Abstract
Introduction
Coronavirus disease 19 (COVID-19), due to severe acute respiratory syndrome-coronavirus 2 (SARS-CoV2), comprises a broad spectrum of clinical presentation ranging from flu-like syndrome to organ failure. The risk of coinfections is high and responsible for a worse prognosis, mainly in the case of bacterial involvement and in the presence of particular comorbidity. We present the clinical, laboratory, radiologic characteristic along with therapeutic management of a patient with COVID-19 and Staphylococcus aureus coinfection.
Case Presentation
A 55-year-old Caucasian woman was admitted to our hospital due to a two-day history of fever and acute dyspnea with severe respiratory failure worsened after the administration of atezolizumab and nab-paclitaxel. Her medical history comprehended a triple negative, BRCA1-related, PD-L1 positive right breast cancer with multiple bone metastasis, causing bone marrow infiltration-related severe pancytopenia. Her physical examination revealed scattered wheezes, rales, and bilateral dry crackles in the middle and lower lung fields and lower limb paresis. The body mass index was 30 kg/m2 and arterial blood gas evaluation revealed a stage III acute respiratory distress syndrome. Microbiological specimens revealed a Staphylococcus aureus positivity from endotracheal aspirate. The chest computed tomography (CT) scan showed the presence of large areas of parenchymal consolidation and aerial bronchogram, bilateral “ground glass” areas reaching the highest extension on the upper and middle zones. The high clinical and radiological suspicion of COVID-19 along with the negative result of nasopharyngeal specimen make necessary an endotracheal aspirate resulting positive for SARS-CoV2. Patient started an antimicrobial treatment and lopinavir-ritonavir plus hydroxychloroquine but, unfortunately, died five days after hospital admission.
Conclusion
The high risk of mortality of our patient was due to viral-bacterial coinfection, advanced cancer status with active immunotherapy. This case highlights the need for a prompt clinical, laboratory, and radiological evaluation to allow a correct diagnosis and start a specific therapy.
Keywords: COVID-19, SARS-CoV2, pneumonia, computed tomography, Staphylococcus aureus, cancer patient
Introduction
Severe acute respiratory syndrome-Coronavirus 2 (SARS-CoV2) is a new onset virus belonging to Coronaviridae family causing flu-like syndrome to organ failure with high risk of coinfections.1
A wide range of computed tomography (CT) findings could be associated with COVID-19 pneumonia including both the interstitial involvement and pulmonary consolidation, respectively associated with a viral or bacterial infection. The early stage of the COVID-19 pneumonia, indeed, is characterized by ground‐glass opacities that progress to a crazy-paving pattern and multifocal patchy consolidation.2–5
Currently, viral and bacterial pathogens are responsible for 38% and 11% of adult community-acquired pneumonia, respectively.6
The 30-day mortality of a viral pneumonia is 7.1% and is associated with older age, viral-bacterial coinfection, underlying malignancy, and shock.7
Coinfection by COVID‐19 and Influenza virus did not significantly worsen the outcome;8 otherwise, viral-bacterial coinfection showed higher mortality rates than unique viral infection up to 60%.7,9
Viral infection could facilitate bacterial superinfection by virus-induced airway damage, promoting bacterial adherence, decreasing mucociliary clearance and impairing the immune system.10 Coronavirus-related pneumonia (no COVID-19) showed higher mortality in cancer than in noncancer patients (24.3% vs 3%),7 and in immunocompromised patients.11
The prognosis of influenza virus infection in cancer patients was affected by many other factors such as bacterial or fungal superinfection, time to diagnosis, and immunosuppression therapy.12
Case Presentation
A 55-year-old Caucasian woman was admitted to our Hospital after a two-day history of fever (38.5°C) and acute dyspnea with severe respiratory distress and room-air oxygen saturation of 70% occurring few hours after the administration of chemoimmunotherapy.
The patient was diagnosed with a triple negative, BRCA1-related, PD-L1 positive right breast cancer four years earlier and was treated with quadrantectomy, radiotherapy and adriamycin-cyclophosphamide and docetaxel-based adjuvant chemotherapy.
The tumor had spread with multiple bone metastasis -skull, vertebral, sternum, scapula, ribs, and femurs- and caused bone marrow infiltration-related severe pancytopenia. She was treated with atezolizumab and nab-paclitaxel as first line chemotherapy.
She suspended, two weeks before, the self-administration of Ganoderma lucidum medicinal mushrooms as an anticancer supplement.
Patient recently received flu vaccination and her remaining medical history comprised chemical diabetes.
The physical examination revealed Glascow Coma Scale 14, body temperature 37.7°C, blood pressure 105/60 mmHg, heart rate 117 beats/minute, respiratory rate 25 breaths/minute, oxygen saturation 90% while receiving a 100% fraction of inspired oxygen (FiO2) on reservoir mask -pH 7.55; pCO2 30 mmHg; pO2 87 mmHg, arterial bicarbonate level of 27.5 mmol/L, arterial lactated level of 21, partial pressure of oxygen (pO2)/FiO2 87 mmHg (stage III Acute Respiratory Distress Syndrome)- BMI 30 kg/m2. Chest examination showed scattered wheezes, rales, and bilateral dry crackles in the middle and lower lung fields; regular cardiac rhythm with paraphonic cardiac sounds. Neurological examination revealed lower limb paresis.
Laboratory tests results included hemoglobin of 6.7 g/dL, hematocrit of 20.2%, platelet count of 18,000/µL, white blood cells of 2210/µL -neutrophil and lymphocytic count of 1720 (77%) and 440/µL (19.9%), respectively- erythrocyte sedimentation rate of 76 mm/h, C-reactive protein of 43.06 mg/dL, procalcitonin of 0.55 ng/dL, and albumin of 2.6 g/dL.
The microbiological sample results for Chlamydia pneumoniae, Mycoplasma pneumoniae, Respiratory Syncytial, Adenoviruses, Coxsackieviruses, Influenza A and B viruses turned out negative, as well as the nasopharyngeal swab for SARS-CoV2. Further tests included negative Streptococcus pneumoniae and Legionella pneumophila urinary antigens, serum galactomannan assay, beta D-glucan assay, immunofluorescence assay for the detection of Pneumocystis jirovecii and blood culture. Quantiferon TB Gold test resulted indeterminate, and Staphylococcus aureus was isolated from endotracheal aspirate.
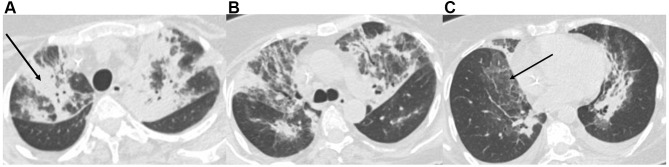
The chest computed tomography (CT) scan showed the presence of large areas of parenchymal consolidation with aerial bronchogram, bilateral “ground glass” areas reaching the highest extension on the upper and middle zones, and a moderate bilateral pleural effusion. Nonspecific sub-centimetric ilo-mediastinal lymph nodes and several metastatic lytic bone lesions were also revealed (Figure 1A–C). The artificial intelligence software (CT deep learning algorithm) estimated a positive predictive value of 64% for COVID-1-related pneumonia.
Figure 1.
Chest computed tomography imaging for the upper (A), medium (B), and lower (C) zones: large areas of parenchymal consolidation with aerial bronchogram (black arrow, A), bilateral “ground glass” areas (black arrow, C), and a moderate bilateral pleural effusion. Nonspecific sub-centimetric ilo-mediastinal lymph nodes.
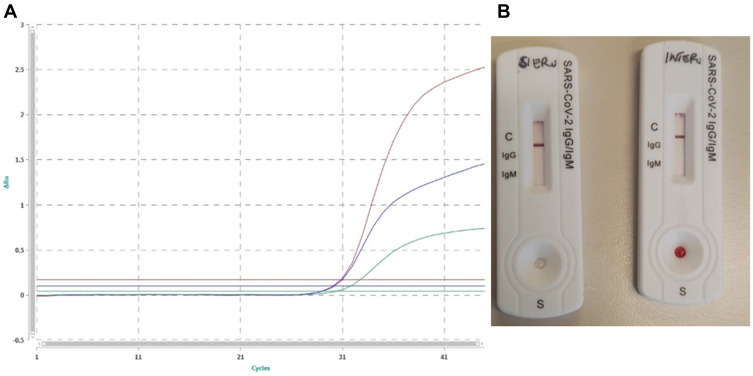
The chest physical examination was suggestive for interstitial pneumonia that was confirmed by the CT scan. Furthermore, based on current epidemiology, the oncologic patient’s frailty suggested the need for an endotracheal aspirate detecting spike protein (S) and envelope (E) genes for SARS-CoV2 molecular test that resulted positive (Figure 2A).
Figure 2.
(A) PCR Real Time SARS-CoV2 on endotracheal aspirate swab. The green curve shows internal control, the red curve shows the gene of PanCoronavirus, the blue curve shows the SARS-CoV2. (B) Immunochromatography performed on the patient’s serum and whole blood showing absence of antiSARS-CoV2 IgM and IgG antibodies.
The bilateral lung consolidation along with the interstitial radiologic pattern and the recent history of G. lucidum administration questioned the real nature of the pneumonia.
However, to date, no data are available on fungal pneumonia due to G. lucidum administration.
The patient started an empiric antimicrobial treatment with vancomycin 2 g/day, meropenem 3 g/day, and levofloxacin 500 mg/day along with amphotericin B therapy (loading dose of 6 mg/kg bid, followed by 300 mg bid) and high flow oxygen supplementation (FiO2100%).
Otherwise, the endotracheal aspirates showed positive results for SARS-CoV2 and for methicillin-sensible S. aureus.13 Antimicrobial therapy was, therefore, de-escalated and lopinavir-ritonavir plus hydroxychloroquine therapy was started. Unfortunately, the patient died five days after hospital admission.
Discussion and Conclusion
Viral infection contributes in bacterial spread providing more adhesion sites, causing cell and tissue structural alteration, and impairing immune system response. Bacterial coinfection was responsible for a worse prognosis characterized by higher disease severity also influenced by viral and bacterial strains, density of bacterial colonization, and the time between viral infection and bacterial coinfection.14
S. aureus, a Gram-positive coccus, may complicate Influenza infection that increase the S. aureus adherence to the host pharyngeal cells. This phenomenon increases patients’ mortality within two to seven days from bacterial coinfection.15
Available evidence does not allow an accurate estimation of the bacterial coinfection prevalence on COVID-19.
In this case, the bacterial coinfection has hidden the typical radiologic feature of COVID-19-related pneumonia.2,3 However, the clinical findings along with the high prevalence of the disease suggested the execution of an endotracheal rather than nasopharyngeal aspirate for SARS-CoV2 that showed a sensitivity of 95% when correctly collected. Interestingly, we performed a qSARS-CoV-2 IgG/IgM cassette rapid test two days after COVID-19 diagnosis showing negative results (Figure 2B). This could be the consequence of a failure of the immunological memory, the patient’s immunosuppression state, or the lower test sensitivity (87.9%).
Respiratory viruses are pathogens of 38% of community-acquired pneumonia, but they can also be responsible for coinfections or bacterial lung superinfections.6 The influenza virus is known to increase the susceptibility to pneumonia caused by S. aureus. Furthermore, this latter caused 4% of sepsis among hospitalized patients with cancer.16
Bacterial superinfection should never be excluded and, in this case, changed the classical radiological alteration lowering the positive predictive value of the artificial intelligence software. Otherwise, immunotherapy could mimic the infectious tomographic pattern, as previously described.2,3
Being the epidemic in Lazio Region represented mostly in big family and rest home clusters, it is important to monitor specific cases, when possible. Clinical diagnosis is a primary and important step sustained by CT and laboratory confirmation.
The reported mortality ranged from 4.3% to 15% in China. The high mortality rate reported in Italy (actually, 11.4%) may be related to an underestimation of the prevalence of the disease related to the quote of asymptomatic population.17–20
We present a case of a breast cancer patient receiving immunotherapy, in which the bacterial coinfection could have covered the radiological diagnosis of Coronavirus pneumoniae, allowed only by the clinical-laboratory and radiological integration.
The high risk of death of our patient related to viral-bacterial coinfection was also increased by the advanced cancer status with active immunotherapy. Moreover, these latter agents may be involved in a more aggressive clinical behavior of viral infections, even if data are still premature and under investigation.
COVID-19 is a systemic disease that can affect several organs or systems (respiratory, cardiac, nervous, gastrointestinal systems, kidney, and skin) with high risk of superinfections and pulmonary embolism, requiring several clinical skills along with a specific radiological and laboratory workup.
Funding Statement
The authors declared that this case has received no financial support.
Abbreviations
COVID-19, coronavirus disease 19; SARS-CoV2, severe acute respiratory syndrome-coronavirus 2; CT, computed tomography; BMI, body mass index.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, SS.
Ethics and Consent Statement
Based on the regulations of the department of research of the University Campus Bio-Medico of Rome, an institutional review board approval is not required for case reports.
Consent for Publication
The patient provided informed consent for publication of the case details and images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ieven M, Coenen S, Loens K, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24:1158–1163. doi: 10.1016/j.cmi.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YJ, Lee ES, Lee YS. High mortality from viral pneumonia in patients with cancer. Infect Dis (Lond). 2019;51:502–509. doi: 10.1080/23744235.2019.1592217 [DOI] [PubMed] [Google Scholar]
- 8.Ding Q, Lu P, Fan Y, et al. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020. doi: 10.1002/jmv.25781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Loeches I, Schultz MJ, Vincent JL, et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017;43:48–58. doi: 10.1007/s00134-016-4578-y [DOI] [PubMed] [Google Scholar]
- 10.Cawcutt K, Kalil AC. Pneumonia with bacterial and viral coinfection. Curr Opin Crit Care. 2017;23:385–390. doi: 10.1097/MCC.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 11.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2017;64:1532–1539. doi: 10.1093/cid/cix160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann B, Lehners N, Brodhun M, et al. Influenza virus infections in patients with malignancies – characteristics and outcome of the season 2014/15. A survey conducted by the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Medical Oncology (DGHO). Eur J Clin Microbiol Infect Dis. 2017;36:565–573. doi: 10.1007/s10096-016-2833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancilleri F, Ciccozzi M, Fogolari M, et al. A case of methicillin-resistant Staphylococcus aureus wound infection: phylogenetic analysis to establish if nosocomial or community acquired. Clin Case Rep. 2018;6:871–874. doi: 10.1002/ccr3.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith AM, McCullers JA. Secondary bacterial infections in influenza virus infection pathogenesis. Curr Top Microbiol Immunol. 2014;385:327–356. doi: 10.1007/82_2014_394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS One. 2009;4:e8540. doi: 10.1371/journal.pone.0008540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres VB, Azevedo LC, Silva UV, et al. Sepsis-associated outcomes in critically Ill patients with malignancies. Ann Am Thorac Soc. 2015;12(8):1185–1192. doi: 10.1513/AnnalsATS.201501-046OC [DOI] [PubMed] [Google Scholar]
- 17.Potere N, Valeriani E, Candeloro M, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24:389. doi: 10.1186/s13054-020-03022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benvenuto D, Giovanetti M, Ciccozzi A, et al. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol. 2020;92:455–459. doi: 10.1002/jmv.25688 [DOI] [PMC free article] [PubMed] [Google Scholar]