Abstract
Optical coherence tomography (OCT) is an imaging technique that can visualize the internal biological structure without X-ray exposure. Swept-source OCT (SS-OCT) is one of the latest version of OCT, wherein the light source is a tunable laser that sweeps near-infrared wavelength light to achieve real-time imaging. The imaging depth of OCT is highly influenced by the translucency of the medium. The medium that does not transmit light and the deeper structure beyond the range of light penetration depth are not relevant for OCT imaging. In OCT, sound enamel is almost transparent at the OCT wavelength range, and enamel and dentin can be distinguished from each other as the dentin–enamel junction (DEJ) appears as a dark border. Demineralized enamel and dentin are imaged as bright zones because of the formation of numerous micro-porosities where the backscatter of OCT signal is increased. In cavitated caries at interproximal or occlusal hidden zone, the upper margin of the cavity reflects the signal showing a distinct bright border in the SS-OCT image. SS-OCT is capable of determining crack penetration depth even when the cracks extended beyond the DEJ. SS-OCT has a high degree of sensitivity and specificity for the detection of dental caries and tooth cracks. SS-OCT is also capable of detecting non-carious cervical lesions and occlusal tooth wear in cross-sectional views to estimate the amount of tooth structure loss.
Keywords: Optical coherence tomography, Diagnosis, Caries, Tooth crack, NCCL, Tooth wear, Age-related changes
1. Introduction
Optical coherence tomography (OCT) is an interferometric technique that can create cross-sectional images of biological structures without X-ray exposure [1]. The imaging mechanism is analogous to ultrasonography [2]. Ultrasonography uses sound to measure the echo and time delay from the deep structures to generate the images, while OCT uses light and measures the backscattered signals from the deep structures [2]. Since the velocity of light is too high to measure the time delay, OCT employs an interferometer to measure the pathway difference of the light and construct the depth profile [1,2]. To create fringe responses of the light, OCT uses coherent light of near-infrared wavelength, where light has its maximum depth of penetration in the biological structures (near-infrared window) [2]. The first in vitro and in vivo images of dental hard and soft tissue with OCT were acquired by Colston et al. [3].
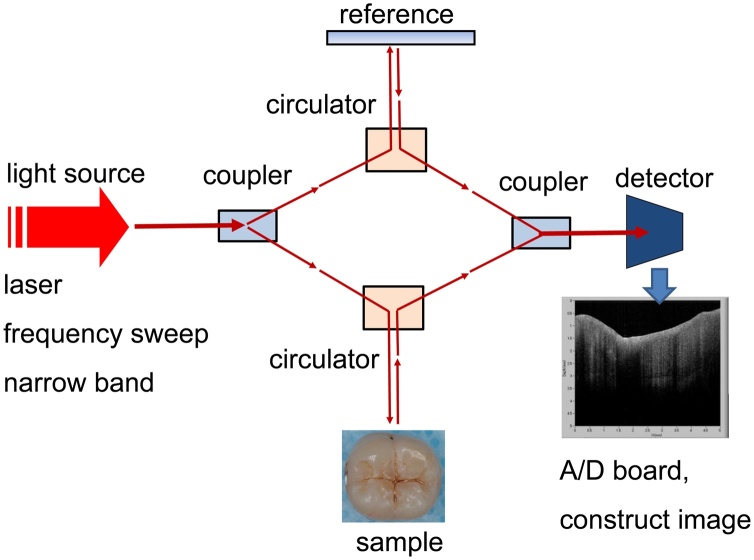
OCT is a non-invasive imaging method that uses light and eliminates the risk of radiation exposure. Thus, OCT is a safe diagnostic method for dental diseases and can be used in pregnant woman and young children. The early OCT systems were based on time-domain (TD) detection in which echo time delays of light were identified by measuring the interference signal as a function of time, while scanning the optical path length of the reference arm [2]. Advances in OCT technology have enabled dramatic increases in image resolution and speed of imaging. Fourier-domain (FD) techniques provide distinct increase in sensitivity as compared to the traditional TD-OCT [2,4]. Swept-source (SS-)OCT is one of the implements of FD-OCT and employs an interferometer with a narrow-linewidth, frequency-sweep laser, and detectors to measure interference versus time (Fig. 1) [2,5]. Recent SS-OCT systems offer cross-sectional images of the internal biological structures in real time with microscopic level resolution (Fig. 2).
Fig. 1.
Schematic illustration of swept source optical coherence tomography (SS-OCT) system.
Near-infrared laser is emitted and divided into a signal and a reference beam. Reference light and backscattered light from the sample are recombined by a second fiber coupler to create an interferogram over time. Fringe responses are detected with a balanced detector, converted to electrical signals, and digitized by an analog-to-digital converter (A/D) board.
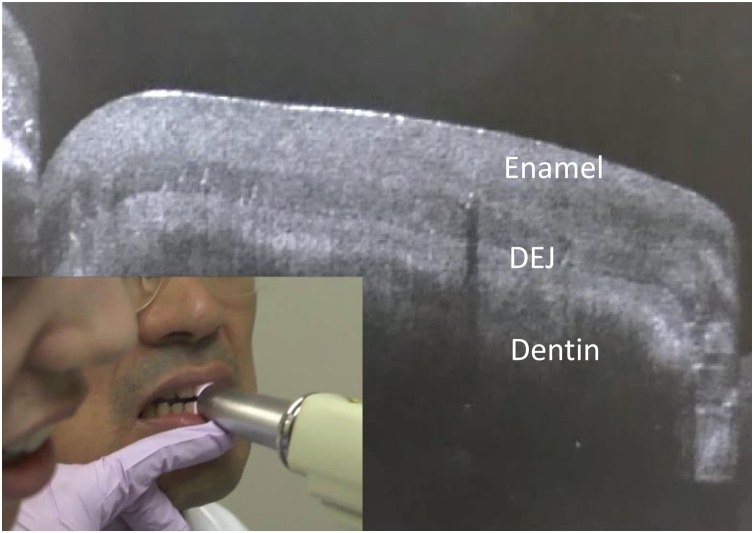
Fig. 2.
Real-time cross-sectional imaging was performed for the anterior tooth using SS-OCT.
Enamel and dentin, as well as dentin-enamel junction (DEJ) appearing as a border between the two structures, are clearly observed using SS-OCT. Imaging situation is shown in the lower left side image.
The imaging depth of OCT is highly influenced by the translucency of the medium [2]. OCT imaging is favorable for an object that permits the penetration of incident light, while the medium that does not transmit light and the deeper structure beyond the range of light penetration depth are not relevant for OCT imaging.
It is well known that enamel exhibits high translucency and dentin allows some transmission of light [6]. Therefore, OCT has the potential to be a new imaging modality in dentistry for the detection of dental caries and other dental hard tissue diseases [3]. Moreover, OCT is capable to capture the aging changes of tooth structure, such as tooth crack or tooth wear, even at the subclinical level [[7], [8], [9], [10]]. Tooth wear is multifactorial disease manifests as erosion, attrition, abrasion, or a combination of these factors [11,12]. Recent increasing of tooth wear in younger generation has raised concern in dental community [13]. These age-related changes often cause serious pulpitis or periapical lesions, and these could endanger the longevity of the tooth [14]. Development of an imaging modality to detect the age-related changes in the early stages is required for the dental health care in elderly people.
2. Dental OCT system
A dental SS-OCT system employs near-infrared laser as a light source with the center wavelength near 1310 nm, because infrared light from 780 to 1550 nm can help in optical imaging of the enamel with weak scattering and absorption in this region [15].
In SS-OCT, the spectrally resolved interference is derived from rapidly sweeping the wavelength of the laser [2]. The axial resolution of SS-OCT is ultimately set by the linewidth of the laser beam [2]. The transverse resolution is determined by the focus spot size on the sample [2]. The high acquisition speed of SS OCT, providing near real-time video-rate imaging while improving the overall signal-to-noise ratio of the acquired images, has made clinical applications of OCT more feasible [16].
The axial resolution of dental OCT system is around 11 μm in air, which corresponds to approximately 7 μm in biological structure with a refractive index of around 1.5. The lateral resolution is around 20 μm which is determined by the objective lens.
3. OCT imaging of intact tooth
In SS-OCT, sound enamel is almost transparent and the whole thickness of enamel can be captured at the SS-OCT wavelength range of approximately 1300 nm [3,15]. Enamel and dentin can be distinguished from each other since the dentin–enamel junction (DEJ) appears as a dark border (Fig. 2). Dentin is composed of 50 vol % inorganic material, 30 vol % organic material and 20 vol % fluid [17]. Most of the organic phase of dentin comprises of type 1 collagen, which is optically non-linear and scatters light [6]. The measurable depth of dentin is smaller than that of enamel because of higher attenuation.
The penetration depth of the signal is significantly influenced by the surface inclination and roughness. Since the light refracts in the direction of the medium by nature, the object with a flat surface and less inclination is more advantageous in OCT imaging. As a result, smooth tooth surface would exhibit better penetration depth for OCT imaging than the occlusal surface [18,19].
4. OCT imaging of dental caries
4.1. Tooth demineralization and enamel caries
In OCT, demineralized enamel and dentin are imaged as bright zone because of the increased backscattered signal. Scattering from the demineralized lesion is considered because of the formation of numerous microporosities within the structure where variation of local refractive index occurred. Hariri et al. measured the refractive indices of enamel and dentin after demineralization and remineralization and showed the linear correlation between the mineral density and refractive indices [20]. Consequently, increased backscattering signal in OCT could be utilized as the criterion for the detection of demineralization.
In OCT, demineralized lesion is imaged as white on a gray scale image by increasing the backscattered signal (Fig. 3, Fig. 4, Fig. 5, Fig. 6) [18,20,21]. This phenomenon is in contrast with the radiography images, where the caries appears radiolucency because of the mineral loss [21,22].. It has been demonstrated that scattering coefficient of demineralized enamel is increased by 2–3 order of magnitude from the baseline before the demineralization [23,24]. Therefore, OCT can discriminate the enamel demineralization clearly even at the incipient stage. If enamel caries has progressed to cavitation, OCT can image the contour of the cavity to show the lesion depth clearly [18].
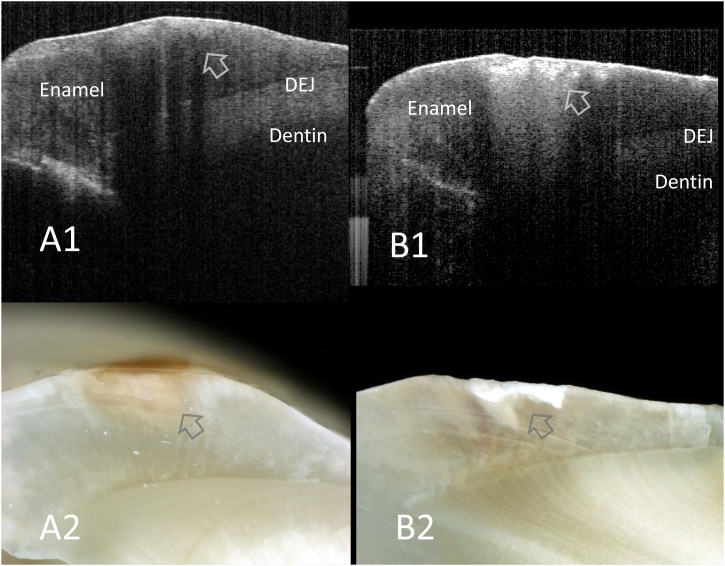
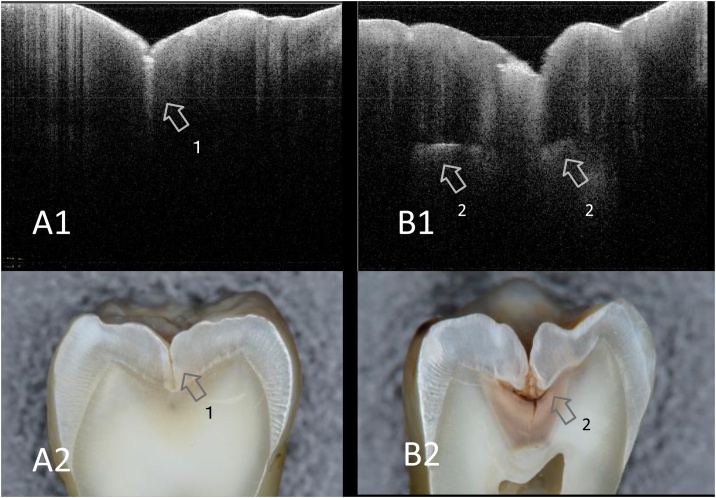
Fig. 3.
SS-OCT images of smooth surface enamel caries obtained noninvasively (A1, B1) and corresponding histological view after cross-sectioning (A2, B2).
In SS-OCT, presence of enamel demineralization is observed as a bright zone because of the increased scattering (arrows). Counter of slight cavitation is also depicted in SS-OCT.
A1, A2: Enamel demineralization without cavitation
B1, B2: Enamel caries with cavitation
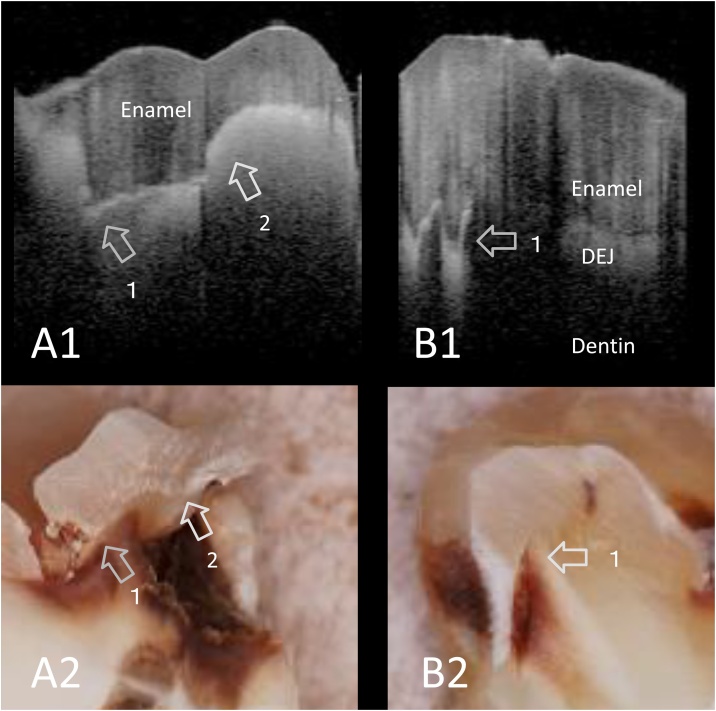
Fig. 4.
SS-OCT images of dentin caries at approximal surface obtained noninvasively (A1, B1) and corresponding histological view after cross-sectioning (A2, B2).
In SS-OCT, dentin caries is imaged as a bright zone. Lesion expanding along the DEJ is imaged as distinct bright line (arrow 1). A strong reflection penetrating into the structure indicates the lesion is “cavitated” (arrow 2).
A1, A2: Superficial dentin caries
B1, B2: Deep dentin caries
Fig. 5.
SS-OCT images of occlusal caries obtained noninvasively (A1, B1) and corresponding histological view after cross-sectioning (A2, B2).
Enamel demineralization at occlusal fissure is imaged as increased brightness within the depth of DEJ (arrow 1). Presence of hidden dentin caries is depicted in SS-OCT as backscattering signal from the lesion boundary (B1). Lesion expand along the DEJ is imaged as a bright line (arrow 2).
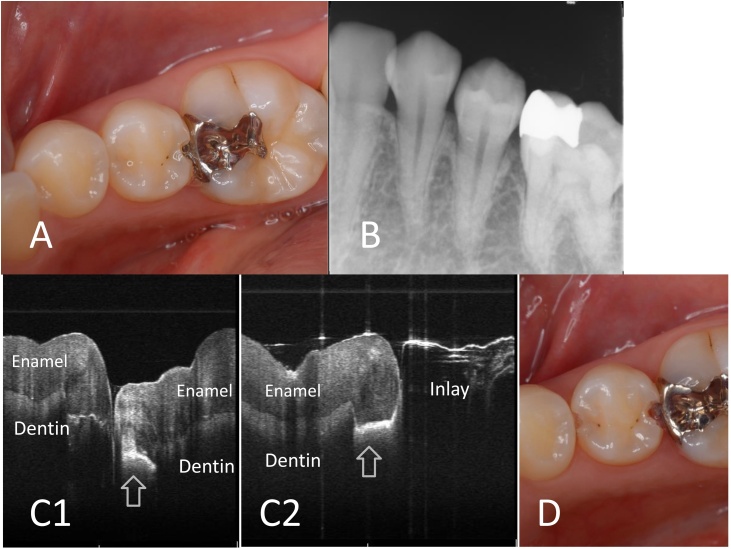
Fig. 6.
Clinical case of approximal caries in lower second premolar.
A. Clinical view. Cavitation of distal enamel is obvious in second premolar; however, detection of caries in mesial proximal surface is not evident.
B. X-ray image. Increase in radiolucency at proximal contacts is not evident in second premolar.
C1. SS-OCT image of approximal contacts between first and second premolars. Scanning from occlusal surface. Distinct white line indicates the presence of caries with cavitation (arrow).
C2. SS-OCT image of approximal contacts between second premolar and first molar. Scanning from occlusal surface. Distinct white line indicates the presence of caries with cavitation (arrow). Cross-sectional imaging of first molar is impossible because of the metal inlay.
D. Clinical view after cavity preparation. Presence of dentin caries in the mesial and distal proximal surfaces is confirmed.
4.2. Dentin caries
In dentin caries, OCT can distinguish the lesion that penetrates into dentin [18]. Since DEJ is clearly displayed in OCT images in many cases, the penetration depth of caries as bright zone can be estimated by the location of DEJ as a reference point (Fig. 4, Fig. 5, Fig. 6) [18]. Despite the enamel exhibits excellent penetration depth of light signal in OCT, the incident light suffers significant attenuation in dentin, which limits the imaging depth for dentin caries in deeper zone. However, if the lesion penetrates into dentin, the carious demineralization expands laterally along DEJ, where a strong reflection of OCT signal occurs (Figs. 4,5) [25]. Hence, we can utilize the presence of the bright signal along the DEJ as the determining criteria for dentin caries. In the case of caries with cavitation that forms a hollow space, the upper margin of the cavity reflects the signal to show a distinct bright border in OCT image (Figs. 4,5) [25]. So far, OCT can detect the hidden caries beneath the seemingly sound surface by the cross-sectional imaging that depicts the cavity border as a white line (Fig. 5) [19,26]. Our previous studies have shown that the high image resolution and penetration depth of SS-OCT is advantageous for the detection of dentin caries [18,21].
4.3. Smooth surface caries
SS-OCT imaging of caries on smooth tooth surface was performed to evaluate the diagnostic accuracy of SS-OCT and compared the results with visual inspection [18]. Extracted human teeth including those with sound enamel and localized enamel discoloration or cavitated caries were used. The teeth were cleaned with prophylactic paste using a low-speed brush cone attached to a rotary instrument, followed by visual inspection and SS-OCT imaging. SS-OCT system used (Santec OCT-2000®, Santec Co., Komaki, Japan) incorporates a high-speed frequency swept external cavity laser with the center wavelength for 1319 nm and the scan range for 112 nm at a 20-kHz sweep rate. SS-OCT image was obtained by the scanning over the area of interest of smooth tooth surface keeping in moist so as to simulate the intraoral condition and to avoid the influence of drying shrinkage. Dentists with 3 years of clinical experience and over 9 years of experience were recruited to evaluate the lesion extent on a 4-rank depth scale as follows:
0: sound surface; 1: enamel demineralization without surface breakdown; 2: enamel caries with cavitation; 3: dentin caries.
Validation was performed by direct observation of the cross-section of the investigation sites using confocal laser scanning microscopy (CLSM). Sensitivity, specificity, and area under receiver operating characteristic curve (ROC; Az value) in each examiner group were calculated for each category.
As the results, SS-OCT showed higher sensitivity than that of visual inspection for the diagnosis of caries of all levels (Fig. 3) (Table 1) [18]. Specificity of SS-OCT was higher for most of the cases, except for dentin caries, where the values of both methods were quite similar. The SS-OCT results for examiners over 9 years clinical experience showed higher sensitivity and Az values for dentin caries and higher specificity and Az values for cavitated enamel caries than 3 year experience examiners. Consequently, SS-OCT appears to be an efficient imaging method for the detection of smooth surface caries and estimation of lesion extent. Moreover, clinical experience and image interpretation skills can improve the utility of OCT as a modality for caries assessment.
Table 1.
Sensitivity, specificity, and Az value for smooth surface caries.
| Sensitivity | ||||
|---|---|---|---|---|
| Caries level | Visual inspection |
SS-OCT |
||
| 3 yrs | <9 yrs | 3 yrs | <9 yrs | |
| Enamel dem | 0.98 | 0.96 | 0.98 | 0.97 |
| Enamel caries | 0.73 | 0.88 | 0.82 | 0.88 |
| Dentin caries | 0.58 | 0.49 | 0.64 | 0.96 |
| Specificity | ||||
|---|---|---|---|---|
| Caries level | Visual inspection |
SS-OCT |
||
| 3 yrs | <9 yrs | 3 yrs | <9 yrs | |
| Enamel dem | 0.67 | 0.78 | 0.89 | 0.98 |
| Enamel caries | 0.63 | 0.62 | 0.76 | 0.97 |
| Dentin caries | 0.84 | 0.97 | 0.90 | 0.95 |
| Az value | ||||
|---|---|---|---|---|
| Caries level | Visual inspection |
SS-OCT |
||
| 3 yrs | <9 yrs | 3 yrs | <9 yrs | |
| Enamel dem | 0.83 | 0.87 | 0.94 | 0.98 |
| Enamel caries | 0.68 | 0.75 | 0.79 | 0.92 |
| Dentin caries | 0.71 | 0.73 | 0.77 | 0.96 |
Enamel dem: enamel demineralization.
Enamel caries: enamel caries with cavitation.
SS-OCT: swept-source optical coherence tomography.
3 yrs: examiners with clinical experience for 3 years.
<9 yrs: examiners with clinical experience over 9 years.
4.4. Occlusal caries
Occlusal fissures of first molars are generally the first sites to be affected by caries in the permanent dentition. Prevalence of occlusal dentin caries which is missed on visual examination is a clinical issue for several decades, because complex occlusal fissure morphology can lead to misdiagnosis and mask the further development of underlining caries. The term “hidden caries” is used to describe the occlusal lesion that is missed on visual inspection but only detected by radiography [27]. However, dental radiographs do not have the sensitivity for detecting early occlusal caries [28]. By the time the lesions appear radiolucent, they have often progressed deep into dentin [28].
Extracted human molar teeth with stained occlusal fissures or small open caries lesions with diameter within 1 mm were collected and used to assess the diagnostic accuracy of SS-OCT for occlusal caries [19]. After cleaning and drying the tooth surface, visual inspection and SS-OCT imaging of occlusal surface were performed. SS-OCT (Santec OCT-2000®) image was obtained by the scanning from occlusal surface. Presence and progression of occlusal caries was classified using 4-rank depth scale as follows;
Udit 0: sound tooth surface; 1: enamel demineralization without cavitation; 2: cavitated enamel caries; 3: dentin caries.
Sensitivity and specificity for the detection of enamel demineralization and dentin caries and Az value of ROC curve were calculated in each category based on the direct observation of sectioned fissures using CLSM.
The results showed that the sensitivity values of SS-OCT (0.98 for enamel demineralization and 0.60 for dentin caries) were higher than that of the visual inspection (Table 2). Specificity for visual inspection was 0.69 for caries lesion and 1.00 for dentin caries, whereas those for the SS-OCT were 0.75 and 0.98, respectively. With respect to the obtained Az value, SS-OCT presented higher values for the detection of both enamel demineralization and dentin caries.
Table 2.
Sensitivity, specificity, and Az value for occlusal caries.
| Sensitivity | ||
|---|---|---|
| Caries level | Visual inspection | SS-OCT |
| Enamel dem | 0.80 | 0.98 |
| Dentin caries | 0.36 | 0.60 |
| Specificity | ||
| Caries level | Visual inspection | SS-OCT |
| Enamel dem | 0.69 | 0.75 |
| Dentin caries | 1.0 | 0.98 |
| Az value | ||
| Caries level | Visual inspection | SS-OCT |
| Enamel dem | 0.74 | 0.86 |
| Dentin caries | 0.68 | 0.80 |
Enamel dem: enamel demineralization.
SS-OCT: swept-source optical coherence tomopgraphy.
Diagnosis of hidden caries is probably one of the most difficult challenges for the dentist clinically. In our study, some of the lesions estimated as initial demineralization in visual inspection were actually deep lesions close to the DEJ. Although limitation of penetration depth of SS-OCT imaging exists, SS-OCT provides some useful information for the detection of hidden lesions. In our study, visual inspection showed 30 locations of no caries (score 0) involving 19 false-negative caries. However in SS-OCT imaging, the number of cases of false-negative lesion with score 0 was only 2 cases. Distinct cavity border imaged as a white line was observed in most of the hidden dentin caries penetrating the dentin (Fig. 5) [25,26].
4.5. Approximal caries
The detection and diagnosis of approximal caries in posterior teeth is difficult because of the restricted access for visual inspection. Although bitewing radiography is well accepted, evidence in epidemiological studies exhibits low sensitivity for the diagnosis of this type of caries [28]. The early and accurate diagnosis of caries enables non-surgical therapy or immediate operative treatment; thereby suppressing the progress of the lesion and preventing tooth loss. A prototype dental OCT system incorporating hand-held probe (Prototype 2, Panasonic Healthcare Co. Ltd. Ehime, Japan) was developed and used in the present study. The spectral bandwidth of the laser was over 100 nm centered at 1330 nm at a 30-kHz sweep rate. SS-OCT imaging for approximal caries was performed from the occlusal aspects of posterior teeth in a real clinical situation [29]. The high-speed frequency-swept laser was projected from occluso-proximal aspect onto the proximal contact areas using a right-angle hand-held probe designed for the imaging of posterior teeth.
Seventy-five non-restored proximal surfaces of premolars and molars were selected from for the 53 healthy adults. The proximal surfaces were captured on bitewing radiographs on E-speed films with a 60-kV X-ray unit, followed by SS-OCT imaging of the proximal area scanned from the occlusal surface (Fig. 6). Six dentists carried out deduction of the radiographs and SS-OCT to evaluate the level of caries progression using a 5-point rank scale as follows:
Udit 0: sound tooth surface; 1: enamel demineralization without cavitation; 2: cavitated enamel caries; 3: superficial dentin caries limited to the outer half of the dentin thickness; 4: deep dentin caries penetrating to the inner half of the dentin.
The test surfaces were then carefully observed, and if the caries was evident, the lesion was accessed and removed mechanically guided by a caries-detecting dye (Caries Detector, Kuraray Noritake Dental Inc., Tokyo, Japan) to facilitate the clinical discrimination. In cases of intact surfaces and non-cavitated enamel demineralization, a preventive treatment was provided with fluoride application followed by observation, without any surgical intervention. The surface was finally scored according to the clinical examination after actual treatment.
Sensitivity and specificity of the dental radiography and SS-OCT were calculated for each caries level, and ROC analysis of Az value was performed. Table 3 shows the obtained values of sensitivity, specificity and area under the ROC curve (Az values) for detection in each diagnostic threshold.
Table 3.
Sensitivity, specificity and Az value for approximal caries.
| Sensitivity | ||||
|---|---|---|---|---|
| Caries level | X-ray |
SS-OCT |
||
| Mean | SD | Mean | SD | |
| Enamel dem | 0.83 | 0.13 | 0.93 | 0.059 |
| Enamel caries | 0.62 | 0.12 | 0.86 | 0.14 |
| Dentin superficial | 0.35 | 0.097 | 0.62 | 0.22 |
| Dentin deep | 0.35 | 0.10 | 0.34 | 0.27 |
| Specificity | ||||
| Caries level |
X-ray |
SS-OCT |
||
| Mean | SD | Mean | SD | |
| Enamel dem | 0.54 | 0.23 | 0.64 | 0.18 |
| Enamel caries | 0.76 | 0.19 | 0.84 | 0.10 |
| Dentin superficial | 0.92 | 0.043 | 0.93 | 0.041 |
| Dentin deep | 0.98 | 0.015 | 0.98 | 0.029 |
| Az value | ||||
| Caries level |
X-ray |
SS-OCT |
||
| Mean | SD | Mean | SD | |
| Enamel dem | 0.69 | 0.065 | 0.78 | 0.10 |
| Enamel caries | 0.69 | 0.065 | 0.85 | 0.062 |
| Dentin superficial | 0.64 | 0.049 | 0.77 | 0.10 |
| Dentin deep | 0.67 | 0.051 | 0.66 | 0.13 |
SD: standard deviation.
Enamel dem: enamel demineralization.
Enamel caries: enamel caries with cavitation.
Dentin superficial: superficial dentin caries limited to the outer half of the dentin thickness.
Dentin deep: deep dentin caries penetrates to the inner half of the dentin.
SS-OCT: swept-source optical coherence tomography.
The sensitivity of SS-OCT for the detection of cavitated enamel caries and dentin caries were significantly higher than that of radiography (Student’s t-test, p < 0.05). For the specificity, no significant difference was found between the values of SS-OCT and radiography, despite SS-OCT showing higher or identical values in all diagnostic thresholds (Student’s t-test, p > 0.05). Considering Az values, the detection rate of SS-OCT for enamel demineralization, cavitated enamel caries, and dentin caries was significantly higher than that of radiography (Student’s t-test, p < 0.05). Consequently, our results showed that SS-OCT was a superior diagnostic tool for the diagnosis of approximal caries over bitewing radiographs for the lesion depth up to the superficial dentin caries.
In this study, no cases of deep caries that required dental pulp treatments were included. In most of the SS-OCT images in this study, the pulp chamber within the tooth did not appear clearly, and it was impossible to observe the distance of the caries from the dental pulp. Currently, radiographic examination is still considered to be necessary in cases that exhibit significant irreversible pulpitis symptoms. Moreover, OCT is not applicable for the imaging of object that does not transmit the incident light of OCT. Meanwhile the detection of caries beneath an existing restoration is important factor for the decision over the replacement of restoration, OCT monitoring is hard to conduct if the restoration is of metallic or opaque material. On the other hand, several lines of evidence have shown that OCT can image the interfacial gaps and caries around the tooth color restorations that transmit the light [[30], [31], [32], [33], [34]]. Further technological development seems necessary for the examination of advanced lesion depth.
5. Tooth crack
A tooth crack initially occurs on the enamel surface due to local stress concentration and grows with increased load and penetrates into the dentin [35,36]. Tooth cracks tend to deteriorate to vertical tooth fractures, and significantly threaten to the viability of the tooth [35]. Detection of tooth crack using current methodology is diagnostically challenging because of the difficulty in locating crack lines. Dental radiographs do not disclose the presence of tooth cracks, despite they can show the subsequent inflammatory bony damage due to the bacterial infection [37]. Visual inspection using transillumination is reported to be valuable with a high level of sensitivity for the detection of tooth cracks [38]. However, transillumination does not provide information on depth, and shows all cracks causing even craze lines to appear as structural tooth cracks. Imaging technology is necessary to quantify the progression of tooth crack with high reliability. Since OCT can create cross-sectional images of the tooth structure non-invasively, we assessed SS-OCT imaging for the diagnosis of tooth cracks and compared the results with transillumination [7].
Seventy-one sites of smooth tooth surfaces visibly with or without naturally occurring tooth cracks were selected from 20 extracted human teeth. The surface of interest was inspected by three dentists using a photocuring unit as transillumination, and the presence of tooth crack was scored on a depth scale as follows:
Udit 1: no crack, intact surface; 2: superficial enamel crack, enamel crack <50% enamel thickness; 3: deep enamel crack, enamel crack >50% enamel thickness but not extending up to the DEJ; 4: whole-thickness enamel crack, 100% enamel crack extending up to the DEJ; 5: dentin crack, crack extending beyond the DEJ.
A prototype dental OCT system incorporating hand-held probe (Prototype 2, Panasonic Healthcare Co. Ltd) was used in the present study. The same criteria used for transillumination were applied to the SS-OCT images of the investigation sites to score the crack progression. The validation method for diagnosis was determined by direct observation of the sectioned surfaces using CLSM. Sensitivity and specificity for the detection of enamel cracks (score 1 versus scores 2–5) and whole-thickness enamel cracks (scores1–3 versus scores 4,5) were calculated from the results obtained from transillumination and SS-OCT.
The tooth cracks on the SS-OCT images were clearly displayed as a bright line because of the increased backscattered signal (Fig. 7, Fig. 8) [7]. Even for the deep cracks extended beyond the DEJ, the whole line could be imaged in SS-OCT (Fig. 8). SS-OCT showed higher sensitivity and Av value for both enamel cracks and whole thickness cracks (Table 4). The reproducibility of visual inspection and SS-OCT imaging, weighted kappa-values of interexaminer agreement were calculated. The reproducibility of SS-OCT and transillumination was calculated using weighted kappa-values of inter-examiner agreement. The reproducibility of SS-OCT was higher than that of transillumination (transillumination, 0.18; SS-OCT, 0.61).
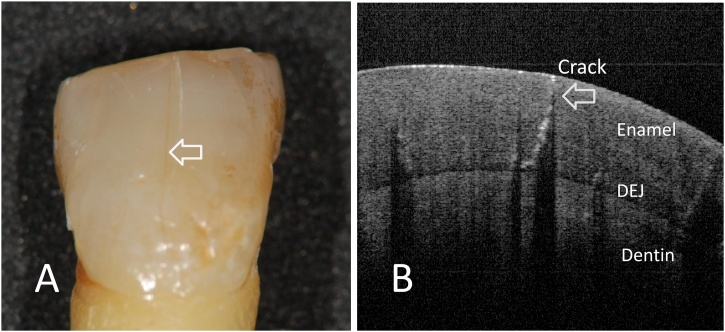
Fig. 7.
Enamel crack. Permission to reprint from [7].
A. Hairline enamel crack is visually found on the buccal surface.
B. SS-OCT image of A. Although the thickness of crack is very thin, the crack is clearly imaged as a white line penetrating into the depth to DEJ (whole thickness enamel crack).
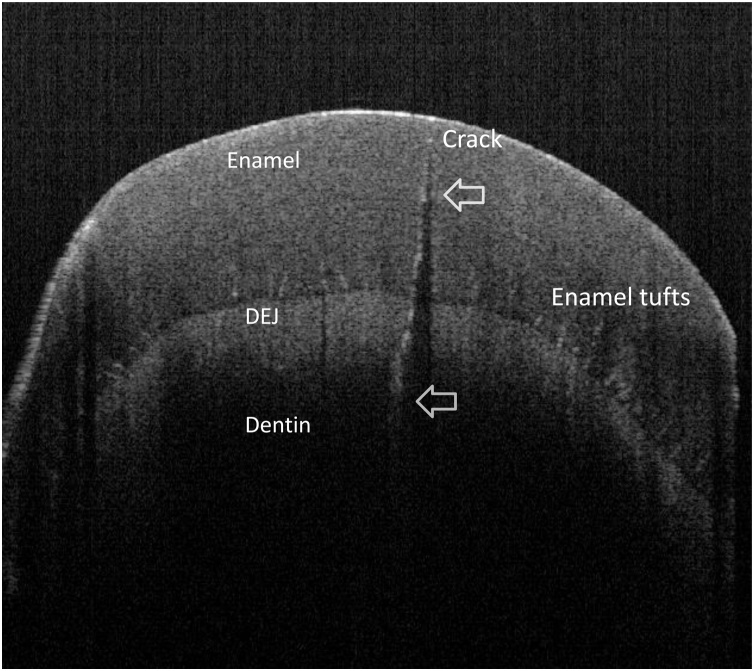
Fig. 8.
SS-OCT image of dentin crack. The crack displayed as white line penetrating into the dentin. Enamel tufts are imaged as tiny white lines near DEJ.
Table 4.
Sensitivity, specificity, Az value, and kappa value for tooth crack.
| Sensitivity | Specificity | Az value | Kappa value | ||
|---|---|---|---|---|---|
| Transillumination | Enamel C | 0.87 | 0.50 | 0.69 | 0.18 |
| whole C | 0.19 | 0.89 | 0.56 | ||
| SS-OCT | Enamel C | 0.95 | 0.75 | 0.85 | 0.61 |
| Whole C | 0.90 | 0.63 | 0.77 | ||
Enamel C: enamel crack.
Whole C: whole thickness enamel crack.
SS-OCT: swept-source optical coherence tomography.
Consequently, our results showed SS-OCT as an effective tool for the detection and diagnosis of tooth cracks. Moreover, inter-examiner reproducibility value (weighted kappa) of SS-OCT was higher than that of transillumination, suggesting that SS-OCT could provide stable and objective information for the diagnosis of tooth crack. Since SS-OCT can construct the image only within the range of near infrared penetration depth, its application is limited to the coronal portion where the laser light can be irradiated. Further development of the technology, such as systems with improved transmission into the soft tissue and special imaging probe for the detection of sub-gingival zone, can improve the diagnostic accuracy for the detection of tooth cracks and enhance its demand in clinical usage.
6. Non-carious cervical lesion (NCCL)
Non-carious cervical lesion (NCCL) is defined as a loss of tooth structure at the cervical region that is unrelated to dental caries [39]. NCCLs are considered to have a multifactorial etiology and various mechanisms including abrasion, erosion, and abfraction [39]. With an increase in the elderly population, the severity and prevalence of NCCLs has increased [40]. Since the progression of NCCL affects on the structural integrity and pulpal vitality of the tooth, adequate management is necessary to protect against the further structural loss.
SS-OCT imaging was performed on NCCLs in vitro and in vivo to evaluate the dimensions of tooth loss, presence of cervical enamel crack, and cervical demineralization [9]. A prototype dental OCT system incorporating hand-held probe (Prototype 2, Panasonic Healthcare Co. Ltd.) was used in the present study. In the in vitro study, demineralization level of NCCL dentin was measured using transverse microradiography (TMR), which showed an average mineral loss of 12.3 % in NCCL of the extracted teeth. Since dentin caries exhibits more than 25 % loss of mineral contents, the level of mineral loss in NCCL was much lower than that in dentin caries. Attenuation coefficient (μt) of SS-OCT signal in the NCCL dentin was also calculated in vitro, and it was linked with the TMR results. The μt value as a threshold for the detection of demineralization was then chosen from the average μt and standard deviation.
In the in vivo study, 242 buccal surfaces were investigated in 35 healthy adults. SS-OCT imaging was performed scanning over the NCCL using hand-held probe. Presence and dimensions of NCCLs, cervical cracking and the degree of demineralization at the exposed cervical dentin were determined using SS-OCT (Fig. 9) [17]. Of the 242 teeth, NCCLs were found in 145, while 97 were intact teeth. Among the teeth with NCCLs, the presence of occlusal attrition was confirmed in 87 (60%) teeth, hypersensitivity in 58 (40%) teeth, cervical enamel cracks in 44 (30.3%) teeth, and dentin demineralization in 100 (69.0%) teeth.
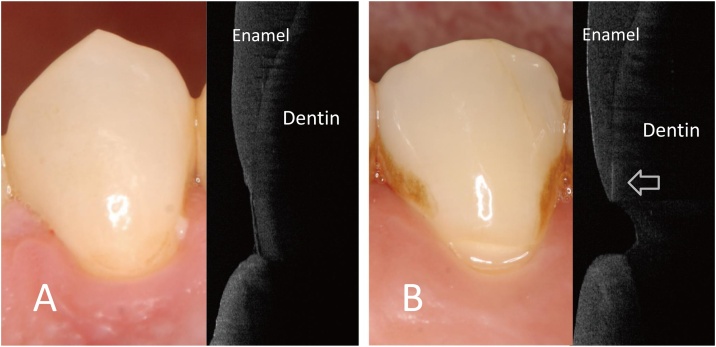
Fig. 9.
Non-carious cervical lesion (NCCL).
A. Without NCCL. Although root surface was exposed because of the gingival recession, there is no loss of tooth structure observed.
B. NCCL. SS-OCT image clearly shows the loss of tooth structure at the root surface. Separation of enamel at DEJ is observed as the bright line along the DEJ (arrow).
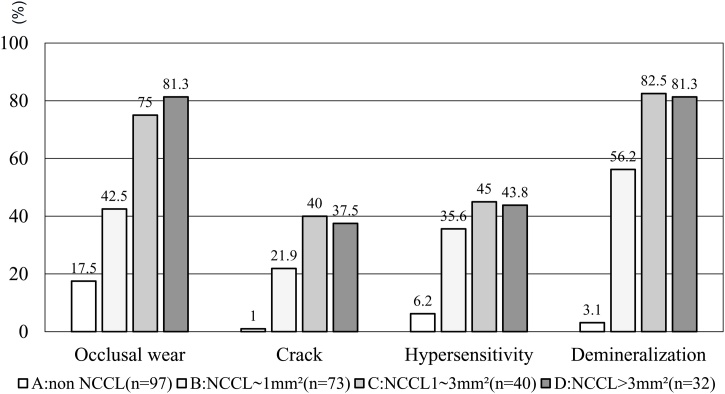
The frequency of occlusal wear, cracking, hypersensitivity, and dentin demineralization in each NCCL size group is summarized in Fig. 10 [9]. Analysis of the dimensions of NCCLs revealed that occlusal wear, hypersensitivity, cervical cracking, and dentin demineralization were significantly more prevalent in NCCL cases, even in cases with small lesions (group B), as compared to the group of intact teeth (group A). Notably, dentin demineralization was observed in 56.2% of small NCCLs (group B), and the ratio increased to >80% in larger NCCLs.
Fig. 10.
Frequency of occlusal wear, cracking, hypersensitivity, and dentin demineralization in NCCL size groups (%). A. Non NCCL. B. NCCL less than 1mm [2]. C. NCCL more than 1mm [2], but less than 3 mm [2]. D. NCCL more than 3 mm [2].
Hence, demineralization of NCCL dentin could be suggested as a cofactor in the formation of NCCL. SS-OCT showed the presence of cervical enamel cracking in NCCLs, and this phenomenon increased with age and seemed to occur due to parafunctional occlusal stress.
7. Occlusal tooth wear
Occlusal tooth wear occurs due to masticatory function and parafunctional tooth contacts. Occlusal tooth wear can deflect the occlusal interferences to cause parafunctional occlusal activity [41]. Moreover, pathologic loss of occlusal surface causes pulpal exposure or near-pulp exposure, if the secondary dentin is not deposited rapidly enough. However, no clinical gold standard method exists for the assessment of occlusal tooth wear to estimate the remaining tooth thickness. Wada et al. measured the loss of occlusal enamel in vivo using SS-OCT and demonstrated that SS-OCT is capable to estimate the remaining enamel thickness for occlusal tooth wear [9]. Alghilan et al. measured the enamel thickness using PS-OCT to show the well agreement of the values with micro-CT results [10]. Majkut et al. performed the in vitro study to measure the remaining dentin thickness in deep caries after the excavation of carious dentin [42]. A prototype dental OCT system (Prototype 2, Panasonic Healthcare Co. Ltd.) was used in the present study. Three-dimensional (3D) images and high resolution two-dimensional (2D) cross-sectional images were obtained by SS-OCT at 1330 nm center wavelength to measure the remaining dentin thickness (RDT) values using image analysis software. After SS-OCT imaging, micro-CT images were obtained to confirm the OCT findings. The results were compared to evaluate whether a linear relationship existed.
In SS-OCT images, the anatomical shape of the pulp horn was visible with higher backscatter intensities at the border distinguishing from the hollow region of the pulp chamber (Fig. 11) [42]. A strong significant correlation was found between the SS-OCT measurements and the micro-CT values. Pulpal horns and pulp chamber roof observation under OCT allowed measurement of the dentin thickness. Consequently, SS-OCT appears to have potential that can be utilized to measure the remaining dentin thickness in distinct tooth wear with dentin involvement. However, in the case of dentin thickness over 2 mm, the pulpal roof was occasionally masked because of the attenuation of SS-OCT signal [42]. It was possible to observe peaks of pulpal horns at around 1.5∼2.0 mm depth in deep dentin structure that allowed in-depth assessment.
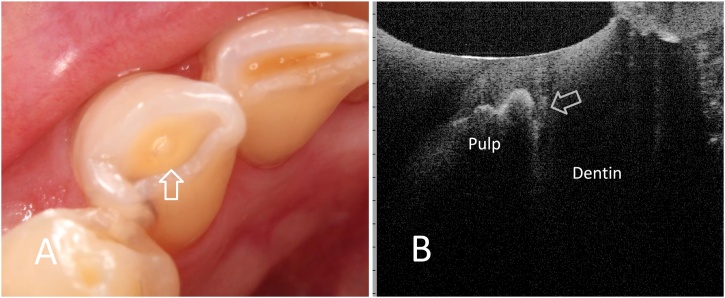
Fig. 11.
Occlusal (incisal) tooth wear.
A. Clinical image of lower incisor with occlusal tooth wear. Semi-translucency zone indicates that remaining dentin is very thin (arrow).
B. SS-OCT image from incisal scan. Location of the pulp hone is clearly depicted under the remaining dentin (arrow).
8. Conclusions
OCT can create cross-sectional images of translucent or semitranslucent biological structures with microscopic level resolution. In dentistry, OCT imaging is effective for the diagnosis of dental caries, tooth cracks, and aging changes of tooth structures such as NCCL and occlusal tooth wear. Advance in modern imaging technology developed new functional OCT systems that provide specific optical characteristics for new biomedical research applications. Further technological evolution will allow to utilize the OCT imaging for diagnosing periodontal disease and oral soft tissue diseases. One of the big advantages of OCT is using light, and it can safely be used in infants and pregnant women without the risk of X-ray radiation exposure. Precise results with high sensitivity and satisfactory specificity indicate the possibility of OCT imaging to be utilized for the diagnosis of other dental diseases.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Acknowledgements
This research project was supported by the Research Grant for Longevity Science (29-3) Ministry of Health, Labor and Welfare, Japan.
References
- 1.Huang D., Swanson E.A., Lin C.P., Schuman J.S., Stinson W.G., Chang W. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto J.G. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol. 2003;21(11):1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 3.Colston B.W., Jr, Everett M.J., Da Silva L.B., Otis L.L., Stroeve P., Nathel H. Imaging of hard- and soft-tissue structure in the oral cavity by optical coherence tomography. Appl Opt. 1998;37(16):3582–3585. doi: 10.1364/ao.37.003582. [DOI] [PubMed] [Google Scholar]
- 4.Leitgeb R., Hitzenberger C., Fercher A. Performance of fourier domain vs. Time domain optical coherence tomography. Opt Express. 2003;11(8):889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 5.Chinn S.R., Swanson E.A., Fujimoto J.G. Optical coherence tomography using a frequency-tunable optical source. Opt Lett. 1997;22(5):340–342. doi: 10.1364/ol.22.000340. [DOI] [PubMed] [Google Scholar]
- 6.Fried D., Glena R.E., Featherstone J.D., Seka W. Nature of light scattering in dental enamel and dentin at visible and near-infrared wavelengths. Appl Opt. 1995;34(7):1278–1285. doi: 10.1364/AO.34.001278. [DOI] [PubMed] [Google Scholar]
- 7.Imai K., Shimada Y., Sadr A., Sumi Y., Tagami J. Noninvasive cross-sectional visualization of enamel cracks by optical coherence tomography in vitro. J Endod. 2012;38(9):1269–1274. doi: 10.1016/j.joen.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Segarra M.S., Shimada Y., Sadr A., Sumi Y., Tagami J. Three-dimensional analysis of enamel crack behavior using optical coherence tomography. J Dent Res. 2017;96(3):308–314. doi: 10.1177/0022034516680156. [DOI] [PubMed] [Google Scholar]
- 9.Wada I., Shimada Y., Ikeda M., Sadr A., Nakashima S., Tagami J. Clinical assessment of non carious cervical lesion using swept-source optical coherence tomography. J Biophotonics. 2015;8(10):846–854. doi: 10.1002/jbio.201400113. [DOI] [PubMed] [Google Scholar]
- 10.Alghilan M.A., Lippert F., Platt J.A., Eckert G.J., González-Cabezas C., Fried D. In vitro longitudinal evaluation of enamel wear by cross-polarization optical coherence tomography. Dent Mater. 2019;35(10):1464–1470. doi: 10.1016/j.dental.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Addy M., Shellis R.P. Interaction between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2006;20:17–31. doi: 10.1159/000093348. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett D.W. The role of erosion in tooth wear: aetiology, prevention and management. Int Dent J. 2005;55(4 Suppl 1):277–284. doi: 10.1111/j.1875-595x.2005.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 13.Truin G.J., van Rijkom H.M., Mulder J., van’t Hof M.A. Caries trends 1996-2002 among 6- and 12-year-old children and erosive wear prevalence among 12-year-old children in the Hague. Caries Res. 2005;39(1):2–8. doi: 10.1159/000081650. [DOI] [PubMed] [Google Scholar]
- 14.Cvek M., Cleaton-Jones P.E., Austin J.C., Andreasen J.O. Pulp reactions to exposure after experimental crown fractures or grinding in adult monkeys. J Endod. 1982;8(9):391–397. doi: 10.1016/S0099-2399(82)80092-7. [DOI] [PubMed] [Google Scholar]
- 15.Fried D., Staninec M., Darling C.L., Lee C., Kang H., Chan K.H. In vivo Near-IR imaging of occlusal lesions at 1310-nm. Proc SPIE Int Soc Opt Eng. 2011 doi: 10.1117/12.878888. 7884(78840b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choma M., Sarunic M., Yang C., Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11(18):2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 17.Marshall G.W., Jr, Marshall S.J., Kinney J.H., Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa H., Sadr A., Shimada Y., Tagami J., Sumi Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J Dent. 2013;41(1):80–89. doi: 10.1016/j.jdent.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Shimada Y., Sadr A., Burrow M.F., Tagami J., Ozawa N., Sumi Y. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. J Dent. 2010;38(8):655–665. doi: 10.1016/j.jdent.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Hariri I., Sadr A., Nakashima S., Shimada Y., Tagami J., Sumi Y. Estimation of the enamel and dentin mineral content from the refractive index. Caries Res. 2013;47(1):18–26. doi: 10.1159/000342416. [DOI] [PubMed] [Google Scholar]
- 21.Natsume Y., Nakashima S., Sadr A., Shimada Y., Tagami J., Sumi Y. Estimation of lesion progress in artificial root caries by swept source optical coherence tomography in comparison to transverse microradiography. J Biomed Opt. 2011;16(7):071408. doi: 10.1117/1.3600448. [DOI] [PubMed] [Google Scholar]
- 22.Takagi S., Liao H., Chow L.C. Effect of tooth-bound fluoride on enamel demineralization/ remineralization in vitro. Caries Res. 2000;34(4):281–288. doi: 10.1159/000016603. [DOI] [PubMed] [Google Scholar]
- 23.Darling C.L., Huynh G.D., Fried D. Light scattering properties of natural and artificially demineralized dental enamel at 1310 nm. J Biomed Opt. 2006;11(3):34023. doi: 10.1117/1.2204603. [DOI] [PubMed] [Google Scholar]
- 24.Jones R.S., Fried D. Remineralization of enamel caries can decrease optical reflectivity. J Dent Res. 2006;85(9):804–808. doi: 10.1177/154405910608500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada Y., Sadr A., Sumi Y., Tagami J. Application of optical coherence tomography (OCT) for diagnosis of caries, cracks, and defects of restorations. Curr Oral Health Rep. 2015;2(2):73–80. doi: 10.1007/s40496-015-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luong M.N., Shimada Y., Araki K., Yoshiyama M., Tagami J., Sadr A. Diagnosis of occlusal caries with dynamic slicing of 3D optical coherence tomography images. Sensors (Basel) 2020;20(6) doi: 10.3390/s20061659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricketts D., Kidd E., Weerheijm K., de Soet H. Hidden caries: what is it? Does it exist? does it matter? Int Dent J. 1997;47(5):259–265. doi: 10.1002/j.1875-595x.1997.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Bader J.D., Shugars D.A., Bonito A.J. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001;65(10):960–968. [PubMed] [Google Scholar]
- 29.Shimada Y., Nakagawa H., Sadr A., Wada I., Nakajima M., Nikaido T. Noninvasive cross-sectional imaging of proximal caries using swept-source optical coherence tomography (SS-OCT) in vivo. J Biophotonics. 2014;7(7):506–513. doi: 10.1002/jbio.201200210. [DOI] [PubMed] [Google Scholar]
- 30.Bakhsh T.A., Sadr A., Shimada Y., Tagami J., Sumi Y. Non-invasive quantification of resin-dentin interfacial gaps using optical coherence tomography: validation against confocal microscopy. Dent Mater. 2011;27(9):915–925. doi: 10.1016/j.dental.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Haak R., Hähnel M., Schneider H., Rosolowski M., Park K.J., Ziebolz D. Clinical and OCT outcomes of a universal adhesive in a randomized clinical trial after 12 months. J Dent. 2019;90 doi: 10.1016/j.jdent.2019.103200. [DOI] [PubMed] [Google Scholar]
- 32.Han S.H., Shimada Y., Sadr A., Tagami J., Kum K.Y., Park S.H. Effect of pretreatment and activation mode on the interfacial adaptation of nanoceramic resin inlay and self-adhesive resin cement. Dent Mater. 2020 doi: 10.1016/j.dental.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y., Shimada Y., Matin K., Sadr A., Sumi Y., Tagami J. Assessment of bacterial demineralization around composite restorations using swept-source optical coherence tomography (SS-OCT) Dent Mater. 2016;32(9):1177–1188. doi: 10.1016/j.dental.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Matsuura C., Shimada Y., Sadr A., Sumi Y., Tagami J. Three-dimensional diagnosis of dentin caries beneath composite restorations using swept-source optical coherence tomography. Dent Mater J. 2018;37(4):642–649. doi: 10.4012/dmj.2017-252. [DOI] [PubMed] [Google Scholar]
- 35.Cameron C.E. Cracked-tooth syndrome. J Am Dent Assoc. 1964;68:405–411. doi: 10.14219/jada.archive.1964.0108. [DOI] [PubMed] [Google Scholar]
- 36.Ellis S.G. Incomplete tooth fracture--proposal for a new definition. Br Dent J. 2001;190(8):424–428. doi: 10.1038/sj.bdj.4800992. [DOI] [PubMed] [Google Scholar]
- 37.Dowker S.E., Davis G.R., Elliott J.C. X-ray microtomography: nondestructive three-dimensional imaging for in vitro endodontic studies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(4):510–516. doi: 10.1016/s1079-2104(97)90155-4. [DOI] [PubMed] [Google Scholar]
- 38.Clark D.J., Sheets C.G., Paquette J.M. Definitive diagnosis of early enamel and dentin cracks based on microscopic evaluation. Journal of esthetic and restorative dentistry : official publication of the American Academy of Esthetic Dentistry [et al] 2003;15(7):391–401. doi: 10.1111/j.1708-8240.2003.tb00963.x. discussion. [DOI] [PubMed] [Google Scholar]
- 39.Piotrowski B.T., Gillette W.B., Hancock E.B. Examining the prevalence and characteristics of abfractionlike cervical lesions in a population of U.S. Veterans. J Am Dent Assoc. 2001;132(12):1694–1701. doi: 10.14219/jada.archive.2001.0122. quiz 726-727. [DOI] [PubMed] [Google Scholar]
- 40.Aw T.C., Lepe X., Johnson G.H., Mancl L. Characteristics of noncarious cervical lesions: a clinical investigation. J Am Dent Assoc. 2002;133(6):725–733. doi: 10.14219/jada.archive.2002.0268. [DOI] [PubMed] [Google Scholar]
- 41.Smith B.G., Knight J.K. An index for measuring the wear of teeth. Br Dent J. 1984;156(12):435–438. doi: 10.1038/sj.bdj.4805394. [DOI] [PubMed] [Google Scholar]
- 42.Majkut P., Sadr A., Shimada Y., Sumi Y., Tagami J. Validation of optical coherence tomography against micro-computed tomography for evaluation of remaining coronal dentin thickness. J Endod. 2015;41(8):1349–1352. doi: 10.1016/j.joen.2015.03.016. [DOI] [PubMed] [Google Scholar]