In the study, we first generated a ME49Δα‐amy mutant and discovered that loss of α‐AMY robustly grew in vitro but contributed to significant virulence attenuation in vivo. The results obtained from our study revealed that ME49Δα‐amy attenuated strain could provide efficient protection against acute infection and chronic infection caused by multiple strains, which signified that ME49Δα‐amy mutant might be a potential vaccine candidate.
![]()
Summary
Individuals with inhibited immunity may develop lethal toxoplasmosis; thus, a safe and effective vaccine is urged to be developed. Toxoplasma gondii (T. gondii) α‐amylase (α‐AMY) is one of the enzymes responsible for starch digestion. In the present study, we first generated a ME49Δα‐amy mutant and discovered that loss of α‐AMY robustly grew in vitro but contributed to significant virulence attenuation in vivo. Therefore, we established a mouse model to explore the protective immunity of Δα‐amy mutant against acute and chronic toxoplasmosis. The results indicated that the survival rates of short‐term or long‐term immunized mice re‐infected with the tachyzoites of multiple T. gondii strains were nearly 100%. ME49Δα‐amy not only could provide protective immunity against tachyzoites infection but also could resist the infection of tissue cysts. Furthermore, we detected that ME49Δα‐amy vaccination could effectively eliminate the proliferation of parasites in mice and prevent the formation of cysts. The significant increases of Th1‐type cytokines, Th2‐type cytokines and specific total IgG and IgG subclasses (IgG2a and IgG1) confirmed efficiency of a combination of cellular and humoral immunity against infection. In conclusion, ME49Δα‐amy attenuated strain can produce strong immune responses to provide efficient protection against toxoplasmosis, which signifies that ME49Δα‐amy mutant may be a potential vaccine candidate.
Introduction
Toxoplasma gondii (T. gondii), a vital obligate intracellular parasitic protozoan, is the causative agent of toxoplasmosis (Kato, 2018). The characteristics of it are worldwide distribution and broad host range that can infect all warm‐blooded animals including humans (Prandovszky et al., 2018; Coutermarsh‐Ott, 2019). T. gondii is considered as a considerably successful parasitic organism, which can persist in the host for the host's entire lifetime and present opportunistic pathogenicity after successful infection (Dubey and Jones, 2008; Montazeri et al., 2018a, 2018b,2018a, 2018b). T. gondii usually can be controlled by immunocompetent hosts. Nevertheless, individuals with inhibited immunity may develop severe clinical symptoms such as encephalitis, ophthalmia, abortion and even death (Williams, 1979; Miedema et al., 2013; Yang et al., 2018). The main epidemic strains of T. gondii are classified as types I, II and III: type I, a robust virulence in mice, type II, predominant strain of human infections in Europe and North America, and type III, known for its low virulence in hosts (Howe and Sibley, 1995; Howe et al., 1997). The diversified population structure of T. gondii strains, complexity of life cycle and diversity of transmission routes result in the difficulties in toxoplasmosis control (Howe and Sibley, 1995; Dubey, 2009; Shwab et al., 2018). At present, drugs against toxoplasmosis are restricted to combinations of pyrimethamine and sulfadiazine. However, this treatment may contribute to numerous side effects, drug residues and no effect on chronic infection (Rajapakse et al., 2013; Montazeri et al., 2015, 2018a, 2018b,2015, 2018a, 2018b).
In recent years, important progresses have been made in the development of vaccines against toxoplasmosis, especially in live‐attenuated vaccines, DNA vaccines, recombinant protein vaccines and live vector‐based vaccines (Wang et al., 2019). Surface proteins (SAGs), dense granules (GRAs), rhoptries (ROPs) and micronemes (MICs) are core component of DNA vaccines and recombinant protein vaccines, which play crucial roles in development of T. gondii and manipulation of the host’s immune responses (Zhang et al., 2015; Pan et al., 2017; Zhang et al., 2018). Although above vaccines alleviated T. gondii infection, none of these were able to effectively eliminate the formation of cysts and provide enough protection. Therefore, it is imperative to develop a safe and efficient vaccine for T. gondii. Live‐attenuated vaccine is one of the most effective approaches to provide immune protection, although numerous attempts have been made to produce attenuated T. gondii vaccines, only one commercial vaccine, Toxovax® based on the live‐attenuated tachyzoite S48 strain, is licensed (Buxton et al., 1991).
The energy metabolism of bradyzoites is different from tachyzoites, and the most typical feature is massive amylopectin accumulated in the cytoplasm (Guimaraes et al., 2003). T. gondii can store glucose in the form of amylopectin that can be found in several life stages (Guimaraes et al., 2003; Guerardel et al., 2005). It has been hypothesized that amylopectin granules may be an energy reserve for the conversion of bradyzoites to tachyzoites (Coppin et al., 2003). Recent studies have proved that a Ca2+‐dependent protein kinase (CDPK2), as a key regulator in amylopectin metabolism, can bind to amylopectin granules and phosphorylate some enzymes involved in amylopectin metabolism. And the deletion of CDPK2 leads to an imbalance of digestion and storage of amylopectin, thereby effecting the development of bradyzoites (Uboldi et al., 2015; Sugi et al., 2017). α‐amylase (α‐AMY) is one of the enzymes responsible for amylopectin digestion, and it may be phosphorylated by CDPK2 (Uboldi et al., 2015). However, the specific biological function of α‐AMY in T. gondii is rarely reported.
In recent years, genome editing techniques have been applied to develop attenuated Toxoplasma vaccines, and some studies have indicated that genetically attenuated strains produced by deletion of certain genes could provide excellent effect against toxoplasmosis (Ismael et al., 2006; Lagal et al., 2015; Wang et al., 2016). In the present study, we first constructed a Δα‐amy mutant designated ME49Δα‐amy and then proved the ability of α‐AMY to degrade amylopectin and detected that the virulence of the mutant in vivo was significantly alleviated. Therefore, we established a mouse model to explore the immune responses of Δα‐amy mutant against acute infection and chronic infection caused by multiple strains. The results obtained from this study revealed that ME49Δα‐amy mutant might be a potential vaccine candidate against various T. gondii strains infections.
Results
Toxoplasma α‐AMY is involved in amylopectin digestion and disruption of α‐AMY attenuates virulence in vivo but grows robustly in vitro
The α‐AMY gene deletion was successfully established using CRISPR/CAS9 genome editing technique. T. gondii α‐AMY gene was completely knocked out in ME49 strain, and the pyrimethamine‐resistant marker DHFR* was inserted by CRISPR/CAS9‐mediated homologous recombination (Fig. 1A). After the α‐AMY‐targeted CRISPR plasmid and the homology template were co‐transferred into the ME49 strain, the single clones were screened by diagnostic PCRs to verify the deletion of the α‐AMY gene and the correct insertion of homologous recombination fragments (Fig. 1B). In addition, the lack of α‐AMY protein expression in ME49Δα‐amy strain was confirmed by Western blotting, which further demonstrated that α‐AMY was successfully knocked out in the ME49 strain (Fig. 1C).
Fig. 1.
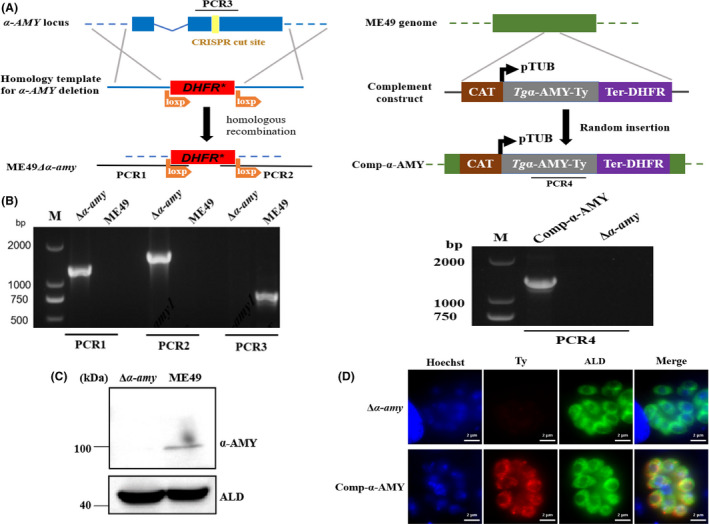
Construction of α‐AMY knockout and complementation strains.
A. Schematic illustration of generation of the ME49Δα‐amy and Comp‐α‐AMY strains via CRISPR/CAS9‐mediated technique.
B. Diagnostic PCRs on a Δα‐amy: DHFR* and Comp‐α‐AMY clones.
C. Western blotting confirmed disruption of α‐AMY in transgenic parasites. Parasite lysates were incubated with anti‐α‐AMY antibody, ALD served as the loading control.
D. The presence of α‐AMY was confirmed by IFA. Samples were stained with mouse anti‐Ty and rabbit anti‐T. gondii ALD.
To identify the function of α‐AMY in amylopectin digestion, the formation of amylopectin was detected by periodic acid‐Schiff (PAS) staining. The uneven and slight accumulation of amylopectin granules was distributed in α‐AMY knockout tachyzoites, whereas the accumulation was further enhanced by bradyzoite culture conditions (Fig. 2A and B). The plaque assay was performed to evaluate the growth of disruption of α‐AMY mutant, and results in Figure 2C and D showed that there was no difference between parental strain and α‐AMY deletion mutant. To explore the effect of ME49 strain loss of α‐AMY on virulence, ICR mice were intraperitoneally injected with 100 tachyzoites of parental strain ME49 and α‐AMY deletion mutant respectively. The survival of infected mice was monitored for 30 days. As shown in Figure 2E, mice infected with ME49 began to show irreversible death from day 11, and the final mortality rate reached 70%. However, the survival of mice infected with ME49Δα‐amy was as high as 90%, and mice did not show significant clinical symptoms in form of locomotor activity and depressive symptoms 30 days post‐observation (Mahmoud et al., 2017). In addition, α‐AMY deletion resulted in significant reduction in the formation of brain cysts (Fig. 2F).
Fig. 2.
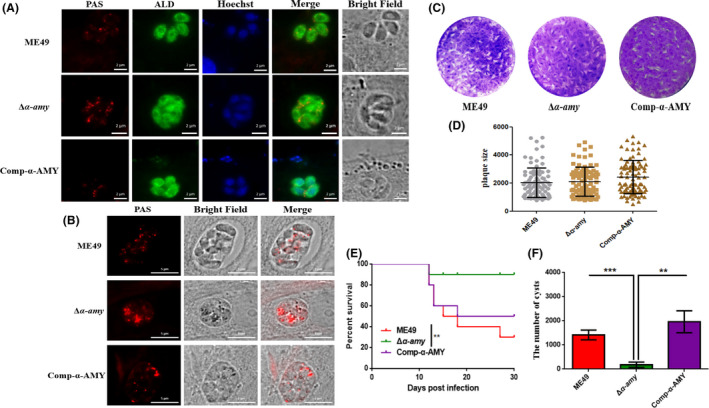
Phenotypic analysis of α‐AMY knockout and complementation strains. A and B. Amylopectin accumulation was detected with PAS staining. Parasites were inoculated to HFF host cells and incubated for 24 h under normal culture conditions for tachyzoites (A) or incubated for 2 h to allow the parasites to invade host cells, followed by incubation under conditions of pH 8.2 medium without CO2 for 4 days for bradyzoites (B). C. Plaque assay of parasites in vitro. Purified indicated tachyzoites were used to infect HFF cells, and 200 parasites were cultured for 12 days before staining to determine the plaque size. D. Relative size of plaques in Fig. 2C. E. Survival curves of mice infected with tachyzoites of indicated strains. ME49 strain and α‐AMY‐deficient mutant were used to infect ICR mice (100 parasites per mouse, 10 mice for each group) by intraperitoneal injection, and the survival of mice was monitored for 30 days. F. Brain cysts of ICR mice infected with corresponding strains. *P < 0.05, Student’s t‐test. **P < 0.01, Mantel‐Cox log‐rank test. The data are presented as the mean ± SEM of three independent experiments.
To determine whether these phenotypic changes were directly caused by α‐AMY deletion, we randomly inserted a C‐terminally Ty‐tagged complementary fragment in ME49 genome (Fig. 1A). Screening PCRs and immunostaining demonstrated the desired integration of the complementing construct and the successful expression of protein (Fig. 1B and D). Phenotypic analysis of the complementation strain indeed rescued the defects in Δα‐amy mutant (Fig. 2). Together, these results indicated that disruption of α‐AMY had no effect on growth in vitro but contributed to virulence attenuation and cysts reduction in mice.
ME49Δα‐amy vaccination protects mice from tachyzoites infection
Considering low virulence of α‐AMY deletion mutant in mice, this feature may indicate that α‐AMY deletion mutant has potential to be a good vaccine candidate. Thus, ICR mice were first vaccinated with 100 ME49Δα‐amy tachyzoites to evaluate the immune protection. After 30 days, the vaccinated mice were challenged with 103 tachyzoites of RHΔhxgprt, ME49, 104 tachyzoites of VEG, C7719 or TgPIG‐WH1 by intraperitoneal injection for 30 days. As expected, non‐immunized mice all died within 9 days after infection with RHΔhxgprt, and ME49, VEG and C7719 infection of mice led to 80%, 40% and 60% mortality rates respectively. Nevertheless, the survival rates of all vaccinated mice were upon 100% (Fig. 3A–D), which showed significant increases when compared with non‐vaccinated mice. These above results illustrated that α‐AMY deletion mutant was able to provide effective protection against acute infection caused by the tachyzoites of T. gondii.
Fig. 3.
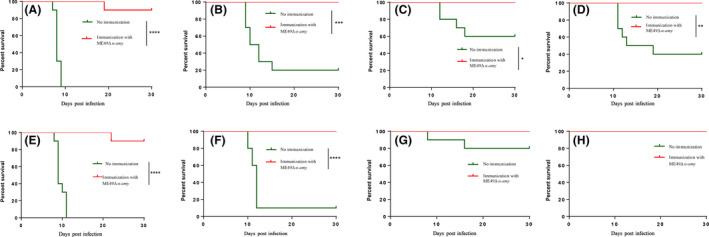
ME49Δα‐amy vaccination protected mice from multiple T. gondii tachyzoites infection. Survival curves of ME49Δα‐amy‐vaccinated mice, 30 days post‐vaccination, intraperitoneally challenged with 103 tachyzoites of RHΔhxgprt (A), 103 tachyzoites of ME49 (B), 104 tachyzoites of VEG (C) or 104 tachyzoites of C7719 (D) strains. 75 days post‐vaccination, vaccinated mice were intraperitoneally challenged with 103 tachyzoites of RHΔhxgprt (E), 103 tachyzoites of ME49 (F), 104 tachyzoites of VEG (G) or 104 tachyzoites of TgPIG‐Wh1 (H). All mice were monitored for 30 days, and non‐vaccinated mice were included as control. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, Mantel‐Cox log‐rank test.
To verify whether ME49Δα‐amy produced longer‐term immune protection against acute toxoplasmosis in mice, ICR mice were immunized with 100 tachyzoites of ME49Δα‐amy for 75 days; then, they were intraperitoneally injected with 103 tachyzoites of type Ⅰ strain RHΔhxgprt, type II strain ME49, 104 tachyzoites of type III stain VEG or DB#3 isolated strain TgPIG‐WH1. Being monitored 30 days later, the survival rates of vaccinated mice re‐infected RHΔhxgprt, ME49 and VEG were 100%, which were consistent with the results of 30 days post‐immunization (Fig. 3E–G). While the mortality rate of non‐vaccinated mice infected attenuated strain VEG was only 20%, there was no significant difference from the immunized group despite the deaths. And both naïve group and vaccinated group re‐infected TgPIG‐WH1 all survived (Fig. 3H). These aforementioned results demonstrated that ME49Δα‐amy could exert short‐term and long‐term protective immunity against acute infection.
ME49Δα‐amy vaccination protects mice from bradyzoites infection
Since ME49Δα‐amy could effectively resist acute infection induced by tachyzoites of T. gondii, we further explored whether it could also take effect against bradyzoites infection that was induced by T. gondii cysts. 30 days immunized later, either naïve mice or immunized mice were orally infected with 50 cysts of ME49 strain. After 30 days of continuous observation, the survival of mice was recorded. Consistent with the results of protective immunity against tachyzoites infection, the final survival rate of naïve mice infected with cysts was only 20%, and yet all vaccinated mice survived after infection (Fig. 4). These results further signified that Δα‐amy mutant could provide good immune protection against bradyzoites infection.
Fig. 4.
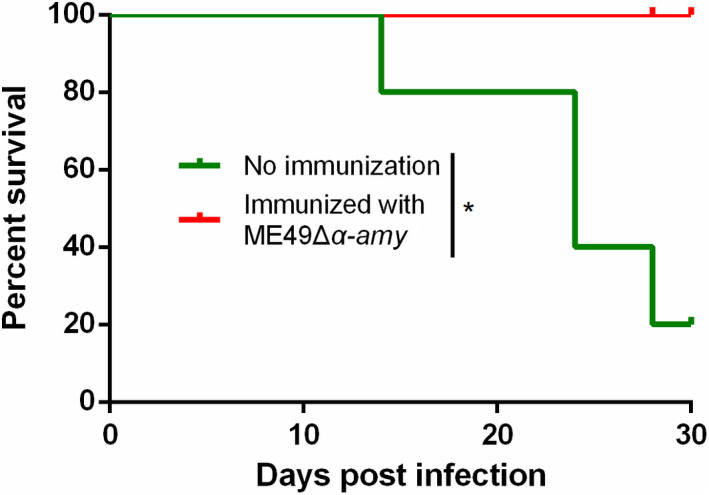
ME49Δα‐amy vaccination protected mice from bradyzoites infection. ME49Δα‐amy vaccination were infected with 50 fresh brain cysts of ME49 strain by oral infection, and all mice were monitored for 30 days (5 mice for each group). *P < 0.05, Mantel‐Cox log‐rank test.
ME49Δα‐amy vaccination contributes to decrease of parasite burden in peritoneal fluids
Due to ME49Δα‐amy vaccination performed efficient protection against acute infection, we probed the possible mechanism of this phenomenon and examined the tachyzoites growth in both naïve mice and immunized mice. After infection with the tachyzoites of RHΔhxgprt for 6 days, the peritoneal fluids were harvested, and parasite burden was measured by qPCR. As shown in Figure 5, abundant parasites were detected in non‐vaccinated infected mice, which almost developed more than 108 parasites. In contrast, the low levels of parasites burden appeared in vaccinated mice infected with RHΔhxgprt (≈102 per mouse), which nearly decreased by 106‐fold compared with non‐immunized control group, suggesting that vaccinated with ME49Δα‐amy could effectively eliminate the proliferation of parasites in mice.
Fig. 5.
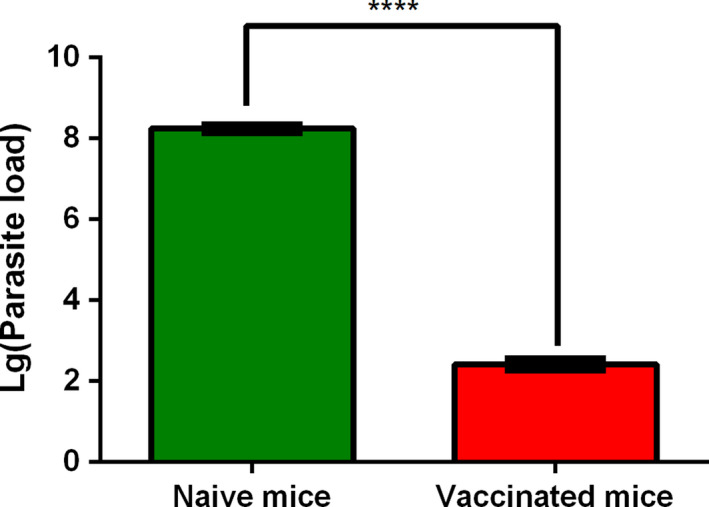
ME49Δα‐amy vaccination effectively reduced the replication of challenging parasites in mice. Naïve or vaccinated mice were challenged with 105 tachyzoites of RHΔhxgprt (5 mice for each group) by intraperitoneal injection, and the parasite loads in peritoneal fluids 6 days post‐infection were determined by quantitative PCR. The data are presented as the mean ± SEM of three independent experiments, ****P < 0.0001, Student’s t‐test.
ME49Δα‐amy immunization provides protection against chronic infection
The typical trait of chronic infection was the persistence of tissue cysts containing bradyzoites, which was considered to be the primary approach of exposure to T. gondii (Pittman and Knoll, 2015; Montazeri et al., 2018a, 2018b,2018a, 2018b). Therefore, study was performed to validate whether Δα‐amy mutant vaccination could provide protection against chronic infection. TgPIG‐WH1, a less virulent strain and prone to forming cysts, was used to re‐infect the immunized mice (Xia et al., 2018a). 30 days post‐infection, the survival rates of both naïve mice and immunized mice infected with TgPIG‐WH1 were 100% (Fig. 2H). The brain tissues of four mice that were randomly picked out from each group were harvested, and the brain cysts number was counted using DBA staining under fluorescence microscope, subsequently. The number of cysts in vaccinated mice group significantly decreased compared with non‐vaccinated mice, although a small amount of brain cysts could still be detected in vaccinated mice, which might be produced by the ME49Δα‐amy infection. Thus, results in Figure 6 revealed that ME49Δα‐amy immunization could effectively prevent the formation of cysts, which demonstrated that ME49Δα‐amy could also exert protective immunity against chronic infection.
Fig. 6.
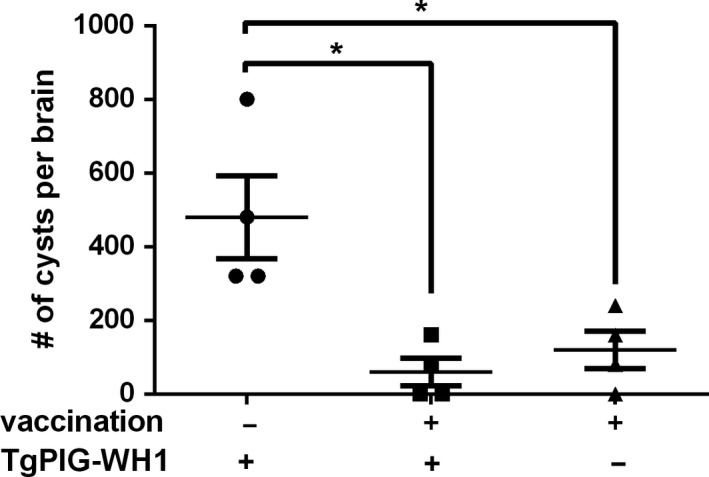
ME49Δα‐amy vaccination prevented mice from the formation of cysts. The survived mice in both naïve and immunized groups challenged with TgPIG‐WH1 were sacrificed, and the number of Toxoplasma cysts in the brain was determined by DBA‐FITC staining under fluorescent microscopy. Data from the analysis of four mice in each group were graphed, *P < 0.05, Student’s t‐test.
Levels of cytokines and T. gondii specific IgG antibodies after ME49Δα‐amy immunization
To elucidate the potential mechanism of protective immunity against T. gondii infection provided by Δα‐amy mutant, the levels of cytokines in serum were measured by ELISA 30 days or 75 days after ME49Δα‐amy vaccination. The results showed that the levels of Th1‐type cytokines (IFN‐γ, IL‐12 and TNF‐α) significantly increased than those in naïve mice (Fig. 7A–C). Similarly, the level of Th2‐type cytokine (IL‐4) of immunized mice at 30 days was notably higher than that of non‐immunized mice. Although the level of IL‐4 in the serum of vaccinated mice still increased after 75 days, there was no significant difference compared with the non‐vaccinated group (Fig. 7D). In addition, we determined the presence of T. gondii specific IgG levels and found that ME49Δα‐amy vaccination elicited high levels of IgG, which developed an incremental trend with the extension of time (Fig. 7E). Then, the levels of specific IgG subclasses (IgG1 and IgG2a isotypes respectively) were detected to evaluate Th1 and/or Th2 response in immunized mice. As shown in Figure 7E, the expression levels of IgG1 and IgG2a 30 and 75 days post‐immunization were significantly increased in vaccinated mice. Together, these results illuminated that vaccination with ME49Δα‐amy in mice was likely to stimulate organism to elicit a mix of Th1/Th2 immune response against the infection.
Fig. 7.
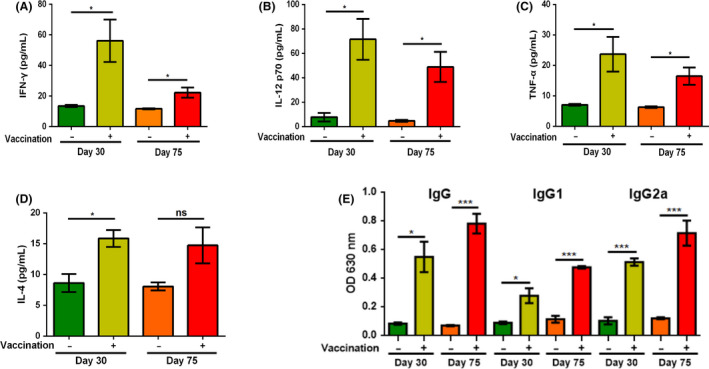
Levels of cytokines and Toxoplasma‐specific IgG in the serum of mice 30 and 75 days post‐vaccination. A–D. Levels of IFN‐γ, IL‐12 p70, TNF‐α and IL‐4 were measured by ELISA. E. Levels of Toxoplasma‐specific total IgG and IgG subclass (IgG1 and IgG2a) measured by indirect ELISA. Serum samples of naïve mice were determined as controls, and three mice from each group were analysed. The data are presented as the mean ± SEM of three mice from each group. ***P < 0.001, *P < 0.05, ‘ns’ indicated as not significant, Student’s t‐test.
Cytokine production by splenocytes of immunized mice
Splenocytes were collected to further assess cellular immune responses and antigen‐specific memory responses induced by soluble Toxoplasma antigen (TSA) in immunized mice. 145 days post‐immunization, the cytokine production of serum samples was measured, and results revealed that there was no significant difference of cytokine levels between immunized mice and naïve mice (Fig. 8A). In contrast, IFN‐γ, TNF‐α, IL‐12 levels of splenocytes stimulated by TSA markedly increased in immunized mice compared with that in non‐immunized mice, although the production of IL‐4 showed an increase, there was no significant difference compared with naïve mice (Fig. 8B–E). Taken these results together, Th1‐type cells‐mediated immune responses triggered robust protection against secondary stimulation.
Fig. 8.
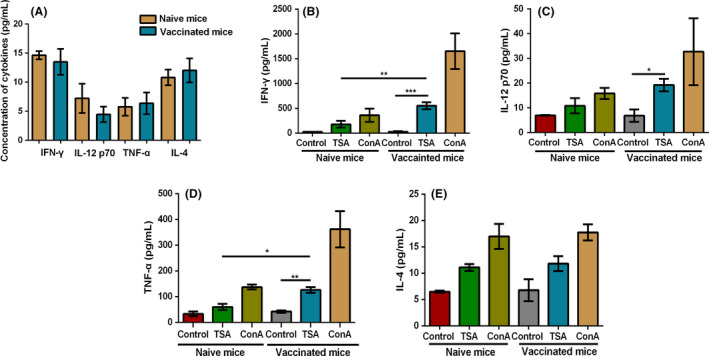
Cytokines production by splenocytes of naïve mice and vaccinated mice after Toxoplasma antigen stimulation.
A. The serum samples of naïve mice and immunized mice were collected to measure the indicated cytokines 145 days post‐immunization.
B–E. The splenocytes of vaccinated mice were harvested 145 days post‐vaccination. Control is the group treated with foetal bovine serum, TSA is the group treated with total Toxoplasma antigen, and ConA is the group treated with concanavalin A as a positive control. The levels of IFN‐γ, IL‐12 p70, TNF‐α and IL‐4 were measured by ELISA. Three mice from each group were analysed, and each treatment condition was repeated three times, ***P < 0.001, **P < 0.01, *P < 0.05, Student’s t‐test.
Discussion
T. gondii can cause persistent infection in both animals and humans as a worldwide distributed intracellular protozoan, and severe toxoplasmosis generally occurs in immunocompromised hosts (Di Cristina et al., 2017; Wang et al., 2017a). The main clinical symptoms of toxoplasmosis are mental illnesses and congenital impairment (Elsheikha, 2008; Sutterland et al., 2015). Currently, the most commonly used drugs for toxoplasmosis cure are pyrimethamine and sulfadiazine; however, these therapeutic drugs take no effect on chronic infection and there may exist risk of side effects in the treated hosts (Pramanik et al., 2019). Recent research has demonstrated that vaccines may be the best choice for toxoplasmosis treatment, and a commercial vaccine for preventing abortion in sheep is available (Dunay et al., 2018). Nevertheless, numerous drawbacks still present in current studies, and it is urgent to excogitate a safe and efficient vaccine. In the present study, a disruption of α‐AMY strain was successfully constructed, and then, a mouse model vaccinated was established to study the protective immunity provided by ME49Δα‐amy against acute and chronic infection.
Amylopectin granule is considered to be an important energy storage material for bradyzoites formation (Coppin et al., 2003; Tomavo, 2015), and α‐AMY is an enzyme involved in the amylopectin metabolism pathway of T. gondii, which may be phosphorylated by CDPK2 (Uboldi et al., 2015; Sugi et al., 2017). However, the specific biological function of α‐AMY is rarely reported. In this study, CRISPR/Cas9 gene editing technology was applied to generate deletion of α‐AMY gene, and results in our research revealed that α‐AMY could degrade amylopectin in both tachyzoites and bradyzoites conditions. In view of a low dose (100) of ME49Δα‐amy tachyzoites contributing to the attenuation of the virulence in mice, thus this dose was used for all subsequent experiments to evaluate the possibility of Δα‐amy mutant resistance to T. gondii challenge.
One of the most effective means of provision of protective immune responses are live‐attenuated vaccines due to the mimic natural infection that can built an approximate microenvironment for antigen processing and presentation (Wang et al., 2019). Based on the phenotypes of effective growth in vitro and low proliferation ability in vivo, α‐AMY‐deficient ME49 strain was thought to be a potential live‐attenuated vaccine against T. gondii. The rapid replication of tachyzoites throughout hosts’ body can cause the outbreak of acute infection, and the severity of an acute infection is affected by environmental factors, the strains of T. gondii and the route of infection (Pittman and Knoll, 2015). The main epidemic strains of T. gondii are classified as types I, II and III, which exert different acute virulence in mice (Howe and Sibley, 1995; Howe et al., 1997), and the main route of host infection with Toxoplasma is to ingest contaminated water and food (Esch and Petersen, 2013). As shown by the results, mice immunized with ME49Δα‐amy appeared no clinical symptom and all survived when challenged with tachyzoites of type II, type III and other natural isolates by intraperitoneal infection, as well as re‐infection with cysts by oral infection, even the protection rate reached 90% and parasite burden in peritoneal fluids dropped to an extremely low level against type I strain challenge, indicating that ME49Δα‐amy vaccination could trigger effective protection to significantly improve survival rates and reduce parasite burden caused by acute T. gondii infection. Tissue cysts can facilely be killed by physical methods, while antiparasitic drugs have no effect on cysts (Coombs and Muller, 2002; Hill and Dubey, 2002). Consequently, the aim of developing an efficient vaccine is to prevent tissue cysts formation. Mice immunized with ME49Δα‐amy could dramatically reduce the formation of brain cysts. Compared with other previously reported vaccines, ME49Δα‐amy could provide a long‐term protective immune response against both acute infection and chronic infection (Huang et al., 2019; Zheng et al., 2019). However, there are some disadvantages of it, such as incomplete reduction of cysts and a certain virulence threat (Fox and Bzik, 2010).
The B‐cell response can be activated and produce numerous specific IgG, which can neutralize the adhesion of T. gondii and then inhibit its ability of infection (Sayles et al., 2000). In the study presented here, we measured specific anti‐T. gondii IgG antibody titres and IgG isotypes in both vaccinated mice and non‐vaccinated mice. Consistent with other attenuated T. gondii vaccines, ME49Δα‐amy vaccination indeed upregulated the production of IgG, IgG1 and IgG2a, which signified that ME49Δα‐amy immunization induced a mixed Th1/Th2‐type immune response. CD8+ T and CD4+ T cells play a crucial role against T. gondii as effector T lymphocytes (Suzuki and Remington, 1988). IL‐12 produced by macrophages, neutrophils and dendritic cells can induce proliferation and differentiation of Th1‐type T cells, which can stimulate natural killer cells and T cells to produce IFN‐γ, a major cytokine against T. gondii (Gazzinelli et al., 1993). In addition to the effective elimination of tachyzoites, IL‐12 and IFN‐γ are also essential for maintaining chronic infection (Yarovinsky, 2014). Other cytokines such as TNF‐α, IL‐2, IL‐4 and IL‐6 play a protective role in T. gondii infection. Then, we analysed the levels of cytokines and found that both Th1‐type cytokines (IFN‐γ, IL‐12 and TNF‐α) and Th2‐type cytokines (IL‐4) significantly increased. Above results further supported the inference that ME49Δα‐amy immunized could simultaneously activate Th1 and Th2 immune responses to secret cytokines against T. gondii. Regarding patterns of cytokines measured in splenocytes harvested from naïve mice or immunized mice after 145 days, a time point, cytokines recovered to normal levels. When the cytokines production of splenocytes isolated from immunized mice strongly increased after stimulation with Toxoplasma‐specific antigen; moreover, these cytokines are mainly based on Th1‐type cytokines. These results potently suggest that ME49Δα‐amy vaccination can induce a combination of Th1/Th2 immune response, whereas Th1‐type cell‐mediated immune response, especially IFN‐γ‐mediated activation, plays a leading role.
In conclusion, the present study demonstrated that α‐AMY was able to degrade amylopectin and deletion of α‐AMY mutant attenuated virulence in vivo but normal proliferation in vitro, which were the bases of a potential vaccine candidate. In addition, a safe dose, 100 tachyzoites of ME49Δα‐amy vaccination provided both short‐term and long‐term protective immunity against challenge with multiple T. gondii classic strains and nature isolates. The results of extreme reduction in the parasite cyst burden in the brain of immunized mice illustrated that vaccination of ME49Δα‐amy could also effectually prevent form chronic infection. And the administration of ME49Δα‐amy was able to stimulate a combination of humoral immune response and cellular immune response to protect animals from further infections of various T. gondii strains. Our findings may reveal the potential of ME49Δα‐amy as an efficient and safe live‐attenuated vaccine candidate. Despite these, the protection of ME49Δα‐amy needs to be improved based on its above deficiencies. It has been proved that the deletion of CDPK2 would lead to abnormal accumulation of abundant amylopectin, which may prevent the establishment of the chronic toxoplasmosis in the mouse model (Uboldi et al., 2015). In addition, live‐attenuated Pru:Δcdpk2 strain could also develop a protective immune response against acute, chronic and congenital toxoplasmosis (Wang et al., 2018). Therefore, in order to abolish the formation of brain cysts in mice, we will consider the deletion of CDPK2 based on ME49Δα‐amy strain in the future studies, making it a safer and more efficacious vaccine in the clinical application. In addition, in biological security of this vaccine requires to be further studied, and the protective efficiency also needs to be assessed in large animals, such as sheep.
Experimental procedures
Animals and parasites
8‐week female ICR mice were acquired from the Hubei Provincial Centre for Diseases Control and Prevention (Wuhan, China). All mice were co‐housed under specific pathogen‐free conditions at 25° under a 12 h light–dark cycle (Wuhan, China). All experimental procedures were approved by the Scientific Ethic Committee of Huazhong Agricultural University (HZAUMO‐2019‐005), and all efforts were made to minimize the suffering of animals.
Tachyzoites of T. gondii type Ⅰ strain RHΔhxgprt, type Ⅱ strain ME49, the mutant strain ME49Δα‐amy, type Ⅲ strain VEG, RFLP genotype ToxoDB #3 strain TgPIG‐WH1 and ToxoDB #9 strain C7719 were propagated in human foreskin fibroblast cells (obtained from ATCC, USA) which were cultured in DMEM supplemented with 2% FBS and were incubated at 37°C and 5% CO2.
Generation of α‐AMY knockout and complementation strains
CRISPR/CAS9‐mediated homologous gene replacement system was used to knockout α‐AMY gene (TGME49_246690) of T. gondii, and the methods were described previously (Mahmoud et al., 2017). Briefly, the α‐AMY targeting CRISPR plasmid and 5H‐DHFR‐3H homologous templates for replacing α‐AMY were co‐transfected into purified tachyzoites of ME49 and then selected with 1 μM pyrimethamine. Positive single clone was identified by diagnostic PCR. The construction of complementation strain was based on the processes of pα‐AMY::α‐AMY‐CAT fragment being transfected into ME49Δα‐amy and selected with 30 μM chloramphenicol, and diagnostic PCR (PCR4) was used to identify positive clones. All primers and plasmids used in this study were listed in Table S1. In addition, a series of techniques used to determine protein expression and parasites growth, such as Western blot, IFA, plaque assays and periodic acid‐Schiff (PAS) staining, were all executed as previously described (Shen and Sibley, 2014; Sugi et al., 2017; Xia et al., 2018a).
Virulence tests in mice
8‐week‐old female ICR mice were infected with 100 freshly egressed tachyzoites of ME49 or ME49Δα‐amy by intraperitoneal injection. Then, all mice were monitored daily to assess mortality for 30 days, and the serum samples of surviving mice were collected for MIC3‐based ELISA test after 30 days post‐infection. The anti‐MIC3 negative mice were left out in subsequent analysis.
Immune protection against tachyzoites infection and bradyzoites infection
Eight‐week female ICR mice were first immunized with ME49Δα‐amy, 30 and 75 days post‐vaccination; then both immunized mice and naïve mice were challenged with 103 tachyzoites of RHΔhxgprt or ME49, 104 tachyzoites of VEG, C7719 or TgPIG‐WH1 by intraperitoneal injection to test protective immunity against tachyzoites infection (10 mice/parasite strain), or 50 fresh brain cysts of ME49 by oral infection to test efficacy against bradyzoites infection (5 mice/group). Subsequently, clinical symptoms and survival of all mice were monitored daily for another 30 days.
Determination of parasite burden in peritoneal fluids by ME49Δα‐amy vaccination
Additionally, vaccinated mice and naïve mice of the same age were intraperitoneally injected with 105 tachyzoites of RHΔhxgprt. After 6 days post‐infection, all the mice were euthanized by inhalation of CO2, and peritoneal fluid was harvested from ME49Δα‐amy‐immunized mice infected with RHΔhxgprt and non‐immunized mice infected with RHΔhxgprt, and total genomic DNA was extracted from peritoneal fluid using the EasyPure Genomic DNA Kit (Tiangen Biotech, Beijing, China) following the manufacturer’s protocols. The method of calculating parasite burden in peritoneal fluids was performed as described previously (Xia et al., 2018b). Briefly, CT value of each sample was measured by quantitative real‐time PCR using corresponding genomic DNA samples, and β‐tubulin gene‐specific quantitative RT‐PCR primers were listed Table S1. Quantitative RT‐PCR was performed on ABI Step one plus real‐time PCR instrument using TB Green qPCR Master Mix (TaKaRa, Japan). Parasite burden in peritoneal fluids was calculated from a standard curve using CT values and Lg (tachyzoite numbers).
The formation of brain cysts after vaccination
Mice immunized with ME49Δα‐amy and naïve mice were infected with 104 tachyzoites of TgPIG‐WH1, and mice immunized but not TgPIG‐WH1 challenged were considered as a control group. After 30 days post‐infection, seropositive mice were euthanized and brain tissues of them were homogenized to measure the number of cysts by DBA‐FITC (Vector Laboratories, Burlingame, CA, USA) staining as described previously (Huskinson‐Mark et al., 1991).
Preparation of soluble Toxoplasma antigen (TSA)
The method of preparing soluble tachyzoite antigens was performed as previously described (Mineo et al., 1993; Bastos et al., 2016). Briefly, freshly egressed T. gondii ME49 tachyzoites were collected, washed in PBS and then lysed by sonication on ice for 30 min. Subsequently, the samples after sonication treatment were centrifuged at 12 000 rcf for 10 min at 4°. The supernatants were collected as TSA that were stored at −80° before use, and the TSA concentration was determined BCA Protein Assay Kit (Beyotime Biotechnology, Beijing, China).
Vaccine‐induced cytokines and antibody levels measurements
Serum samples of immunized mice and naïve mice were gathered for cytokines and antibody levels detection 30 or 75‐days post‐infection. The effect of vaccine‐induced cytokine production was measured using mouse IFN‐γ, TNF‐α, IL‐12p70 and IL‐10 ELISA kits (BioLegend, San Diego, CA, USA) according to the manufacturer’s protocols. The levels of T. gondii specific total immunoglobulin G (IgG) and subclasses of IgG antibodies IgG2a and IgG1 as indicators of type 1T‐helper (Th1) and type 2T‐helper (Th2) responses, respectively, were analysed using ELISA assay as described previously (Wang et al., 2017a; Huang et al., 2019). A total of 100 μl soluble tachyzoite antigens (5 μg ml−1) were coated in a 96‐well plate and incubated at 4°C overnight. Then, the plate was washed 3 times and blocked with 1% BSA at 37°C for 1 h. After washing 3 times, 100 μl serum samples were added to appropriate wells at 37°C for 45 min. Subsequently, 100 μl of HRP conjugated goat anti‐mouse IgG secondary antibodies (1:5000 dilutions, Beyotime Biotechnology, Beijing, China), anti‐mouse IgG1(1:10 000, Abcam, Cambridge, UK) and anti‐mouse IgG2a (1:10 000, Abcam) were added to each well and incubated at 37°C for 30 min. Finally, tetramethylbenzidine as substrate was incubated to visualize the immunoreaction, and the OD value was determined at 630 nm using a microplate reader.
Cytokines in splenocyte supernatants after Toxoplasma antigen treatment
The spleens and serum samples of naïve mice and vaccinated mice were harvested 145 days vaccination later, and the splenocyte suspensions were acquired as previously described (Elsheikha, 2008; Wang et al., 2017b). In brief, spleens were washed in Roswell Park Memorial Institute medium (RPMI) 1640 (Life Technologies, Rockville, MD, USA), and then the spleen was pushed through a nylon net. Subsequently, red cell lysis solution (Biosharp, Beijing, China) was added into the compound to remove the red blood cells. The purified suspensions were plated in 96‐well plates and cultured in RPMI 1640 supplemented with 10% FBS. The suspensions in either non‐vaccinated mice or vaccinated mice were treated in groups as follows: (i) control: suspensions without any treatment as negative controls; (ii) TSA: suspensions were stimulated with total T. gondii soluble antigens (TSA, final concentration 50 µg ml−1); (iii) ConA: suspensions were stimulated with 5 µg ml−1 concanavalin A (LifeTechnologies, Rockville, MD, USA) as positive control. After 3 days treatment, the supernatants were gathered to measure the cytokine production using ELISA.
Statistical analysis
All data were performed with graphpad prism 6 (GraphPad Software, La Jolla, CA, USA) and presented as the mean ± SEM. The statistical differences between groups were determined using Student’s t‐test or two‐way analysis of variance as indicated in the figure legends. A value of P < 0.05 was defined as statistically significant. Cumulative mortality was graphed as Kaplan–Meier survival plots and analysed by Mantel‐Cox log‐rank test.
Supporting information
Table S1. Primers used in the study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31802182) and the Independent Technology Innovation Fund Project of Huazhong Agricultural University (2662020DKPY015).
Microbial Biotechnology (2020) 13(6), 2057–2069
Funding information
This work was supported by the National Natural Science Foundation of China (Grant No. 31802182) and the Independent Technology Innovation Fund Project of Huazhong Agricultural University (2662020DKPY015).
References
- Bastos, L.M. , Macêdo, A.G. Jr , Silva, M.V. , Santiago, F.M. , Ramos, E.L. , Santos, F.A. , et al (2016) Toxoplasma gondii‐derived synthetic peptides containing B‐ and T‐cell epitopes from GRA2 protein are able to enhance mice survival in a model of experimental toxoplasmosis. Front Cell Infect Microbiol 6: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton, D. , Thomson, K. , Maley, S. , Wright, S. , and Bos, H.J. (1991) Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet Rec 129: 89–93. [DOI] [PubMed] [Google Scholar]
- Coombs, G.H. , and Muller, S. (2002) Recent advances in the search for new anti‐coccidial drugs. Int J Parasitol 32: 497–508. [DOI] [PubMed] [Google Scholar]
- Coppin, A. , Dzierszinski, F. , Legrand, S. , Mortuaire, M. , Ferguson, D. , and Tomavo, S. (2003) Developmentally regulated biosynthesis of carbohydrate and storage polysaccharide during differentiation and tissue cyst formation in Toxoplasma gondii . Biochimie 85: 353–361. [DOI] [PubMed] [Google Scholar]
- Coutermarsh‐Ott, S. (2019) Toxoplasma gondii as a model of in vivo host‐parasite interactions. Methods Mol Biol 1960: 237–247. [DOI] [PubMed] [Google Scholar]
- Di Cristina, M. , Dou, Z. , Lunghi, M. , Kannan, G. , Huynh, M.H. , McGovern, O.L. , et al (2017) Toxoplasma depends on lysosomal consumption of autophagosomes for persistent infection. Nat Microbiol 2: 17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, J.P. (2009) History of the discovery of the life cycle of Toxoplasma gondii . Int J Parasitol 39: 877–882. [DOI] [PubMed] [Google Scholar]
- Dubey, J.P. , and Jones, J.L. (2008) Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 38: 1257–1278. [DOI] [PubMed] [Google Scholar]
- Dunay, I.R. , Gajurel, K. , Dhakal, R. , Liesenfeld, O. , and Montoya, J.G. (2018) Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev 31: e00057‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikha, H.M. (2008) Congenital toxoplasmosis: priorities for further health promotion action. Public Health 122: 335–353. [DOI] [PubMed] [Google Scholar]
- Esch, K.J. , and Petersen, C.A. (2013) Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev 26: 58–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, B.A. , and Bzik, D.J. (2010) Avirulent uracil auxotrophs based on disruption of orotidine‐5ʹ‐monophosphate decarboxylase elicit protective immunity to Toxoplasma gondii. Infect Immun 78: 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli, R.T. , Hieny, S. , Wynn, T.A. , Wolf, S. , and Sher, A. (1993) Interleukin 12 is required for the T‐lymphocyte‐independent induction of interferon gamma by an intracellular parasite and induces resistance in T‐cell‐deficient hosts. Proc Natl Acad Sci USA 90: 6115–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerardel, Y. , Leleu, D. , Coppin, A. , Lienard, L. , Slomianny, C. , Strecker, G. , et al (2005) Amylopectin biogenesis and characterization in the protozoan parasite Toxoplasma gondii, the intracellular development of which is restricted in the HepG2 cell line. Microbes Infect 7: 41–48. [DOI] [PubMed] [Google Scholar]
- Guimaraes, E.V. , de Carvalho, L. , and Barbosa, H.S. (2003) An alternative technique to reveal polysaccharides in Toxoplasma gondii tissue cysts. Mem Inst Oswaldo Cruz 98: 915–917. [DOI] [PubMed] [Google Scholar]
- Hill, D. , and Dubey, J.P. (2002) Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect 8: 634–640. [DOI] [PubMed] [Google Scholar]
- Howe, D.K. , Honore, S. , Derouin, F. , and Sibley, L.D. (1997) Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol 35: 1411–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, D.K. , and Sibley, L.D. (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172: 1561–1566. [DOI] [PubMed] [Google Scholar]
- Huang, S.Y. , Chen, K. , Wang, J.L. , Yang, B. , and Zhu, X.Q. (2019) Evaluation of protective immunity induced by recombinant calcium‐dependent protein kinase 1 (TgCDPK1) protein against acute toxoplasmosis in mice. Microb Pathog 133: 103560. [DOI] [PubMed] [Google Scholar]
- Huskinson‐Mark, J. , Araujo, F.G. , and Remington, J.S. (1991) Evaluation of the effect of drugs on the cyst form of Toxoplasma gondii . J Infect Dis 164: 170–171. [DOI] [PubMed] [Google Scholar]
- Ismael, A.B. , Dimier‐Poisson, I. , Lebrun, M. , Dubremetz, J.F. , Bout, D. , and Mevelec, M.N. (2006) Mic1‐3 knockout of Toxoplasma gondii is a successful vaccine against chronic and congenital toxoplasmosis in mice. J Infect Dis 194: 1176–1183. [DOI] [PubMed] [Google Scholar]
- Kato, K. (2018) How does Toxoplama gondii invade host cells? J Vet Med Sci 80: 1702–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagal, V. , Dinis, M. , Cannella, D. , Bargieri, D. , Gonzalez, V. , Andenmatten, N. , et al (2015) AMA1‐deficient Toxoplasma gondii parasites transiently colonize mice and trigger an innate immune response that leads to long‐lasting protective immunity. Infect Immun 83: 2475–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud, M.E. , Fereig, R. , and Nishikawa, Y. (2017) Involvement of host defense mechanisms against Toxoplasma gondii infection in anhedonic and despair‐like behaviors in mice. Infect Immun 85: e00007‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema, F. , Hazenberg, M.D. , Tesselaar, K. , van Baarle, D. , de Boer, R.J. , and Borghans, J.A. (2013) Immune activation and collateral damage in AIDS pathogenesis. Front Immunol 4: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo, J.R. , McLeod, R. , Mack, D. , Smith, J. , Khan, I.A. , Ely, K.H. , and Kasper, L.H. (1993) Antibodies to Toxoplasma gondii major surface protein (SAG‐1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol 150: 3951–3964. [PubMed] [Google Scholar]
- Montazeri, M. , Daryani, A. , Ebrahimzadeh, M. , Ahmadpour, E. , Sharif, M. , and Sarvi, S. (2015) Effect of propranolol alone and in combination with pyrimethamine on acute murine toxoplasmosis. Jundishapur J Microbiol 8: e22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri, M. , Mehrzadi, S. , Sharif, M. , Sarvi, S. , Shahdin, S. , and Daryani, A. (2018a) Activities of anti‐Toxoplasma drugs and compounds against tissue cysts in the last three decades (1987 to 2017), a systematic review. Parasitol Res 117: 3045–3057. [DOI] [PubMed] [Google Scholar]
- Montazeri, M. , Mehrzadi, S. , Sharif, M. , Sarvi, S. , Tanzifi, A. , Aghayan, S.A. , and Daryani, A. (2018b) Drug resistance in Toxoplasma gondii . Front Microbiol 9: 2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, M. , Lyu, C. , Zhao, J. , and Shen, B. (2017) Sixty Years (1957–2017) of research on toxoplasmosis in China—an overview. Front Microbiol 8: 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman, K.J. , and Knoll, L.J. (2015) Long‐term relationships: the complicated interplay between the host and the developmental stages of Toxoplasma gondii during acute and chronic infections. Microbiol Mol Biol Rev 79: 387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik, P.K. , Alam, M.N. , Chowdhury, D.R. , and Chakraborti, T. (2019) Drug resistance in protozoan parasites: an incessant wrestle for survival. J Glob Antimicrob Resist 18: 1–11. [DOI] [PubMed] [Google Scholar]
- Prandovszky, E. , Li, Y. , Sabunciyan, S. , Steinfeldt, C.B. , Avalos, L.N. , Gressitt, K.L. , et al (2018) Toxoplasma gondii‐induced long‐term changes in the upper intestinal microflora during the chronic stage of infection. Scientifica 2018: 2308619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse, S. , Chrishan Shivanthan, M. , Samaranayake, N. , Rodrigo, C. , and Deepika Fernando, S. (2013) Antibiotics for human toxoplasmosis: a systematic review of randomized trials. Pathog Glob Health 107: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayles, P.C. , Gibson, G.W. , and Johnson, L.L. (2000) B cells are essential for vaccination‐induced resistance to virulent Toxoplasma gondii . Infect Immun 68: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, B. , and Sibley, L.D. (2014) Toxoplasma aldolase is required for metabolism but dispensable for host‐cell invasion. Proc Natl Acad Sci USA 111: 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwab, E.K. , Saraf, P. , Zhu, X.Q. , Zhou, D.H. , McFerrin, B.M. , Ajzenberg, D. , et al (2018) Human impact on the diversity and virulence of the ubiquitous zoonotic parasite Toxoplasma gondii. Proc Natl Acad Sci USA 115: E6956–E6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi, T. , Tu, V. , Ma, Y. , Tomita, T. , and Weiss, L.M. (2017) Toxoplasma gondii requires glycogen phosphorylase for balancing amylopectin storage and for efficient production of brain cysts. MBio 8: e01289‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterland, A.L. , Fond, G. , Kuin, A. , Koeter, M.W. , Lutter, R. , van Gool, T. , et al (2015) Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta‐analysis. Acta Psychiatr Scand 132: 161–179. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , and Remington, J.S. (1988) Dual regulation of resistance against Toxoplasma gondii infection by Lyt‐2+ and Lyt‐1+, L3T4+ T cells in mice. J Immunol 140: 3943–3946. [PubMed] [Google Scholar]
- Tomavo, S. (2015) Too much sugar puts a parasite in jeopardy. Cell Host Microbe 18: 641–643. [DOI] [PubMed] [Google Scholar]
- Uboldi, A.D. , McCoy, J.M. , Blume, M. , Gerlic, M. , Ferguson, D.J. , Dagley, L.F. , et al (2015) Regulation of starch stores by a Ca(2+)‐dependent protein kinase is essential for viable cyst development in Toxoplasma gondii . Cell Host Microbe 18: 670–681. [DOI] [PubMed] [Google Scholar]
- Wang, J.L. , Elsheikha, H.M. , Zhu, W.N. , Chen, K. , Li, T.T. , Yue, D.M. , et al (2017a) Immunization with Toxoplasma gondii GRA17 deletion mutant induces partial protection and survival in challenged mice. Front Immunol 8: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.L. , Huang, S.Y. , Behnke, M.S. , Chen, K. , Shen, B. , and Zhu, X.Q. (2016) The past, present, and future of genetic manipulation in Toxoplasma gondii . Trends Parasitol 32: 542–553. [DOI] [PubMed] [Google Scholar]
- Wang, J.L. , Li, T.T. , Elsheikha, H.M. , Chen, K. , Cong, W. , Yang, W.B. , et al (2018) Live Attenuated Pru:Δcdpk2 strain of Toxoplasma gondii protects against acute, chronic, and congenital toxoplasmosis. J Infect Dis 218: 768–777. [DOI] [PubMed] [Google Scholar]
- Wang, Z.D. , Liu, H.H. , Ma, Z.X. , Ma, H.Y. , Li, Z.Y. , Yang, Z.B. , et al (2017b) Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta‐analysis. Front Microbiol 8: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.L. , Zhang, N.Z. , Li, T.T. , He, J.J. , Elsheikha, H.M. , and Zhu, X.Q. (2019) Advances in the development of anti‐Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol 35: 239–253. [DOI] [PubMed] [Google Scholar]
- Williams, T.D. (1979) Presumed ocular toxoplasmosis: some clinical observations. Am J Optom Physiol Opt 56: 633–641. [PubMed] [Google Scholar]
- Xia, N. , Yang, J. , Ye, S. , Zhang, L. , Zhou, Y. , Zhao, J. , et al (2018a) Functional analysis of Toxoplasma lactate dehydrogenases suggests critical roles of lactate fermentation for parasite growth in vivo. Cell Microbiol 20: e12794. [DOI] [PubMed] [Google Scholar]
- Xia, N. , Zhou, T. , Liang, X. , Ye, S. , Zhao, P. , Yang, J. , et al (2018b) A lactate fermentation mutant of toxoplasma stimulates protective immunity against acute and chronic toxoplasmosis. Front Immunol 9: 1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Wang, L. , Xu, D. , Tang, D. , Li, S. , Du, F. , et al (2018) Risk assessment of etanercept in mice chronically infected with Toxoplasma gondii . Front Microbiol 9: 2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky, F. (2014) Innate immunity to Toxoplasma gondii infection. Nat Rev. Immunol 14: 109–121. [DOI] [PubMed] [Google Scholar]
- Zhang, N.Z. , Gao, Q. , Wang, M. , Elsheikha, H.M. , Wang, B. , Wang, J.L. , et al (2018) Immunization with a DNA vaccine cocktail encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 genes protects mice against chronic toxoplasmosis. Front Immunol 9: 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N.Z. , Wang, M. , Xu, Y. , Petersen, E. , and Zhu, X.Q. (2015) Recent advances in developing vaccines against Toxoplasma gondii: an update. Expert Rev Vaccines 14: 1609–1621. [DOI] [PubMed] [Google Scholar]
- Zheng, B. , Lou, D. , Ding, J. , Zhuo, X. , Ding, H. , Kong, Q. , and Lu, S. (2019) GRA24‐based DNA vaccine prolongs survival in mice challenged with a virulent Toxoplasma gondii strain. Front Immunol 10: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in the study.


