Highlights
-
•
Morel Lavallée Lesion is an elusive diagnosis & can cause a life-threatening state.
-
•
Diagnosis of Morel Lavallée Lesion is clinical, but aided by several modalities.
-
•
There are several treatment options for Morel Lavallée Lesion.
-
•
Morel Lavallée Lesion should be treated when diagnosed.
Keywords: Morel-Lavallée lesion, Degloving, Crushing injury, Case report
Abstract
Introduction
Morel-Lavallée lesion (MLL) is an uncommon entity, by which shearing forces result in a closed degloving lesion. This can result in an infected hematoma and lead to a life-threatening situation.
Presentation of case
We present a case of a 59-year-old patient who presented to our emergency department. This patient had a crushing injury, and later was found to have an infected MLL. The patient was treated with surgical drainage, and 2 split thickness skin grafts. The patient fully healed.
Discussion
We review the current literature regarding MLL and diagnostic tools in order to accurately and rapidly diagnose this often-missed entity. Special emphasis is given to the treatment of MLL, with the current knowledge as reflected in the literature.
Conclusions
It is important for caregivers to know the diagnostic steps and pitfalls of this elusive diagnosis in order to diagnose and treat MLL quickly, before it turns into a life-threatening state for the patient.
1. Introduction
Morel Lavallée Lesions (MLL) are uncommon shearing injuries resulting in separation of the skin and subcutaneous tissue from the underlying fascia - closed internal degloving injuries, classically occurring around the greater trochanter (30.4%), pelvis (18.6%), thigh (20.1%), knee joint (15.7%), gluteal region (6.4%), lumbosacral area (3.4%), abdominal area (1.4%), lower leg (1.5%) and on the head (0.5%) [1], but can appear elsewhere as well [2].
MLL typically occur in the setting of high-speed motor vehicle accident and direct crush to the area [3,4]. Shearing forces cause bone, muscle and deep fascia shear one way and superficial layers such as the superficial fascia, subcutaneous fat and skin, shear the opposite direction, causing perforating arteries and lymphatic vessels to tear [3].
The detachment of the tissues creates a unique potential space that is filled with hemolymphatic and serotic fluid, blood, necrotic fat and lymphatic tissue, leading to cavity formation. Metabolic and inflammatory products within the fluid then potentiate cellular permeability and further exacerbate leakage into the space created. Later granular tissue forms in the periphery of the cavity and may result in fibrotic pseudo capsule formation [5]. The pseudo capsule, in turn, prevents resorption thus causing chronic fluid accumulation with high risk for infection of tissue necrosis. Since the mechanism of injury is often traumatic, bacterial infection is a concern especially when an open lesion of the skin is also present [6]. The collection of blood products and necrotic material occurs within the space created in the lesion potentially result in abscesses, cellulitis or osteomyelitis [5,7].
This work has been reported in line with the SCARE criteria [8].
2. Case report
A 59 years-old male, with no prior medical history, was injured in his right thigh as a result of a crushing injury during his work as a fisherman. The thigh was crushed between his ship and a rope for 15 min. The patient was transported to a nearby hospital. Fracture was ruled out and the patient was transferred to our hospital, since his home is closer to our center.
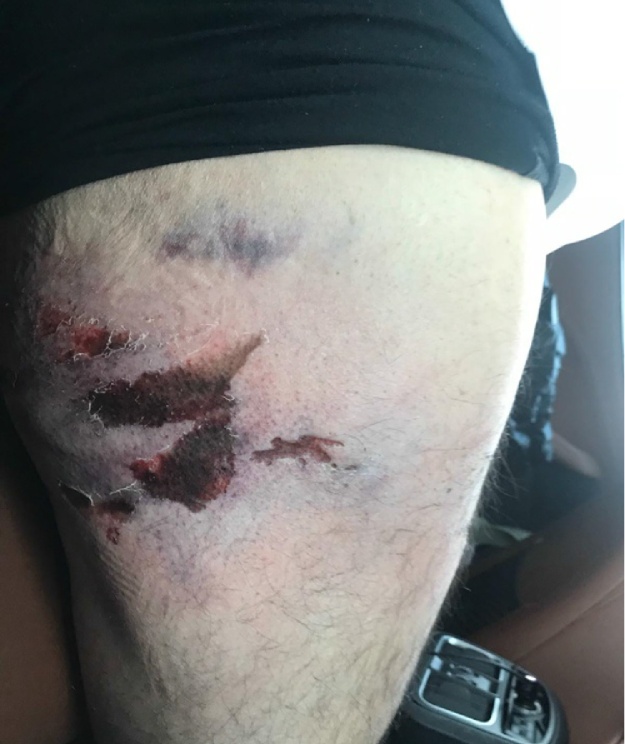
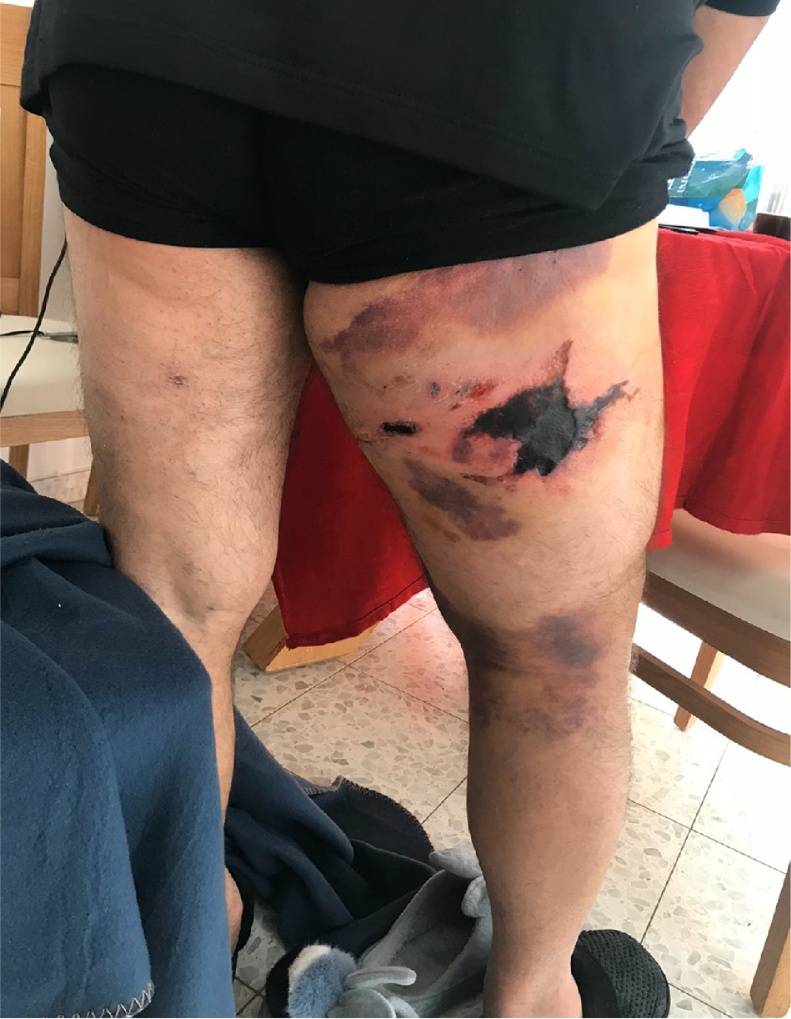
In the emergency department (ED) an abrasion was noted (Fig. 1). Vascular investigation showed patent vessels. X-ray imaging showed no fracture. In computed tomography (CT) there was a significant sub-dermal edema, with fat opacity. The patient was hospitalized for 5 days. Upon his discharge (Fig. 2), a dry superficial necrotic skin area was noted, with no fluctuation or infection.
Fig. 1.
First presentation at our hospital, 7 h after injury.
Fig. 2.
Status at first discharge, 5 days after injury.
One week after discharge, during a routine follow-up, the patent mentioned a fever 2 days prior to the follow-up. He was treated with Augmentin (amoxicillin-clavulanic acid) 875 mg BID. In his exam, there was mild cellulitis noted.
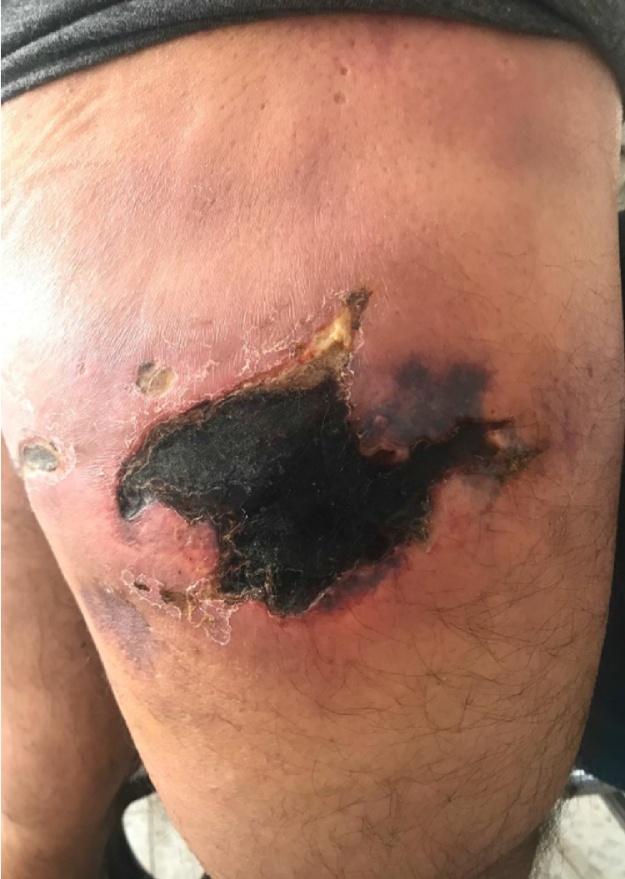
One week later, during the second follow-up, a 3rd year resident physician from our department was called to consult. An area of skin necrosis, roughly 10 × 15 cm, was noted. The was a significant swelling and fluctuation (Fig. 3).
Fig. 3.
Two weeks after first discharge.
The patient rushed to ED, where drainage took place. Approximately 3 L of blood, necrotic muscles and skin were drained. The patient was hospitalized in our department.
During the day of arrival, laboratory findings showed leukocytosis of 21 K, with CRP of 15. The patient underwent CT, which showed hamstring necrosis and a new subdermal collection in the right perineum, continues with the collection in the right thigh. The patient was taken to the operating room (OR).
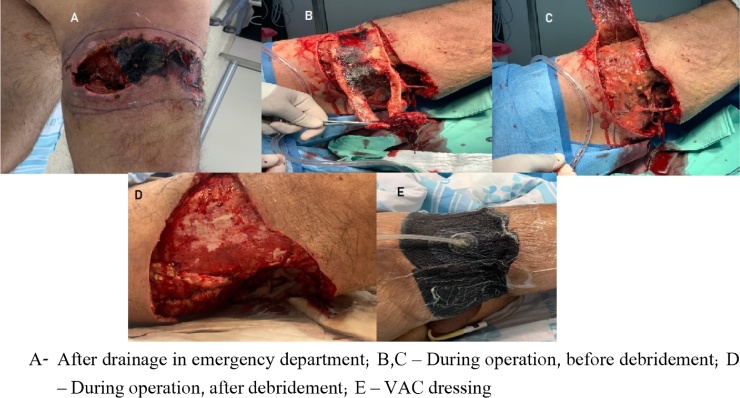
In the OR, the patient underwent extensive debridement of the hamstring’s region to the deep fascia. The patient was dressed with vacuum assisted closure (VAC) dressing. Culture swabs taken and an empiric treatment of Rocephin and Clindamycin initiated. Fig. 4 presents the patient before, during and after surgery.
Fig. 4.
After drainage, during and post operation.
A – After drainage in emergency department; B,C – During operation, before debridement; D – During operation, after debridement; E – VAC dressing.
Due to cultures showing S. aureus and P. aeruginosa the treatment was changed to Ciprofloxacin and Cefazoline.
The patient underwent 2 split thickness skin grafts. The first graft failed due to a different P. aeruginosa found in the wound bed. The second graft succeeded and the patient was discharged to his home.
During follow-up in the outpatient clinic, the patient was dressed with Aquacel® AG (ConvaTec, Oklahoma City, OK, USA) bandage until fully healed. Signs of hypertrophic scar were noted, and the patient was referred to physical therapist for silicone dressing.
3. Review of literature
3.1. Diagnosis
MLL are often delayed or misdiagnosed due to delayed presentation or the presence of distracting injuries, such as in the incidence of multi-trauma. Diagnosis of MLL is mainly a clinical diagnosis and should be taken into account in every traumatic setting. Suspicion should arise in the case of localized swelling, especially with fluctuation. After clinical suspicion imaging studies should be performed. Magnetic resonance imaging (MRI) is the modality of choice since it allows for excellent soft tissue evaluation and high construct resolution. Ultrasound (US) can be done as first step and usually demonstrates the lesion as an anechoic area. Doppler US can be used to rule out other diagnoses and suspicion of vascular compromise such as deep vein thrombosis (DVT) [5,[9], [10], [11], [12]]. CT is not recommended but can demonstrate fluid accumulation. It should be noted that in cases of late diagnosis and imaging, imaging studies might mislead and be interpreted as soft tissue mass or neoplasm. MLL are considered chronic lesion when the lesion contains a capsule [13].
3.2. Treatment
Treatment options range from conservatively to surgically. Nonsurgical management has been recommended if the lesions are remote from a skeletal injury, no fluctuation is found on palpation and the patient has no obvious pain or discomfort [2,8,14]. Others advocated management according to the volume of the cavity created in the lesion, as well as its chronicity [6]. Conservative therapy by compression, activity cessation and/or rest, followed by close monitoring, is considered reasonable in the acute phase for lesions of limited size.
Cases in which lesions are large, chronic or none-responsive to conservative management needle aspiration or drainage should be applied. Needle aspiration should be applied for MLL with a volume of less than 50 mL, since MLL with volumes higher than this have shown to recur. Therefore, aspiration of more than 50 mL of fluid is an indication for operative intervention [4].
Early percutaneous drainage with debridement, irrigation and suction drainage also appears to be safe and effective. In a series of 19 patients, all patients underwent treatment within 3 days of injury. Drainage was usually completed through 2 incisions - one over the most distal aspect of the lesion and one over the most superior and posterior extent of the MLL. Debridement followed by a hemovac drain placed within the lesion. It was concluded that early percutaneous drainage is an excepted initial therapy strategy [15].
Simple open incision drainage of the collection is a common treatment method. Usually done with a 2 cm midline incision over the proximal aspect of the fluid collection, evacuation of fluid and irrigation with normal saline 0.9%/betadine solution. The wound can be closed with a VAC sponge to eliminate the dead space [5].
Series of 13 patients with lower limb MLL showed the efficacy of nose ring drainage technique, combined with compression elastic bandaging [16]. The placing of drainage was in the OR, under general or spinal anesthesia, within 35 days from injury. All bacterial cultures of aspired fluid were negative. All 13 patients healed without complications and obtained a good cosmetic effect. The drains were removed only when there was no more drainage from the incision site. The continuous drainage and adequate pressure provided appropriate conditions for fast recovery of the cavity and reduction in the recurrence rate [16].
Another option is the use of liposuction. That was demonstrated in treatment of a 33-years old woman who presented with a large MLL in her left thigh, 3 weeks after involvement in a motor vehicle accident. Fluid collection was confirmed by x-ray and CT. Liposuction of the cavity and seroma wall was performed and a temporary drain, oral antibiotics and thigh compression were administered post-operatively. No fluid collection was evident in a 1- and 6-weeks follow-up examinations [17].
The use of sclerosing agents was described as a method to close potential space and cavity formed in the lesion. Sclerosing agents cause cell destruction within the periphery of the lesion and induces fibrosis. Doxycycline has shown efficacy in lesions up to 700 mL in volume, with the mean volume of treated lesions being approximately 400 mL [18]. The use of talc to treat chronic MLL was described in 4 patients - all presented with lesion in the thigh or buttock area that persisted for more than 3 months. Talc was administered under fluoroscopic guidance and suction drainage was applied for 12 days. All persistent pseudocysts showed an immediate cessation of fluid accumulation in the treated space without recurrence [19]. Other agents such as bleomycin, tetracycline and erythromycin have been utilized but are not considered ideal agents [20].
There are no mentions in current literature of specific indications for skin grafting or flap transplantation as specific treatment for MLL. It is therefore should be considered in cases of open wound.
4. Conclusion
MLL is an uncommon entity, which can result in a life or organ threatening situation. It is important for the caretakers, in ED and community clinics, to be aware of this entity, it's complications and treatment options.
Declaration of Competing Interest
All authors state no conflict of interest.
Funding
No funding was received for this research.
Ethical approval
This study is exempt from ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author’s contribution
Pikkel YY – writing the paper.
Hasan MJ – Treatment of patient, supplying figures.
Ben-Yehuda Raz D – writing the paper.
Ben-Naftaly Y – Treatment of patient.
Duek OS – Treatment of patient.
Ullman Y – Treatment of patient.
Registration of research studies
N/A.
Guarantor
Yoav Y. Pikkel.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Vanhegan I.S., Dala-Ali B., Verhelst L., Mallucci P., Haddad F.S. The Morel-Lavallée lesion as a rare differential diagnosis for recalcitrant bursitis of the knee: case report and literature review. Case Rep. Orthop. 2012;2012 doi: 10.1155/2012/593193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scolaro J.A., Chao T., Zamorano D.P. The Morel-Lavallée lesion: diagnosis and management. J. Am. Acad. Orthop. Surg. 2016;24(10):667–672. doi: 10.5435/JAAOS-D-15-00181. [DOI] [PubMed] [Google Scholar]
- 3.Hudson D.A., Knottenbelt J.D., Krige J.E. Closed degloving injuries: results following conservative surgery. Plast. Reconstr. Surg. 1992;89(5):853–855. doi: 10.1097/00006534-199205000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson T.P., Zielinski M.D., Jenkins D.H., Schiller H.J. The Mayo Clinic experience with Morel-Lavallée lesions: establishment of a practice management guideline. J. Trauma Acute Care Surg. 2014;76(2):493–497. doi: 10.1097/TA.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 5.Mooney M., Gillette M., Kostiuk D., Hanna M., Ebraheim N. Surgical treatment of a chronic Morel-Lavallée lesion: a case report. J. Orthop. Case Rep. 2020;9(6):15. doi: 10.13107/jocr.2019.v09.i06.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vander Doelen T., Manis A. Conservative management of Morel-Lavallée lesion: a case study. J. Can. Chiropr. Assoc. 2019;63(3):178. [PMC free article] [PubMed] [Google Scholar]
- 7.Zairi F., Wang Z., Shedid D., Boubez G., Sunna T. Lumbar Morel-Lavallée lesion: case report and review of the literature. Orthop. Traumatol. Surg. Res. 2016;102(4):525–527. doi: 10.1016/j.otsr.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Tejwani S.G., Cohen S.B., Bradley J.P. Management of Morel-Lavallée lesion of the knee: twenty-seven cases in the national football league. Am. J. Sports Med. 2007;35(7):1162–1167. doi: 10.1177/0363546507299448. [DOI] [PubMed] [Google Scholar]
- 10.Diviti S., Gupta N., Hooda K., Sharma K., Lo L. Morel-Lavallée lesions-review of pathophysiology, clinical findings, imaging findings and management. J. Clin. Diagn. Res.: JCDR. 2017;11(4):TE01. doi: 10.7860/JCDR/2017/25479.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellado J.M., Bencardino J.T. Morel-Lavallée lesion: review with emphasis on MR imaging. Magn. Reson. Imaging Clin. 2005;13(4):775–782. doi: 10.1016/j.mric.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert B.C., Bui-Mansfield L.T., Dejong S. MRI of a Morel-Lavellée lesion. AJR Am. J. Roentgenol. 2004;182(5):1347. doi: 10.2214/ajr.182.5.1821347. [DOI] [PubMed] [Google Scholar]
- 13.Shen C., Peng J.P., Chen X.D. Efficacy of treatment in peri-pelvic Morel-Lavallée lesion: a systematic review of the literature. Arch. Orthop. Trauma Surg. 2013;133(5):635–640. doi: 10.1007/s00402-013-1703-z. [DOI] [PubMed] [Google Scholar]
- 14.Nair A.V., Nazar P.K., Sekhar R., Ramachandran P.V., Moorthy S. Morel-Lavallée lesion: a closed degloving injury that requires real attention. Indian J. Radiol. Imaging. 2014;24(3):288. doi: 10.4103/0971-3026.137053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng S., Tornetta P., III Percutaneous management of Morel-Lavallée lesions. JBJS. 2006;88(1):92–96. doi: 10.2106/JBJS.E.00021. [DOI] [PubMed] [Google Scholar]
- 16.Li P., Ning X., Jia L., Du G., Jiang S., Gong Z. A minimally invasive incision and loop drainage technique for the treatment of lower limb Morel-Lavallée lesions: Nose ring drainage technique. Injury. 2020;51(2):570–573. doi: 10.1016/j.injury.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Kalaria S.S., Boson A., Griffin L.W. Liposuction treatment of a subacute Morel-Lavallée lesion: a case report. Wounds: A Compend. Clin. Res. Pract. 2020;32(4):E23–E26. [PubMed] [Google Scholar]
- 18.Penaud A., Quignon R., Danin A., Bahe L., Zakine G. Alcohol sclerodhesis: an innovative treatment for chronic Morel-Lavallée lesions. J. Plast. Reconstr. Aesthetic Surg. 2011;64(10):e262–e264. doi: 10.1016/j.bjps.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Luria S., Applbaum Y., Weil Y., Liebergall M., Peyser A. Talc sclerodhesis of persistent Morel-Lavallée lesions (posttraumatic pseudocysts): case report of 4 patients. J. Orthop. Traumatol. Rehabil. 2006;20(6):435–438. doi: 10.1097/00005131-200607000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Greenhill D., Haydel C., Rehman S. Management of the Morel-Lavallée lesion. Orthop. Clin. North Am. 2016;47(January (1)):115–125. doi: 10.1016/j.ocl.2015.08.012. PMID: 26614926. [DOI] [PubMed] [Google Scholar]