cannot determine the impact of taking HCQ on clinical outcomes. There were no clinical side effects of obvious arrhythmia in this study.
Previous studies showed that age, male sex, time from illness onset to hospitalization, invasive mechanical ventilation, and severe disease are associated with persistent existence of SARS-CoV-2 [2,10]. Here, administration of HCQ <5 days from diagnosis (OR: 0.111, 95% CI: 0.034 - 0.367, P = 0.001) was the only protective factor for prolonging of viral shedding in COVID-19 patients. Our data reveals that early administration of HCQ is associated with shorter viral shedding duration and a higher rate of viral clearance after 30 days, despite the initial assessment of high disease severity.
In a recent study, it was demonstrated that severe COVID-19 patients tend to have high viral load and prolonged viral shedding period, which correspond to poor clinical outcomes [10,11]. Our results show that early HCQ administration leads to rapid clearance of viral load, and thus could help improve the clinical outcomes. Contrary to general concerns, glucocorticoid was not associated with delayed viral clearance.
In conclusion, after the diagnosis clinicians should consider early administration of HCQ, especially in severe COVID-19 patients. It suggested that the inhibitory and suppressive effect of HCQ on the virus and cytokine storm is an appropriate first-line treatment to COVID-19 infections in the absence of available treatments. Randomized, controlled trials of HCQ in COVID 19 patients are needed.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (grant no. 2018R1A2A3075013 and NRF-2016M3C7A1913845).
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: JHA, KSH, JH, HNK, WL.
- Data curation: JHA, KSH, JGJ, JH, JHL, HNK, WL.
- Formal analysis: JHA, KSH, JGJ, HNK, WL.
- Writing - original draft: JHA, WL, HNK.
- Writing - review & editing: JHA, WL.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu K, Chen Y, Yuan J, Yi P, Ding C, Wu W, Li Y, Ni Q, Zou R, Li X, Xu M, Zhang Y, Zhao H, Zhang X, Yu L, Su J, Lang G, Liu J, Wu X, Guo Y, Tao J, Shi D, Yu L, Cao Q, Ruan B, Liu L, Wang Z, Xu Y, Liu Y, Sheng J, Li L. Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa351. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, She J, Wang Y, Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa451. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, Park JS, Kim GJ, Sung H, Roh KH, Kim JS, Kim HS, Lee ST, Seong MW, Ryoo N, Lee H, Kwon KC, Yoo CK. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84:465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 6.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2016638. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du X, Yu X, Li Q, Li X, Qin T, Luo Q, Wang M, Jiang M, Bai L, Wang X, Pan Y. Duration for carrying SARS-CoV-2 in COVID-19 patients. J Infect. 2020;81:e78–9. doi: 10.1016/j.jinf.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]