Abstract
mRNA-lipoplex vaccines are currently being explored in phase II clinical trials for the treatment of patients with advanced solid tumors. Mechanistically, these mRNA-lipoplex vaccines are characterized by the induction of type I interferon (IFN) centered innate responses. Earlier studies have identified type I IFNs as major regulators of the T cell response instigated by mRNA-lipoplex vaccines. However, stimulatory or, in contrast, profound inhibitory effects of type I IFNs were described depending on the study. In this mouse study, we demonstrated that the opposing roles of type I IFN signaling on the magnitude of the vaccine-evoked T cell responses is dependent on the route of mRNA-lipoplex administration and is regulated at the level of the T cells rather than indirectly through modulation of dendritic cell function. This study helps to understand the double-edged sword character of type I IFN induction upon mRNA-based vaccine treatment and may contribute to a more rational design of mRNA vaccination regimens.
Keywords: mRNA, mRNA-lipoplex, type I IFN, mRNA vaccination, T cel responses
Graphical Abstract
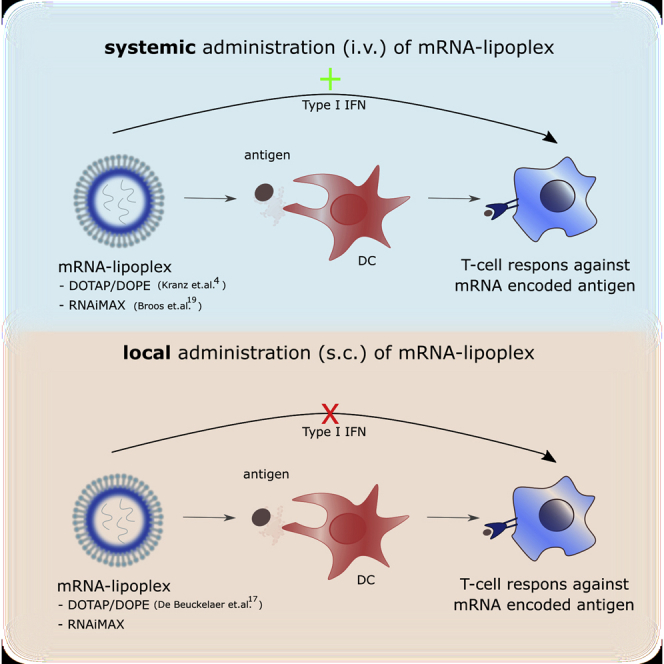
Van Hoecke et al. showed that the route of mRNA-lipoplex injection determines whether type I IFN signaling suppresses or rather stimulates the magnitude of the mRNA vaccine-evoked T cell responses. Moreover, this effect operates at the level of the T cells rather than the dendritic cell.
Introduction
In-vitro-transcribed mRNA has emerged as a promising new class of biologics in a wide variety of treatment areas.1, 2, 3, 4, 5, 6, 7, 8, 9, 10 In-vitro-transcribed mRNA indeed constitutes an extremely versatile platform, enabling one to encode any therapeutic protein of interest, which can be expressed in the cytosol, as a membrane protein, or secreted in the extracellular environment. In the context of vaccination, mRNA vaccines have shown great potential to evoke cytolytic CD8+ T cells, as well as antibody responses, making them particularly attractive modalities for therapeutic cancer vaccine development.11, 12, 13, 14
mRNA is a hydrophilic, negatively charged macromolecule with limited capacity to cross the hydrophobic membranes of cells. Therefore, packaging the mRNA in a nanoparticle format that enables efficient uptake by cells and endosome-to-cytosol translocation is key to success.15,16 mRNA-lipoplexes, in which mRNA is electrostatically complexed into liposomes that contain a cationic lipid and a fusogenic helper lipid, are currently explored as therapeutic cancer vaccines in several clinical studies (e.g., ClinicalTrials.gov: NCT02410733 and NCT02316457). Subcutaneous (s.c.) immunization with mRNA-lipoplexes composed of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)/dioleoylphosphatidylethanolamine (DOPE) was first demonstrated to yield cytolytic T cell responses in mice, albeit of limited potency and magnitude.17,18 More recently, Kranz et al.4 reported that intravenous (i.v.) mRNA-lipoplex vaccination represents a superior route of administration compared with the s.c. or intradermal (i.d.) route, with the induction of high-level T cell responses that showed profound antitumor efficacy in syngeneic tumor models. These promising efficacy data, alongside adequate safety in non-human primate studies, have fueled several clinical studies in patients with metastatic melanoma (ClinicalTrials.gov: NCT03897881 and NCT03480152) and non-small cell lung cancer (ClinicalTrials.gov: NCT03908671 and NCT03948763). Induction of strong T cell responses was also reported by Broos et al.19 upon i.v. immunization of mice with alternative but closely related mRNA-lipoplexes based on RNAiMAX. In all of these studies, type I interferons (IFNs) were identified as the most prominent cytokines elicited early upon mRNA-lipoplex administration.4,17, 18, 19
Type I IFNs constitute a large family of cytokines consisting of the well-studied IFN-α and IFN-β and the less characterized IFN-ε, IFN-τ, IFN-κ, IFN-ω, IFN-δ, and IFN-ζ. Upon recognition of microbial nucleic acids by innate RNA sensors, type I IFN production is initiated.20 Subsequently, these type I IFNs bind to the heterodimeric IFN-α/β receptor (IFNAR) complex consisting of the IFNAR1 and IFNAR2 chains.21 An exception to this is IFN-β that was reported to also bind to IFNAR1 homodimers. Upon IFNAR signaling, the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway is activated.21,22
In-vitro-transcribed mRNA triggers the same innate RNA sensors that have evolved to recognize microbial RNAs, hence evoking the type I IFN signature that is typically associated with viral infections. Type I IFNs are pleiotropic antiviral cytokines that can affect nearly every step of the immune response to mRNA vaccination, ranging from mRNA expression over dendritic cell (DC) activation to T cell differentiation. Not surprisingly, type I IFNs hence were found to be central mediators of T and B cell response evoked to mRNA vaccines. More recently, Zhong et al.23 found that type I IFNs elicited after i.d. vaccination with a self-amplifying RNA against Zika virus negatively modulated the B and T cell responses.
In the context of mRNA lipoplex vaccination, both beneficial and detrimental effects of type I IFN on the vaccine-elicited T cell response were reported. Type I IFNs were reported to be important for the induction of antigen-specific T cell responses following i.v. administration of mRNA-lipoplex vaccines,4,19 yet in sharp contrast, type I IFN signaling was associated with potent suppression of T cell responses upon s.c. and i.d. mRNA vaccination.17,24
With the current study, we intend to shed light on the mechanisms underlying this apparently opposing role of type I IFN in the context of systemic versus topical immunization with mRNA-lipoplexes. We first confirmed the opposing roles of type I IFN on T cell immunity upon i.v. versus s.c. administration, using the same mRNA and lipoplexes (DOTAP/DOPE and RNAiMAX) as described in earlier studies, thereby excluding lab-to-lab variability as a potential underlying cause of these apparently conflicting reports. We next investigated the contribution of IFNAR signaling on the level of the DCs or T cells by using mice that lack the type I IFN receptor (IFNAR) selectively in CD11c+ cells (CD11c-Cre+/−IFNARfl/fl mice) or T cells (CD4-Cre+/−IFNARfl/fl mice). We were able to demonstrate that IFNAR signaling exerts its inhibitory versus stimulatory role directly at the level of the T cells and not indirectly by modulating antigen expression and function of DCs.
Results
Induction of Type I IFN Reporter Gene Expression upon Local and Systemic Injection of Two Types of mRNA-Lipoplexes
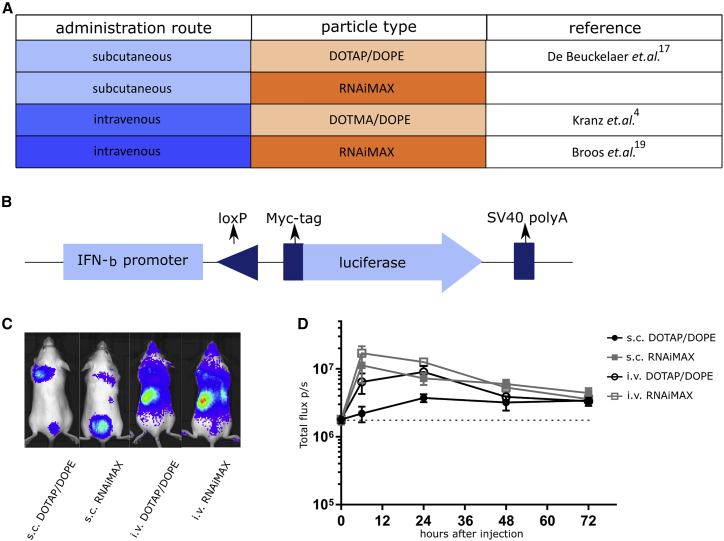
To formally exclude that the reported discrepancies on the role of type I IFN were caused by differences in mRNA format or lipoplex manufacturing, we performed a head-to-head comparison of s.c. and i.v. vaccination using mRNA from a single commercial provider and using identically prepared batches of mRNA-lipoplexes (both DOTAP/DOPE and RNAiMAX). To shed light on the seemingly opposing roles that type I IFN can exert on the magnitude of the T cell response following immunization with mRNA-lipoplexes, we first analyzed the intensity and kinetics of the induction of a reporter gene that is under the transcriptional control of the IFN-β promoter for the two kinds of lipoplexes (DOTAP/DOPE versus RNAiMAX) and two delivery routes (s.c. and i.v.) using an IFN-β reporter mouse strain25 (Figures 1A and 1B). Heterozygous reporter mice (IFN-β+/Δβ-luc) were used to retain the feedforward loop of type I IFN production. Ten micrograms of CleanCap OVA mRNA was packed with either DOTAP/DOPE or RNAiMAX and injected i.v. or s.c. at the tail base of the mice. Regardless of the particle type used and the route of mRNA-lipoplex administration, we observed expression of the reporter gene that peaked 6–24 h after injection (Figures 1C and 1D). After s.c. injection the RNAiMAX formulation tended to be more innate stimulating than the DOTAP/DOPE formulation.
Figure 1.
mRNA-Lipoplexes Induce a Type I IFN Response In Vivo Independently of the Used Particle or Route of Administration
(A) Tabular view of the four possible combinations of mRNA-particles and route of immunization that were tested based on the reports of Broos et al.,19 Kranz et al.,4 and De Beuckelaer et al.17 (B) A schematic representation of the IFN-β reporter transgene. By using a Cre-Lox system, the myc-tagged luciferase gene is under the control of the IFN-β promoter in one allele of the IFN-β gene.25 (C and D) IFN-β+/Δβ-luc mice were injected s.c. or i.v. with 10 μg CleanCap OVA mRNA wrapped in DOTAP/DOPE or RNAiMAX lipoparticles. (C) Whole-body luminescence was measured 24 h after injection of mRNA-lipoplexes. (D) Whole-body luminescence was measured at 0, 6, 24, 48 or 72 h after injection of mRNA-lipoplexes. Data are shown as a mean ± SD of five mice. The dotted line represents the background intensity.
The Route of Administration Governs the Impact of Type I IFN on the Induced T Cell Response
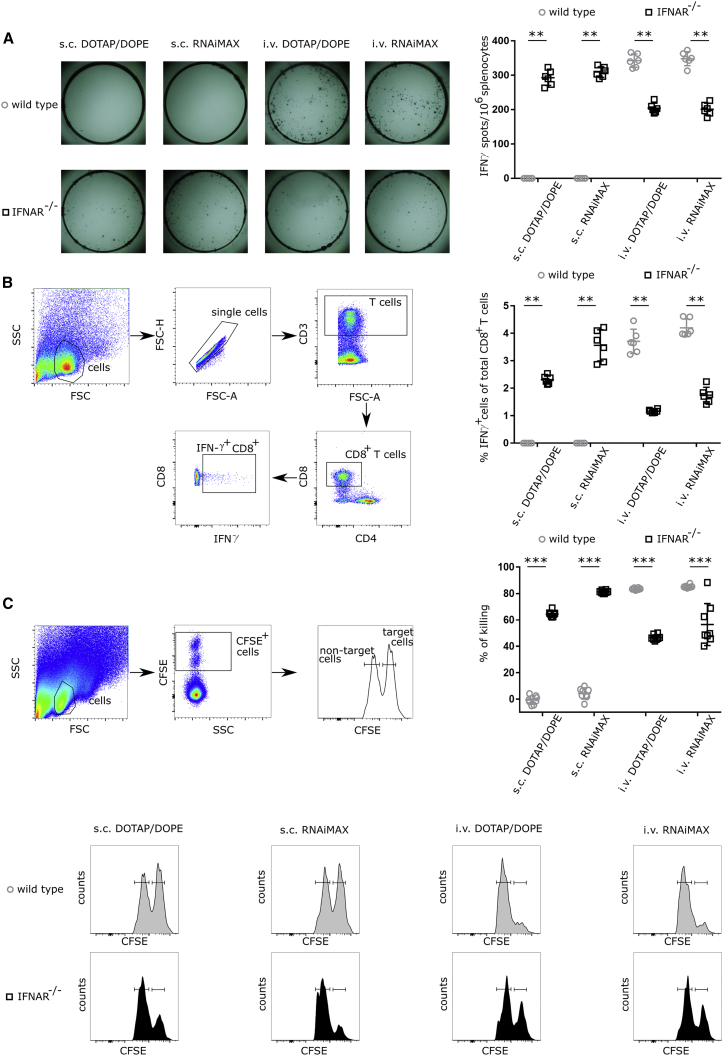
The impact of type I IFN signaling on the magnitude and functionality of the generated T cell response was assessed through comparative immunization studies in wild-type C57BL6 mice and in mice lacking the common IFNAR1 (IFNAR−/−).
Mice were immunized with mRNA encoding ovalbumin (OVA) in a prime-boost-schedule. mRNAs contained a Cap1 structure through co-transcriptional incorporation of CleanCap (TriLink). No modified nucleotides were incorporated. Two weeks after the second immunization, the T cell responses were analyzed using an IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay, an intracellular cytokine staining (ICS) specific for IFN-γ, and an in vivo killing assay.
As depicted in Figures 2A and 2B, IFN-γ-secreting cells were undetectable in splenocytes isolated from wild-type mice after s.c. immunization with DOTAP/DOPE or RNAiMAX-based lipoplexes. Conversely, IFNAR−/− mice displayed high numbers of OVA-specific IFN-γ-secreting T cells after s.c. immunization with both lipoplexes. These results confirm the profound inhibitory effect of type I IFNs on T cell responses induced by mRNA-lipoplexes as reported by De Beuckelaer et al.17 and Pollard et al.18 Upon i.v. administration of OVA encoding mRNA-lipoplexes, high numbers of OVA-specific CD8+ T cells were readily detectable. IFNAR deficiency in this case led to a partial reduction in the magnitude of the evoked response, indicating a positive impact of type I IFN signaling upon i.v. mRNA-lipoplex administration and in line with previous reports of Broos et al.19 and Kranz et al.4 (Figures 2A and 2B). We further analyzed the impact of IFNAR signaling on the cytolytic capacity of the evoked CD8+ T cell response for the different immunization routes and mRNA lipoplexes using an in vivo killing assay.26 Whereas loss of type I IFN clearly enabled the cytolytic capacity of the induced T cells in the case of s.c. immunization, its loss was associated with a reduced T cell stimulatory effect in the case of i.v. immunization (Figure 2C).
Figure 2.
The Outcome of Type I IFN Signaling on the Induced T Cell Response Depends on the Route of mRNA-Lipoplex Administration
Wild-type and IFNAR−/− mice were immunized s.c. or i.v. with 10 μg CleanCap OVA mRNA-lipoplexes (DOTAP/DOPE or RNAiMAX). Two weeks after the last immunization, the induced T cell response was determined. (A and B) Spleens were isolated, and the splenocytes were stimulated in vitro with OVA peptide for 16 h. (A) The number of OVA-specific interferon-γ (IFN-γ)-producing splenocytes (SFCs) was determined by enzyme-linked immunosorbent spot (ELISpot). Data in the graph on the right represent individual mice (n = 8 per group) and the mean. (B) OVA-specific T lymphocytes in the spleens of mRNA-lipoplex-immunized mice were measured by flow cytometry using an intracellular staining against IFN-γ. Data in the graph represent individual mice (n = 8 per group) and the mean +/− SD. (C) A mixture of CFSE-labeled cells pulsed with control (CFSElow) or OVA peptide (CFSEhigh) were adoptively transferred in a 1:1 ratio to the indicated mRNA-lipoplex-immunized mice. Specific killing was measured 2 days later by flow cytometry. Data in the top right graph represent individual mice (n = 8 per group) and the mean. Representative flow cytometry plots are shown below. Statistical analyses on datasets were performed by Mann-Whitney test. Asterisks indicate statistical significance (∗∗p < 0.05, ∗∗∗p < 0.01).
Taken together, these experiments confirm that the differential impact of type I IFN on the T cell response4,17,19 primarily depends on the route of the mRNA-lipoplex administration and is not caused by lab-to-lab variability nor by the type of lipoplex.
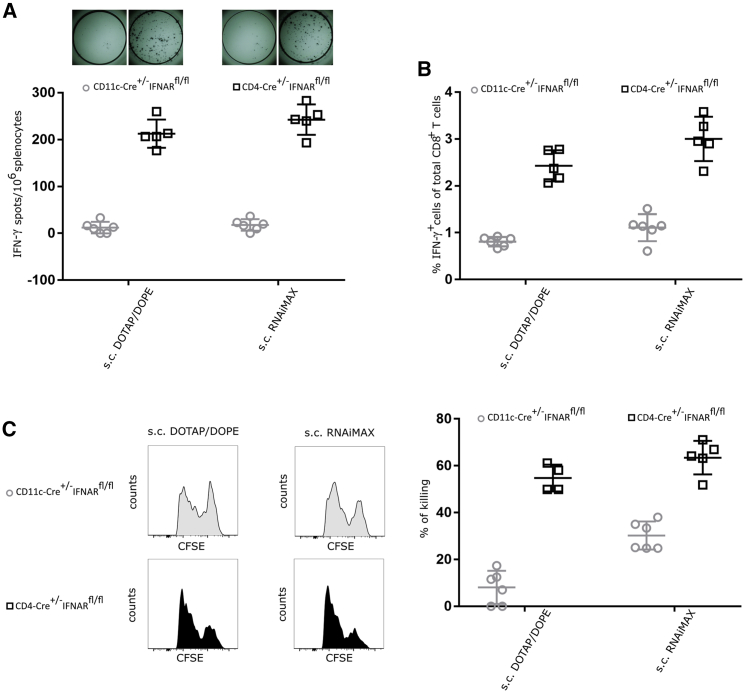
IFNAR Signaling in T Cells Interferes with T Cell Immunity upon s.c. Immunization
As shown above, in the case of s.c. delivery, type I IFN signaling clearly exerts a negative impact on the magnitude and functionality of the T cell response. Theoretically, type I IFN could affect T cell immunity directly at the level of the T cell by regulating T cell differentiation and survival, or indirectly at the level of DCs by regulating antigen presentation.27, 28, 29, 30, 31 To unravel whether this negative role of type I IFN is determined by IFNAR signaling at the level of DCs, T cells, or both, we performed comparative immunization in wild-type mice and in mice lacking IFNAR selectively in CD11c+ cells (CD11c-Cre+/−IFNARfl/fl mice) or T cells (CD4-Cre+/−IFNARfl/fl mice). An identical prime-boost immunization regimen was performed as described above.
Similar to wild-type mice (shown in Figure S1), CD11c-Cre+/−IFNARfl/fl mice showed very weak antigen-specific T cell responses in response to s.c. vaccination, meaning that the impact of IFNAR signaling at the level of the DC is fairly limited (Figures S1 and 3). In contrast, CD4-Cre+/−IFNARfl/fl mice displayed strongly elevated T cell responses compared with wild-type mice (Figures S1 and 3). Taken together, these data indicate that upon s.c. administration of mRNA-lipoplexes, type I IFN signaling hampers the generation of antigen-specific T cells at the level of the T cell.
Figure 3.
Type I IFN Signaling on T Cells Suppresses the Overall Outcome of the T Cell Response upon s.c. Delivery of mRNA-Lipoplexes
CD11c-Cre+/−IFNARfl/fl and CD4-Cre+/−IFNARfl/fl mice were immunized s.c. with 10 μg CleanCap OVA mRNA-lipoplexes (DOTAP/DOPE or RNAiMAX). (A and B) Two weeks after the last immunization the induced T cell responses were determined. Spleens were isolated, and the splenocytes were stimulated in vitro with OVA peptide for 16 h. (A) The number of OVA-specific IFN-γ-secreting splenocytes was determined by ELISpot. Data represent individual mice and the mean (five or six mice per group). (B) OVA-specific T lymphocytes were measured by flow cytometry using intracellular staining for IFN-γ. Data represent individual mice and the mean +/− SD (five or six mice per group). (C) A mixture of CFSE-labeled cells pulsed with control (CFSElow) or OVA peptide (CFSEhigh) was adoptively transferred in a 1:1 ratio to mRNA-lipoplex-immunized mice (s.c. route). Specific killing was analyzed 2 days later by flow cytometry. Data represent individual mice and the mean +/− SD (five or six mice per group).
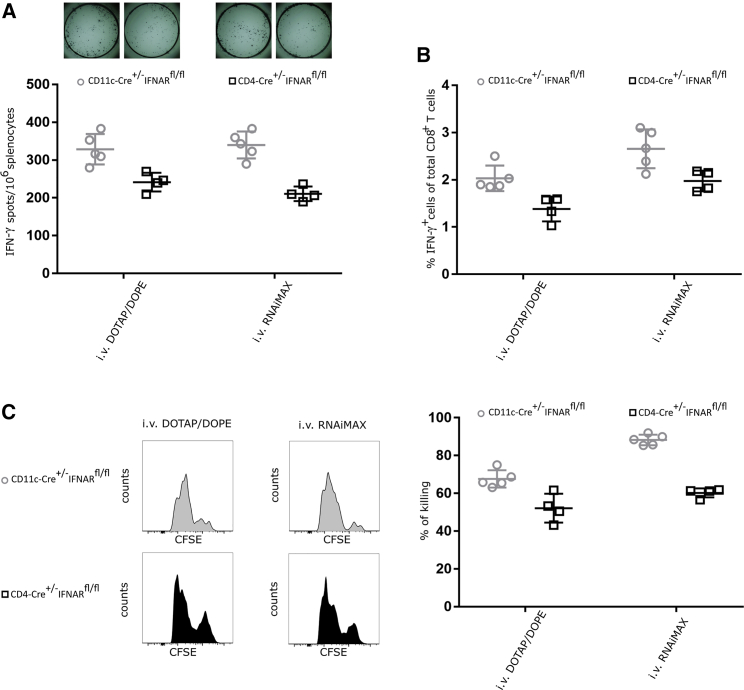
IFNAR Signaling in T Cells Promotes T Cell Immunity upon i.v. Delivery
In contrast with s.c. immunization, type I IFN signaling promotes T cell responses following i.v. administration of mRNA-lipoplexes. To address whether these stimulatory effects are situated at the level of the DC and/or T cell, we now performed comparative immunization experiments for the i.v. vaccination setting. This comparison revealed that, irrespective of the type of particle (DOTAP/DOPE versus RNAiMAX), loss of IFNAR signaling in DCs did not abrogate the T cell response (Figures 4 and S2).19 In contrast, loss of IFNAR signaling in T cells significantly reduced the magnitude and cytolytic functionality of evoked CD8 T cell responses. This shows that type I IFN, also after i.v. mRNA lipoplex injection, mainly acts at the level of the T cell and not at the level of the DC (Figures 4 and S2).
Figure 4.
Type I IFN Signaling on T Cell Enhances the Overall Outcome of the T Cell Response upon i.v. Delivery of mRNA-Lipoplexes
CD11c-Cre+/−IFNARfl/fl and CD4-Cre+/−IFNARfl/fl mice were immunized i.v. with 10 μg CleanCap OVA mRNA-lipoplexes (DOTAP/DOPE or RNAiMAX). Two weeks after the last immunization, the induced T cell responses were determined. (A and B) Spleens were isolated and in vitro stimulated with OVA peptide for 16 h. (A) The number of OVA-specific IFN-γ-secreting splenocytes was determined by an ELISpot. Data represent individual mice and the mean (four or five mice per group). (B) Functionality of OVA-specific T lymphocytes was measured by flow cytometry using intracellular staining for IFN-γ. Data represent individual mice and mean +/− SD (four or five mice per group). (C) A mixture of CFSE-labeled cells pulsed with control (CFSElow) or OVA peptide (CFSEhigh) was adoptively transferred in a 1:1 ratio to mRNA-lipoplex-immunized mice (i.v. route). Specific killing was measured 2 days later by flow cytometry. Data represent individual mice and the mean +/− SD (four or five mice per group).
Discussion
mRNA-lipoplex vaccines have emerged as appealing therapeutic modalities to treat patients with advanced cancers.1, 2, 3,32,33 Earlier studies in mice demonstrated that s.c. administration of mRNA-lipoplexes elicits antigen-specific CD8+ T cells of limited strength and antitumor efficacy.17 More recently, two independent studies showed that i.v. administration of mRNA-lipoplexes resulted in high-magnitude T cell responses and prominent antitumor efficacy, a finding that resulted in several early-stage clinical studies and a phase II trial (ClinicalTrials.gov: NCT03815058) with mRNA-lipoplexes to address safety and efficacy.4,19 Mechanistically, this superior efficacy of i.v. vaccination has been attributed to a systemic targeting of DCs in the spleen, alongside the establishment of a type I IFN centered innate response. Interfering with type I IFN signaling by using IFNAR-deficient mice34 or by antibody-mediated IFNAR blockade indeed resulted in a partial reduction of the magnitude and functionality of the vaccine-elicited CD8 T cell response.4,19 Surprisingly, following s.c. administration of mRNA-lipoplexes, our group previously found that loss of type I IFN signaling was associated with a strongly enhanced cytolytic CD8 T cell response.17,35 Taken together, these reports suggested a complex and opposing role of type I IFN in the regulation of T cell immunity to mRNA vaccines, urging further studies to better comprehend the contribution of type I IFN actions in inducing a cellular immune response by a mRNA-lipoplex vaccine.
To exclude the possibility that the discrepancies between these studies originate from differences in either mRNA, lipoplex format, or experimental conditions, we performed head-to-head comparative immunization experiments in wild-type and IFNAR−/− mice using identical mRNA and two types of lipoplex formulations for each immunization route. These experiments confirmed previous reports,4,17,19 with profound inhibitory effect of type I IFNs signaling upon s.c. administration and stimulatory actions following i.v. administration of the mRNA-lipoplex vaccines. Type I IFNs can affect T cell responses directly, through IFNAR triggering on T cells, and also indirectly, by modulating antigen expression and antigen presentation by DCs.27, 28, 29, 30, 31
By comparing immunization studies in mice that are selectively deficient in IFNAR signaling in T cells (CD4-Cre+/−IFNARfl/fl mice) or in CD11c+ cells (CD11c-Cre+/−IFNARfl/fl mice), we were able to demonstrate that type I IFN signaling regulates the mRNA-lipoplex-induced T cell response predominantly at the T cell level. Selective IFNAR deficiency in T cells reduced the strength of the vaccine-elicited T cell response to a similar extent as full IFNAR deficiency upon i.v. mRNA-lipoplex administration. In this case, type I IFNs thereby act as “signal 3” cytokines that promote T cell differentiation into cytolytic effectors.29,36,37 However, upon s.c. administration, IFNAR deficiency in T cells instead dramatically expanded the strength of the T cell response, showing that the anti-proliferative/pro-apoptotic functions of type I IFN on T cells prevail in this case. This opposing role of type I IFN on T cell function is reminiscent of what has been reported in the context of viral infections.38 Mechanistic studies have revealed that the relative kinetics of IFNAR triggering to T cell receptor (TCR) activation control T cell fate upon type I IFN exposure. If IFNAR triggering precedes TCR, a pro-apoptotic and anti-proliferative transcription program entails. Such a chain of events is likely to unfold in the case of s.c. mRNA-lipoplex immunization, in which type I IFN production occurs almost instantly, yet TCR triggering is delayed until DCs that have internalized the mRNA-lipoplexes have reached the draining lymph nodes from the peripheral tissue. If, however, IFNAR and TCR signaling coincide, type I IFNs act as stimulatory cytokines that promote T cell proliferation, differentiation, and survival. Upon systemic administration, mRNA expression immediately takes place in DCs present in the spleen,19,39 the large secondary lymphoid organ where large numbers of T cells reside, thereby bypassing the need for time-dependent DC migration. Therefore, concomitant TCR activation and type I IFN signaling now can occur in T cells, evoking a T cell-stimulatory transcriptional program. Remarkably, Honke et al.40 showed that expression of the gene encoding the protein Usp18 in macrophages led to lower type I IFN responsiveness. Consequently, expression of USP18 in certain immune cells could also be a possible explanation for the success of the i.v. administration route of non-modified mRNA lipoplexes in spite of type I IFN induction.
In summary, this study contributes to our understanding on the regulation of T cell responses directed against mRNA-encoded antigens that can be elicited by mRNA-lipoplex vaccines. Moreover, these findings enable a more rational design of safer and potentially more potent mRNA-lipoplex vaccines, through the targeted modulation of type I IFN signaling. Despite the strong immunogenicity observed in rodents upon i.v. vaccination, systemic administration of mRNA-lipoplexes comes with an increased safety risk, including potential infusion reactions, liver toxicity, and systemic inflammatory responses. Local administration would be a safer bet, provided it can approach the high immunogenicity seen after i.v. administration. Given the strong T cell responses we have observed upon s.c. immunization of CD4-Cre+/−IFNARfl/fl mice, strategies that interfere with type I IFN induction and/or with IFNAR signaling at the level of T cells could become an appealing avenue to reach this goal.
Materials and Methods
Mice
Seven- to ten-week-old mice, all on C57BL/6 background, were used for all experiments. Wild-type C57BL/6 mice were purchased from Janvier (Le Genest Saint Isle, France). IFN-β reporter mice25 and knockout strains, including mice lacking the common IFNAR1 (IFNAR−/−), CD11c-Cre+/−IFNARfl/fl mice, and CD4-Cre+/−IFNARfl/fl mice, were bred in-house under specific pathogen-free (SPF) conditions. All mice were housed under SPF conditions in individually ventilated cages, in a temperature-controlled environment with 12-h light/dark cycle, with food and water ad libitum. All experiments were approved by the local ethical committee for animal experiments of Ghent University (Ghent, Belgium) (ECD17/54k).
Immunizations and Injections of mRNA-Lipoplexes
CleanCap OVA and luciferase mRNA were purchased from Trilink Biotechnologies (respectively, L-7610 and L-7602). One microgram of mRNA was packaged in 2 μL of Lipofectamine RNAiMAX (Life Technologies). Packaging was performed according to the manufacturer’s instructions. Alternatively, the mRNA was complexed with DOTAP/DOPE lipids in a N/P ratio (the ratio of positively-chargeable polymer amine [N = nitrogen] groups to negatively-charged nucleic acid phosphate [P]) of 1 (Avanti Polar lipids, Alabaster, AL, USA). Ten micrograms of mRNA packed in lipoplexes was injected i.v. (total volume of 100 μL) or s.c. at the tail base (total volume of 40 μL) in a 2-week interval.
In Vivo Imaging of IFN-β Promoter Induction
Heterozygous luciferase reporter mice (IFN-β+/Δβ-luc) were injected s.c. or i.v. with 10 μg OVA-mRNA complexed with DOTAP/DOPE liposomes or RNAiMAX liposomes. Whole-body radiance was measured at 0, 6, 24, 48 or 72 h after injection. The mice were sedated and monitored using an IVIS Lumina II imaging system. Photon flux was quantified using the Living Image 4.4 software (all from Caliper Life Sciences, Hopkinton, MA, USA).
ELISpot
Two days after the second administration, mice were sacrificed and their spleens were isolated and passed through 70-μm nylon strainers (BD Biosciences, San Diego, CA, USA) to obtain single-cell suspensions. Red blood cells were lysed using NH4Cl solution. A total of 2.5 × 105 cells were cultured for 24 h in wells of anti-IFN-γ (Diaclone, Besancon, France) pre-coated 96-well plates in the presence of 10 μg/mL OVA257–264 (SIINFEKL) peptide (12-5743-82; Thermo Fisher). Spots were analyzed according to the manufacturer’s instructions using an ELISpot reader (A. ELVIS, Hannover, Germany).
ICS and Flow Cytometry after Ex Vivo Restimulation
Two days after the second mRNA-lipoplex administration, mice were sacrificed, their spleens were isolated aseptically, and splenocytes were prepared. To obtain single-cell suspensions, we passed spleens through 70-μm nylon strainers (BD Biosciences, San Diego, CA, USA). After red blood cell lysis with NH4Cl solution, 5 × 105 splenocytes were plated in 1 mL of culture medium (RPMI-1640 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 0.4 mM Na-pyruvate, nonessential amino acids, 100 U/mL penicillin, and 0.1 mg/mL streptomycin) supplemented with OVA257–264 (SIINFEKL) peptide (12-5743-82; Thermo Fisher) at 4 μg/mL. After 6 h of peptide restimulation, 1 μL GolgiPlug (brefeldin A; BD, Erembodegem, Belgium) was added to 1 mL culture medium for the measurement of cytokine production by ICS. The Cytofix/Cytoperm kit (BD) was used according to the manufacturer’s protocol. In brief, 16 h after addition of GolgiPlug, cells were stained with a live/dead marker (eBioscience fixable viability dye eFluor 450, 65-0866-14, 1:1,000; Thermo Fisher) and with fluorochrome-labeled antibodies against major histocompatibility complex class II (MHC class II) (MHC class II-eFluor450, 48-5321-82, 1:500; Thermo Fisher), CD3 (CD3-alexa fluor 488 [AF488], 557666, 1:250; BD Pharmingen), CD4 (CD4-phycoerythrin [PE]-cy5, 561836, 1:300; BD Pharmingen), and CD8 (CD8-PE-cy7, 25-0081-81, 1:300; Thermo Fisher). Cells were fixed/permeabilized using the Cytofix/Cytoperm kit (BD) and stained for IFN-γ (IFN-γ-allophycocyanin [APC]; 554413, 1:100; BD Pharmingen). Cells were then analyzed using an LSR Fortessa (BD) with FlowJo software (Tree Star, Ashland, OR, USA).
In Vivo Killing Assay
Splenocytes from naive wild-type mice were pulsed with 1 μg/mL MHC class I OVA peptide or HIV-1 Gag peptide as a control before labeling with 5 or 0.5 μmol/L Carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen), respectively. Labeled cells were mixed at a 1:1 ratio, and a total of 1.5 × 107 cells were adoptively transferred into immunized mice 2 weeks after boost. Splenocytes from host mice were analyzed 2 days later by flow cytometry after staining with α-F4/80 (BD Biosciences) to exclude auto-fluorescent macrophages. Percentage antigen-specific killing was determined using the following formula: (1 − (%CFSEhi cells/%CFSElow cells)treated mice/(%CFSEhi cells/% CSFElow cells)non-treated mice) × 100. The experiments were performed on a triple-laser (B-V-R) LSR-II (Becton Dickinson, San Jose, CA, USA) and analyzed using the FlowJo software (Tree Star).
Author Contributions
L.V.H., , N.S., S.D.K., X.S. and S.V.L. planned the study. L.V.H., K.R., M.B., S.V.L. performed the experiments. Z.Z. performed the experiment in the IFN-B reporter mice. L.V.H. and S.V.L. were involved in data analysis and interpretation and wrote the manuscript. All authors reviewed and approved the manuscript before submission.
Conflicts of Interest
S.D.K. is currently an employee of eTheRNA immunotherapies NV.
Acknowledgments
We thank Prof. Ulrich Kalinke (Institute for Experimental Infection Research, Hannover Medical School, Hannover, Germany) for providing the CD11c-Cre+/−IFNARfl/fl and CD4-Cre+/−IFNARfl/fl mice, as well as Prof. Roosmarijn E. Vandenbroucke (VIB-UGent Center for inflammation Research, Belgium) for providing IFNAR−/− mice. L.V.H. is a junior assistant of the Department of Biomedical Molecular Biology of Ghent University. Funding by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant No. 648124) and from Ghent University (Grant No. BOF2015/GOA/031) is acknowledged with gratitude. Z.Z. acknowledges funding from China Scholarship Council (CSC) (Grant No. 201607650018).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.09.004.
Supplemental Information
References
- 1.Circelli L., Petrizzo A., Tagliamonte M., Heidenreich R., Tornesello M.L., Buonaguro F.M., Buonaguro L. Immunological effects of a novel RNA-based adjuvant in liver cancer patients. Cancer Immunol. Immunother. 2017;66:103–112. doi: 10.1007/s00262-016-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rausch S., Schwentner C., Stenzl A., Bedke J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum. Vaccin. Immunother. 2014;10:3146–3152. doi: 10.4161/hv.29553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheel B., Aulwurm S., Probst J., Stitz L., Hoerr I., Rammensee H.G., Weller M., Pascolo S. Therapeutic anti-tumor immunity triggered by injections of immunostimulating single-stranded RNA. Eur. J. Immunol. 2006;36:2807–2816. doi: 10.1002/eji.200635910. [DOI] [PubMed] [Google Scholar]
- 4.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 5.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson Ö., Thompson J. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richner J.M., Jagger B.W., Shan C., Fontes C.R., Dowd K.A., Cao B., Himansu S, Caine E.A., Nunes B.T.D., Medeiros D.B.A. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell. 2017;170:273–283.e12. doi: 10.1016/j.cell.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beane J.D., Lee G., Zheng Z., Mendel M., Abate-Daga D., Bharathan M., Black M., Gandhi N., Yu Z., Chandran S. Clinical Scale Zinc Finger Nuclease-mediated Gene Editing of PD-1 in Tumor Infiltrating Lymphocytes for the Treatment of Metastatic Melanoma. Mol. Ther. 2015;23:1380–1390. doi: 10.1038/mt.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mock U., Machowicz R., Hauber I., Horn S., Abramowski P., Berdien B., Hauber J., Fehse B. mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Res. 2015;43:5560–5571. doi: 10.1093/nar/gkv469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Lint S., Heirman C., Thielemans K., Breckpot K. mRNA: From a chemical blueprint for protein production to an off-the-shelf therapeutic. Hum. Vaccin. Immunother. 2013;9:265–274. doi: 10.4161/hv.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 12.Brito L.A., Kommareddy S., Maione D., Uematsu Y., Giovani C., Berlanda Scorza F., Otten G.R., Yu D., Mandl C.W., Mason P.W. Self-amplifying mRNA vaccines. Adv. Genet. 2015;89:179–233. doi: 10.1016/bs.adgen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Weide B., Garbe C., Rammensee H.G., Pascolo S. Plasmid DNA- and messenger RNA-based anti-cancer vaccination. Immunol. Lett. 2008;115:33–42. doi: 10.1016/j.imlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Rittig S.M., Haentschel M., Weimer K.J., Heine A., Muller M.R., Brugger W., Horger M.S., Maksimovic O., Stenzl A., Hoerr I. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 2011;19:990–999. doi: 10.1038/mt.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Midoux P., Pichon C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines. 2015;14:221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 16.Paunovska K., Sago C.D., Monaco C.M., Hudson W.H., Castro M.G., Rudoltz T.G., Kalathoor S., Vanover D.A., Santangelo P.J., Ahmed R. A Direct Comparison of in Vitro and in Vivo Nucleic Acid Delivery Mediated by Hundreds of Nanoparticles Reveals a Weak Correlation. Nano Lett. 2018;18:2148–2157. doi: 10.1021/acs.nanolett.8b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Beuckelaer A., Pollard C., Van Lint S., Roose K., Van Hoecke L., Naessens T., Udhayakumar V.K., Smet M., Sanders N., Lienenklaus S. Type I Interferons Interfere with the Capacity of mRNA Lipoplex Vaccines to Elicit Cytolytic T Cell Responses. Mol. Ther. 2016;24:2012–2020. doi: 10.1038/mt.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard C., Rejman J., De Haes W., Verrier B., Van Gulck E., Naessens T., De Smedt S., Bogaert P., Grooten J., Vanham G., De Koker S. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. 2013;21:251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broos K., Van der Jeught K., Puttemans J., Goyvaerts C., Heirman C., Dewitte H., Verbeke R., Lentacker I., Thielemans K., Breckpot K. Particle-mediated Intravenous Delivery of Antigen mRNA Results in Strong Antigen-specific T-cell Responses Despite the Induction of Type I Interferon. Mol. Ther. Nucleic Acids. 2016;5:e326. doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins D.J., Vogel S.N. Space and time: New considerations about the relationship between Toll-like receptors (TLRs) and type I interferons (IFNs) Cytokine. 2015;74:171–174. doi: 10.1016/j.cyto.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Weerd N.A., Vivian J.P., Nguyen T.K., Mangan N.E., Gould J.A., Braniff S.J., Zaker-Tabrizi L., Fung K.Y., Forster S.C., Beddoe T. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat. Immunol. 2013;14:901–907. doi: 10.1038/ni.2667. [DOI] [PubMed] [Google Scholar]
- 22.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Z., Portela Catani J.P., Mc Cafferty S., Couck L., Van Den Broeck W., Gorlé N., Vandenbroucke R.E., Devriendt B., Ulbert S., Cnops L. Immunogenicity and Protection Efficacy of a Naked Self-Replicating mRNA-Based Zika Virus Vaccine. Vaccines (Basel) 2019;7:E96. doi: 10.3390/vaccines7030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepini T., Pulichino A.M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J. Induction of an IFN-Mediated Antiviral Response by a Self-Amplifying RNA Vaccine: Implications for Vaccine Design. J. Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lienenklaus S., Cornitescu M., Zietara N., Łyszkiewicz M., Gekara N., Jabłónska J., Edenhofer F., Rajewsky K., Bruder D., Hafner M. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J. Immunol. 2009;183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 26.Durward M., Harms J., Splitter G. Antigen specific killing assay using CFSE labeled target cells. J Vis Exp. 2010;45:2250. doi: 10.3791/2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker B.S., Rautela J., Hertzog P.J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 28.Le Bon A., Durand V., Kamphuis E., Thompson C., Bulfone-Paus S., Rossmann C., Kalinke U., Tough D.F. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J. Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 29.Tough D.F. Modulation of T-cell function by type I interferon. Immunol. Cell Biol. 2012;90:492–497. doi: 10.1038/icb.2012.7. [DOI] [PubMed] [Google Scholar]
- 30.Hervas-Stubbs S., Perez-Gracia J.L., Rouzaut A., Sanmamed M.F., Le Bon A., Melero I. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 31.Zitvogel L., Galluzzi L., Kepp O., Smyth M.J., Kroemer G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 32.Van Lint S., Goyvaerts C., Maenhout S., Goethals L., Disy A., Benteyn D., Pen J., Bonehill A., Heirman C., Breckpot K., Thielemans K. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661–1671. doi: 10.1158/0008-5472.CAN-11-2957. [DOI] [PubMed] [Google Scholar]
- 33.Van Hoecke L., Van Lint S., Roose K., Van Parys A., Vandenabeele P., Grooten J., Tavernier J., De Koker S., Saelens X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018;9:3417. doi: 10.1038/s41467-018-05979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 35.Pollard D.A., Rockman M.V. Resistance to germline RNA interference in a Caenorhabditis elegans wild isolate exhibits complexity and nonadditivity. G3 (Bethesda) 2013;3:941–947. doi: 10.1534/g3.113.005785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiesel M., Crouse J., Bedenikovic G., Sutherland A., Joller N., Oxenius A. Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur. J. Immunol. 2012;42:320–329. doi: 10.1002/eji.201142091. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal P., Raghavan A., Nandiwada S.L., Curtsinger J.M., Bohjanen P.R., Mueller D.L., Mescher M.F. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J. Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crouse J., Kalinke U., Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 39.Van der Jeught K., De Koker S., Bialkowski L., Heirman C., Tjok Joe P., Perche F., Maenhout S., Bevers S., Broos K., Deswarte K. Dendritic Cell Targeting mRNA Lipopolyplexes Combine Strong Antitumor T-Cell Immunity with Improved Inflammatory Safety. ACS Nano. 2018;12:9815–9829. doi: 10.1021/acsnano.8b00966. [DOI] [PubMed] [Google Scholar]
- 40.Honke N., Shaabani N., Cadeddu G., Sorg U.R., Zhang D.E., Trilling M., Klingel K., Sauter M., Kandolf R., Gailus N. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat. Immunol. 2011;13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.