Sulfane sulfur activates actinorhodin production and sporulation in Streptomyces coelicolor. A gene cluster controlling intracellular sulfane sulfur levels is identified. Genetically modifying this gene cluster can change sulfane sulfur levels, thereby altering the actinorhodin production and sporulation.
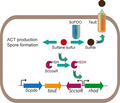
Summary
Sulfane sulfur, including polysulfide and persulfide, is a newly identified cellular component present in microorganisms; however, its physiological functions are unclear. Streptomyces coelicolor M145 is a model strain of actinomycetes, which produces several polyketides, including actinorhodin. Herein, we found that both exogenously added and endogenously generated sulfane sulfur increased the actinorhodin production and accelerated spore formation of S. coelicolor M145. This bacterial species carries a natural gene circuit containing four genes that encode a CsoR‐like transcription factor (ScCsoR), persulfide dioxygenase (ScPDO), rhodanese and a sulfite transporter, which were shown to be responsible for sensing and removal of excessive sulfane sulfur. ScCsoR was observed to bind to the promoters of the four genes, thus repressing their transcription. Sulfane sulfur modified Cys37 of ScCsoR, and the modified ScCSoR did not bind to the promoters, thereby activating the transcription of ScPDO. The deletion of ScCsoR decreased cellular sulfane sulfur, while the deletion of ScPDO increased its levels. The increased sulfane sulfur promoted actinorhodin production and sporulation. This study unveiled a natural gene circuit for maintaining sulfane sulfur homeostasis in bacteria. Further, we identified the trigger effect of sulfane sulfur on actinorhodin production, presenting a new approach for activating polyketide gene clusters in actinomycetes.
Introduction
Sulfane sulfur, presenting as persulfide (HSSH and RSSH) and polysulfide (HSSnH, Sn, RSSnH, RSSnR, n ≥ 2), is a common component found in mammalian cells, (Fukuto et al., 2018). It plays important physiological roles in cytoprotection, anti‐inflammatory activities and angiogenesis (Lau and Pluth, 2019) and is produced by various enzymes. Sulfide: quinone oxidoreductase (SQR) oxidizes H2S to hydrogen polysulfide (HSnH). Cystathionine beta‐lyase (CBS), cystathionine gamma‐lyase (CSE), 3‐mercaptopyruvate sulfurtransferase (3‐MST) and cysteinyl‐tRNA synthetase 2 (CARS2) produce sulfane sulfur from cysteine and its derivatives. Sulfane sulfur can be oxidized by persulfide dioxygenase (PDO; Hildebrandt and Grieshaber, 2008; Liu et al., 2014; Shen et al., 2015) or be reduced to sulfide by cellular thiols, thioredoxin and glutaredoxin (Hou et al., 2019).
Sulfane sulfur and its metabolizing enzymes have been studied in microorganisms as well, demonstrating that it is a key intermediate of H2S oxidation pathway (Luebke et al., 2014; Li et al., 2017b; Shimizu et al., 2017). Sulfane sulfur also modifies some gene regulators, a process now termed as protein S‐sulfhydration or persulfidation, to activate the expression of SQR and/or other H2S metabolizing enzymes. Four types of such gene regulators (CstR, BigR/SqrR, FisR and OxyR) have been identified (Giedroc, 2017; de Lira et al., 2018; Hou et al., 2019; Shimizu and Masuda, 2020). A recent research indicates that sulfane sulfur is involved in the regulation of pathogenicity in Staphylococcus aureus (Peng et al., 2017), suggesting that it has other physiological functions in bacteria.
Actinomycetes are remarkable producers of polyketide drugs, including erythromycin A, rifamycin, chromomycin and tetracyclines (Robertsen and Musiol‐Kroll, 2019). Advancements in microbial genomics indicate that earlier discoveries cite only a small fraction of polyketides in actinobacteria, representing approximately 10% of their biosynthetic capacity (Nett et al., 2009; Shen, 2015; Blin et al., 2019). Additionally, programmes related to isolation of natural products have significantly decreased since the end of the 21st century (Li and Vederas, 2009; Wright, 2017). One of the reasons responsible for the dearth of research on this subject is the fact that in actinobacteria, many biosynthetic gene clusters are regulated via unknown mechanisms. Their activation is on an ‘as‐needed’ basis; however, identifying the need is difficult (Bibb, 2013; Barka et al., 2016).
Streptomyces coelicolor M145 is a model strain for studying actinomycetes. It produces several polyketide compounds (Bentley et al., 2002; Hopwood, 2007), including blue‐pigmented actinorhodin (ACT; Bystrykh et al., 1996), red‐pigmented undecylprodigiosin and yellow‐pigmented coelimycin P2 (Liu et al., 2013; Chen et al., 2016). Among them, ACT has served as an outstanding example for genetic and biochemical investigations of polyketide metabolism, including the seminal work on polyketide biosynthetic gene clusters and the generation of hybrid polyketides (Strohl and Connors, 1992; Khosla et al., 1993). Although transcription regulation of the ACT biosynthetic gene cluster has been intensively studied, the intracellular signal that activates ACT production is still unclear (Liu et al., 2013; Mak and Nodwell, 2017).
In this study, we found that exogenous sulfane sulfur could function as a signal to activate ACT production in S. coelicolor M145. Endogenous sulfane sulfur was detected in this bacterium, and a natural gene circuit controlling its level was identified. Genetically editing the gene circuit changed the intracellular sulfane sulfur levels, ACT production and morphological development of S. coelicolor M145. The gene circuit contains elements that can sense and control intracellular sulfane sulfur level, to maintain it in a homeostatic state like a thermostat. This study unveiled new physiological roles of sulfane sulfur that may provide a new strategy for activating cryptic polyketide gene clusters in actinomycetes.
Results
Treating S. coelicolor with sulfane sulfur promoted its ACT production and spore formation
We used both sulfide (in the form of sodium hydrosulfide [NaHS]) and sulfane sulfur (in the form of S8) to treat S. coelicolor M145. These chemicals were mixed with the yeast‐beef‐peptone (YBP) agar and poured on a culture plate. Next, S. coelicolor M145 spores were spread on the surface of the plate, and the plate was incubated at 30 °C for 6 days. Addition of sulfide marginally increased ACT production, while addition of S8 significantly increased the production in the bacterial cells compared with that of untreated control cells (Fig. 1A and B). The highest increase was observed with the addition of 50 μM S8, about fourfold higher than the control. The addition of S8 also influenced morphological development of S. coelicolor M145, as evidenced by more spores being produced on the S8‐containing plate (Fig. 1C). These results demonstrated that sulfane sulfur can act as a stimulus to affect ACT production and spore formation of S. coelicolor M145.
Fig. 1.
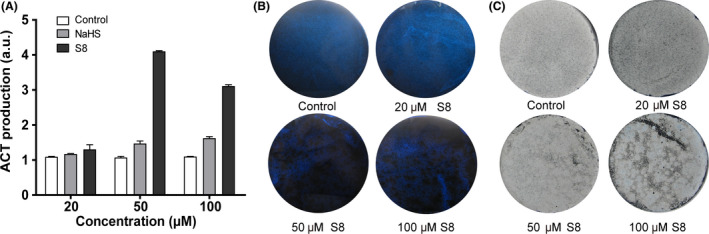
Using NaHS and S8 to treat S. coelicolor M145.
Different concentrations of NaHS or S8 (20, 50 and 100 µM) were added to agar plates before inoculation of S. coelicolor M145 spores. Control was the plate without addition of the chemicals. After inoculation, the plates were incubated at 30 °C for 6 days and then subjected to analysis.
A. Total ACT production of S. coelicolor M145. Data were from three repeats.
B. S8 increased ACT production of S. coelicolor M145. Images were captured from the reverse side of the plates.
C. S8 affected spore formation of S. coelicolor M145. Images were captured from the front side of the plates.
Endogenously produced sulfane sulfur also promoted ACT production and spore formation
Persulfide dioxygenase oxidizes glutathione persulfide (GSSH) and can lower cellular sulfane sulfur (Xia et al., 2017). The S. coelicolor M145 genome contains a gene coding for a hypothetical protein, SCO0618, containing an N‐terminal PDO‐like domain and a C‐terminal rhodanese domain (Fig. 2A). This indicates that SCO0618 might be a type III PDO. We compared it with three representative type III PDOs, Zunongwangia profunda SM‐A87 (ZpPDOIII, ADF52140.1), Staphylococcus aureus (SaPDOIII, WP_000465474.1) and Bacillus cereus ATCC 10876 (BcPdoIII, EEK49737.1) by using ClustalW (Fig. S1). To test whether this protein catalyses sulfane sulfur oxidation, we cloned its ORF into a pET15b vector and transformed the recombinant plasmid into E. coli BL21(DE3) for expression. The enzyme thus obtained was purified with an N‐terminal His‐tag using affinity chromatography and was confirmed via SDS‐PAGE (Fig. S2). Subsequent enzymatic assays indicated that it oxidized sulfane sulfur. When glutathione persulfide (GSSH) was used as a substrate, its V max reached 1.19 ± 0.2 μmol O2 min−1 mg−1 and K m value was 94.25 ± 54.61 μmol (Fig. 2B and C). Hereafter, we refer to this enzyme as ScPDO.
Fig. 2.
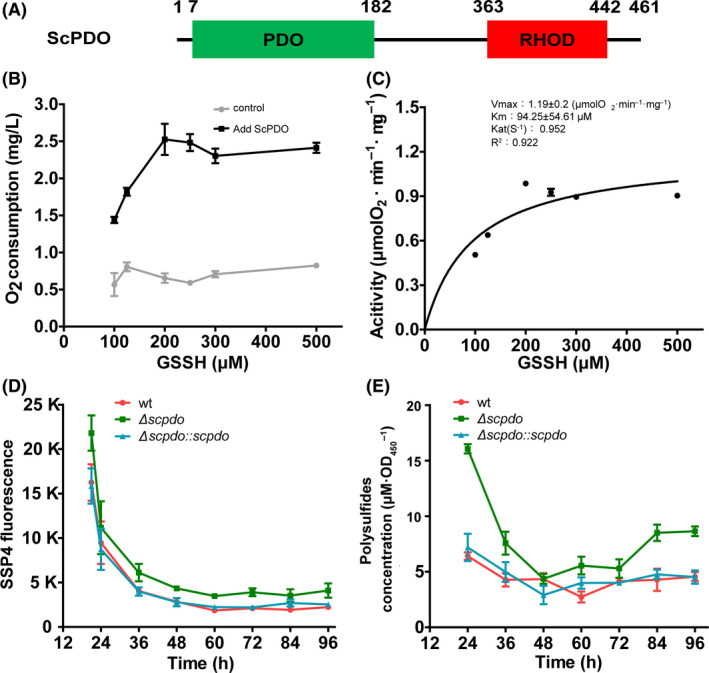
ScPDO had PDO activity, and its deletion increased intracellular sulfane sulfur.
A. ScPDO had two hypothetical domains, PDO and RHOD.
B and C. ScPDO displayed persulfide dioxygenase activity by oxidizing GSSH into sulfite. (B) Persulfide dioxygenase activity was confirmed by detecting O2 consumption. ScPDO (1 µM)_and 0–500 µM GSSH were mixed in 3 ml KPi buffer (100 mM, pH 7.4) at 25 °C, and O2 consumption was examined using an Orion RDO meter. Black line, reaction mixture with ScPDO; grey line, reaction mixture without ScPDO. (C) Catalysing kinetics of ScPDO was calculated using the Michaelis–Menten equation as reported previously (Liu et al., 2014).
D and E. Detection of intracellular sulfane sulfur of wt, ΔScpdo and ΔScpdo::Scpdo strains with SSP4 and HPLC. High SSP4 fluorescence indicated high sulfane sulfur concentration in the cells. For B–D, data are expressed from three independent repeats and shown as mean ± SD.
We deleted the Scpdo gene in S. coelicolor M145. S. coelicolor M145 wild type (wt) and the mutant ΔScpdo did not display a significant difference in growth on agar plates. We subsequently detected its intracellular sulfane sulfur levels using a fluorescence‐based chemical probe SSP4 using a procedure described by Chen and colleagues (2013). The mutant ΔScpdo accumulated a higher amount of sulfane sulfur, especially at the early exponential phase, than did wt (Fig. 2D). To confirm these results, we used an HPLC‐based method to quantify the intracellular sulfane sulfur in both ΔScpdo and wt (Ran et al., 2019). The mutant accumulated 16.06 ± 0.41 μM·OD450 −1 intracellular sulfane sulfur at 24 h, while wt accumulated 6.41 ± 0.34 μM·OD450 −1 (Fig. 2E). We also constructed a complemented strain (ΔScpdo:: Scpdo) by introducing an Scpdo‐expression plasmid into ΔScpdo, in which the Scpdo expression was controlled by a constitutive promoter, kasOp* (Bai et al., 2015). This strain produced similar amounts of intracellular sulfane sulfur as wt. These results indicated that ScPDO was indeed responsible for sulfane sulfur oxidation, preventing the accumulation of sulfane sulfur inside the cells. Notably, in all three strains, sulfane sulfur accumulation showed a similar trend; the level of sulfane sulfur was the highest at the exponential phase, then started to decrease and finally stabilized at the stationary phase (Fig. 2D and E).
We observed that ΔScpdo produced more ACT than wt, especially from 60 to 96 h (Fig. 3A and B). Quantitative analysis indicated that the ACT production by ΔScpdo was about two‐ to sevenfold higher than that of wt depending on the cultivation time. In addition, ΔScpdo generated spores significantly earlier than wt and ΔScpdo:: Scpdo (Fig. 3C). However, the ΔScpdo:: Scpdo strain exhibited a similar trend of ACT production as wt.
Fig. 3.
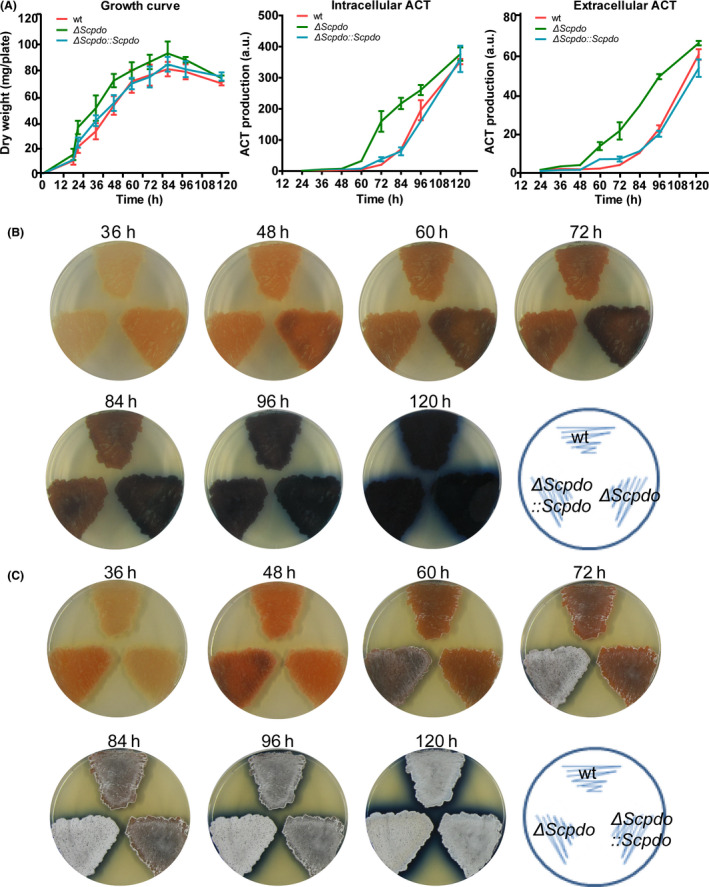
Deletion of ScPDO increased ACT production and spore formation of S. coelicolor M145.
A. Growth and ACT production of the strains in agar plates. Both intracellular and extracellular ACT production were quantified. Dry weight of mycelia (mg plate−1) was quantified and used to represent the growth curve of S. coelicolor M145. The error bars are the standard deviation of the data obtained from three replicates. For comparison, equal amounts of spores of wt, ΔScpdo and ΔScpdo:: Scpdo strains were inoculated on the YBP agar medium and cultured at 30 °C for 5 days. Intracellular ACT was quantified using only the mycelia, and extracellular ACT was quantified using only the medium, as reported previously (Kieser et al., 2000). Data are expressed from three independent repeats.
B and C. ΔScpdo strain showed higher ACT production and spore formation than wt and ΔScpdo:: Scpdo strains; images were captured from reverse and front sides of the plates respectively.
Identification of a transcription factor governing intracellular sulfane sulfur levels
Genome background analysis indicated that there is a hypothetical transcription factor gene (sco0620) adjacent to Scpdo, which encodes a CsoR‐like_DUF156 protein. Hereafter, we refer to this protein and its encoding gene as ScCsoR and SccsoR respectively. To test whether ScCsoR is involved in the regulation of intracellular sulfane sulfur levels, we constructed a SccsoR deletion strain ΔSccsoR and a complemented strain ΔSccsoR:: SccsoR. SSP4 test showed that ΔSccsoR accumulated slightly less sulfane sulfur than wt, whereas ΔSccsoR:: SccsoR produced slightly more sulfane sulfur than wt. The production differences were more apparent with the HPLC test (Fig. 4A). Moreover, ΔSccsoR produced less ACT than wt and its sporulation was also significantly delayed (Fig. 4B).
Fig. 4.
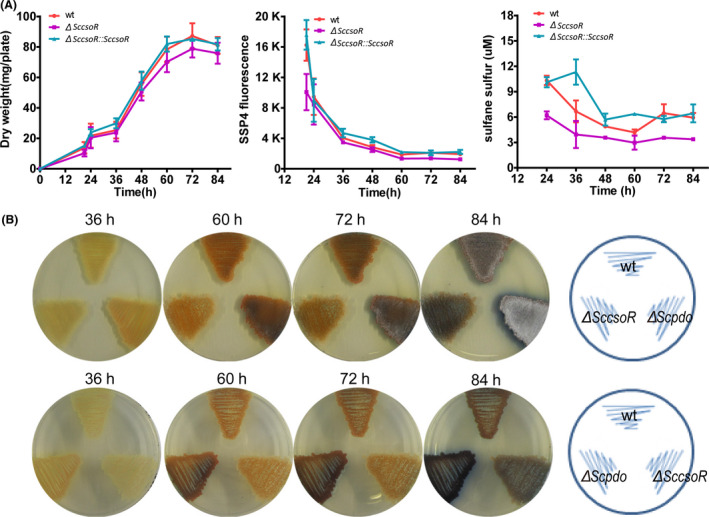
Deletion of ScCsoR decreased intracellular sulfane sulfur and decreased both ACT production and spore formation.
A. Growth and sulfane sulfur production of S. coelicolor M145. The dry weight of mycelia (mg/plate) was quantified and used to represent the growth curve of S. coelicolor M145. For comparison, equal amount spores of wt, ΔSccsoR and ΔSccsoR:: SccsoR strains were inoculated on YBP agar medium and cultured at 30 °C for 84 h. Intracellular sulfane sulfur of S. coelicolor M145 strains was detected using both SSP4 and HPLC. Low SSP4 fluorescence indicated low sulfane sulfur concentration in the cells. Data were from three independent repeats and shown as average ± SD.
B. The ΔSccsoR strain showed lower ACT production and spore formation than wt and ΔScpdo strains. Photographs were taken from reverse and front sides of the plates.
To test whether ScCsoR functions via regulating the expression of Scpdo, we analysed the Scpdo transcription levels in both ΔSccsoR and wt using RT‐qPCR. The level of Scpdo mRNA in ΔSccsoR was much higher than that in wt, with ΔSccsoR exhibiting 100‐ to 480‐fold higher levels than wt depending on the growth time (Fig. 5). These results suggested that ScCsoR negatively controlled the expression of Scpdo.
Fig. 5.
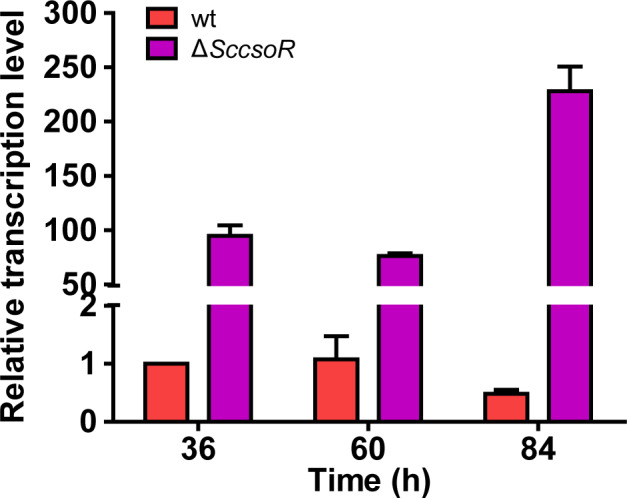
The Scpdo gene showed higher transcription level in ΔSccsoR strain than in wt strain. RT‐qPCR analysis was performed to compare the transcriptional level of Scpdo in wt and ΔSccsoR strains. RNA was harvested at 36, 60 and 84 h in YBP agar medium. hrdB transcription was used as the internal control for normalization. Three independent measurements were carried out; error bars indicate standard deviation.
ScCsoR is a transcription repressor that binds to the upstream sequence of Scpdo promoter
To investigate the mechanism by which ScCsoR controls the expression of Scpdo, we expressed SccsoR gene in E. coli BL21(DE3) and obtained the purified ScCsoR protein. A 5'‐rapid amplification of cDNA ends (RACE) experiment indicated that Scpdo transcription started at −12 bp relative to the start codon GTG. Subsequently, a 256‐bp DNA fragment containing the promoter and partial ORF sequence of Scpdo was synthesized (Fig. 6A). Purified ScCsoR showed significant affinity to this DNA fragment, as judged by an EMSA experiment (Fig. 6B). To determine the exact binding site of ScCsoR, we synthesized four short DNA fragments (f1–f4), with each fragment containing a segment of the 256‐bp DNA (Fig. 6A). EMSA experiments indicated that ScCsoR preferred to bind f2 and f3 fragments (Fig. 6C). We used the Multiple EM for Motif Elicitation (MEME; http://meme‐suite.org/) toolbox to analyse the f2 and f3 fragments and identified a 16‐bp segment containing a pair of palindromic sequences (ATACCn6GGTAT). The 16‐bp segment showed a high similarity to the binding sites of Geobacillus thermodenitrificans CsoR (Coyne and Giedroc, 2013; Chang et al., 2014) and Mycobacterium tuberculosis RicR (Festa et al., 2011; Shi et al., 2014; CsoR‐like Transcription factor; Fig. 6D), suggesting that it is the binding site of ScCsoR. There are two ScCsoR binding sites in the Scpdo promoter. We designated them as bsΙ and bsΠ, located at ‐ 48 and ‐ 9 sites, respectively, from the transcription start site. For further confirmation, we examined the binding affinity of ScCsoR to a 79‐bp DNA fragment containing bsI, bsΠ, as well as to an intergenic sequence located between them. A competitor DNA that did not contain these sequences was used as the control. ScCsoR bound to the 79‐bp DNA but not to the competitor DNA (Fig. 7A); in addition, when the 79‐bp DNA fragment was mixed with 50‐fold (mole ratio) of the competitor DNA, ScCsoR still bound specifically to this fragment.
Fig. 6.
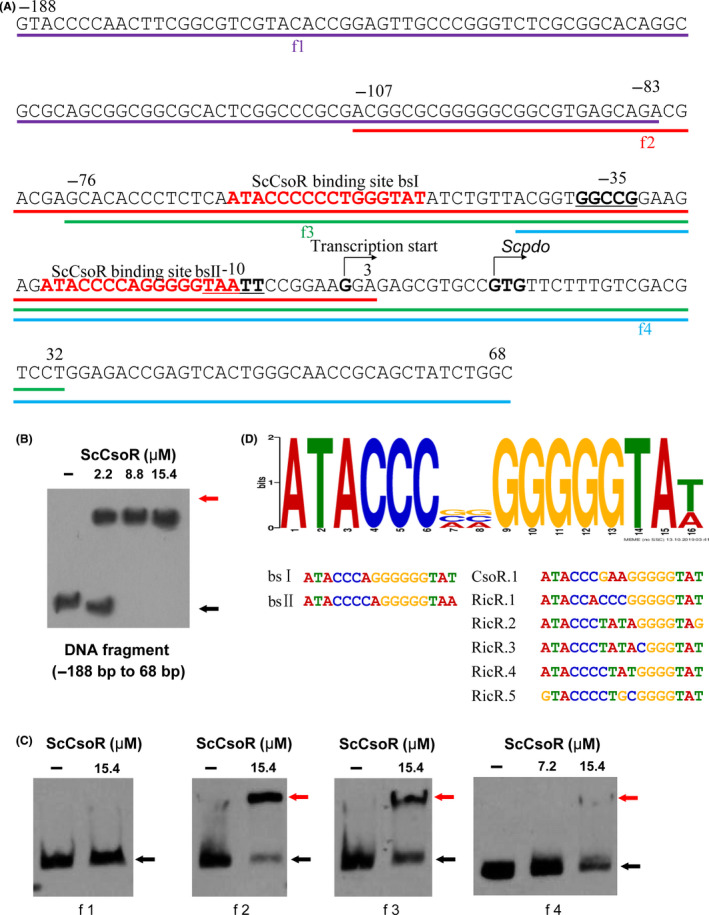
Characterization of the Scpdo promoter.
A. DNA sequence of Scpdo promoter. The transcription start site of Scpdo was identified using 5’‐RACE. GTG is the start codon. The DNA fragments used for EMSA analysis were denoted as f1 to f4.
B. EMSA analysis of the binding affinity of ScCsoR (in reduced form) to Scpdo promoter. DNA probe (1 nM) was incubated with different amounts of ScCsoR (0, 2.2, 8.8, 15.4 µM). Black arrow indicates the free DNA probe, and red arrow indicates ScCsoR‐DNA complex.
C. EMSA analysis of the ScCsoR (in reduced form) binding affinity to different parts of Scpdo promoter. The DNA probe used in (B) was divided into four DNA fragments (f1 to f4), and other conditions were the same as in (B). Only f2 and f3 fragments exhibited obvious band shifts after incubation with ScCsoR, suggesting that ScCsoR was bound to the promoter region of Scpdo gene.
D. Multiple EM for Motif Elicitation analysis of Scpdo promoter. The binding sequences of CsoR from Geobacillus thermodenitrificans and RicR from Mycobacterium tuberculosis, and sequences of f2, f3 fragments were used as inputs for MEME analysis. A 16‐nt consensus sequence with reverse palindrome was identified (ATACCn6GGTAT).
Fig. 7.
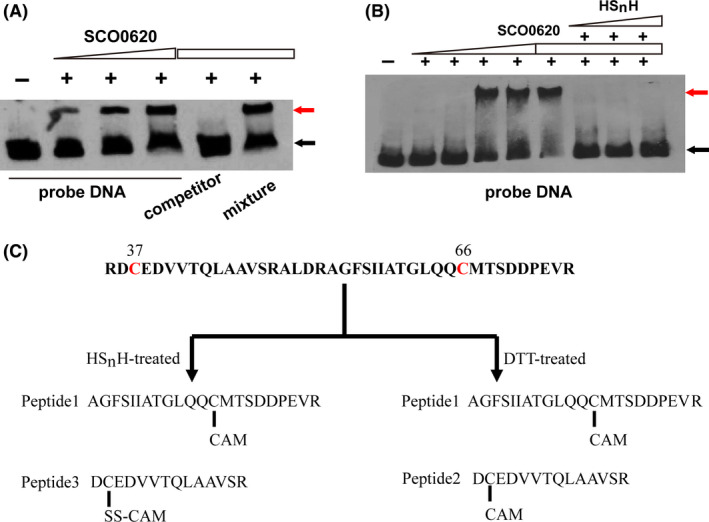
Hydrogen polysulfide (HSnH) affected the binding affinity of ScCsoR to Scpdo promoter.
A. ScCsoR exhibited binding to the probe DNA containing bsΙ and bsΠ, even in the presence of competitor DNA. DNA probe (1 nM) was incubated with different amounts of ScCsoR (0, 2.2, 8.8, 15.4 µM). Black arrow indicates the free DNA probe, and red arrow indicates the shifted DNA probe. 50‐fold excess of unlabelled competitor DNA and its mixture with probe DNA were also tested.
B. HSnH detached ScCsoR from the probe DNA. DNA probe (1 nM) was incubated with different concentrations of ScCsoR (0, 0.5, 1.0, 2.0, 4.0, 8.0, 8.0, 8.0, 8.0 µM). Different amounts of HSnH (0, 1, 2, 3 mM) were added to the reaction system.
C. LC‐MS/MS analysis of HSnH‐ and DTT‐treated ScCsoR. Details of the MS data are shown in Figs S3–S5.
ScCsoR detaches from Scpdo promoter in the presence of sulfane sulfur
We performed an EMSA experiment in the presence of hydrogen polysulfide (HSnH). The binding affinity of ScCsoR was significantly decreased by HSnH (Fig. 7B). Thus, these results indicated that sulfane sulfur reacted with ScCsoR, leading to its detachment from the DNA binding site.
Previous studies have demonstrated that transcription factors CstR, BigR/SqrR, FisR and OxyR all use their cysteine residues to react with sulfane sulfur (Giedroc, 2017; Li et al., 2017b; Hou et al., 2019). ScCsoR contains two cysteine residues, Cys37 and Cys66. To explore which one of these is required to sense sulfane sulfur, we treated the purified ScCsoR with HSnH and analysed the treated protein with LC‐MS/MS. As a reference, DTT‐treated ScCsoR was also analysed following the same protocol. Two peptides were found in the DTT‐treated sample: peptide 1 contained Cys66, and peptide 2 contained Cys37 (Fig. 7C; Figs S3 and S4). Their thiol groups were directly blocked by acetamide (CAM), indicating that these two peptides were not modified. Peptide 1 was also found in the HSnH‐treated sample, and a third peptide, peptide 3, which contained Cys37‐SS‐CAM modification, was found in the HSnH‐treated sample but not in the DTT‐treated sample (Fig. S5), indicating that Cys37‐SH could be modified to Cys37‐SSH when ScCsoR was treated with sulfane sulfur.
We then constructed three ScCsoR mutants: ScCsoR‐C37S, ScCsoR‐C66S and ScCsoR‐C37S‐C66S, in which the cysteine residue was mutated to serine. EMSA results indicated that these three mutants could still bind to the probe DNA; however, HSnH could not detach them from the probe DNA (Fig. S6). These results indicated that both Cys37 and Cys66 were involved in the sulfane sulfur sensing process of ScCsoR.
ScCsoR is a regulator of a sulfane sulfur oxidation gene circuit
We found that the 16‐bp binding site is also present in the intergenic region of SccsoR and sco0621 promoters (Fig. S7); the latter encode a hypothetic protein containing a Rhod homology domain (Motl et al., 2017). Hereafter, we refer to this gene as Scrhod. EMSA experiments confirmed that ScCsoR could bind to the intergenic region (Fig. 8A). Moreover, RT‐qPCR experiments indicated that the expression levels of both SccsoR and Scrhod were increased in the sulfane sulfur over‐accumulation strain (ΔScpdo; Fig. 8B and C). In addition, Scrhod expression was also increased because of the deletion of SccsoR (Fig. 8D). These results indicated that both the transcription of SccsoR and Scrhod was regulated by ScCsoR.
Fig. 8.
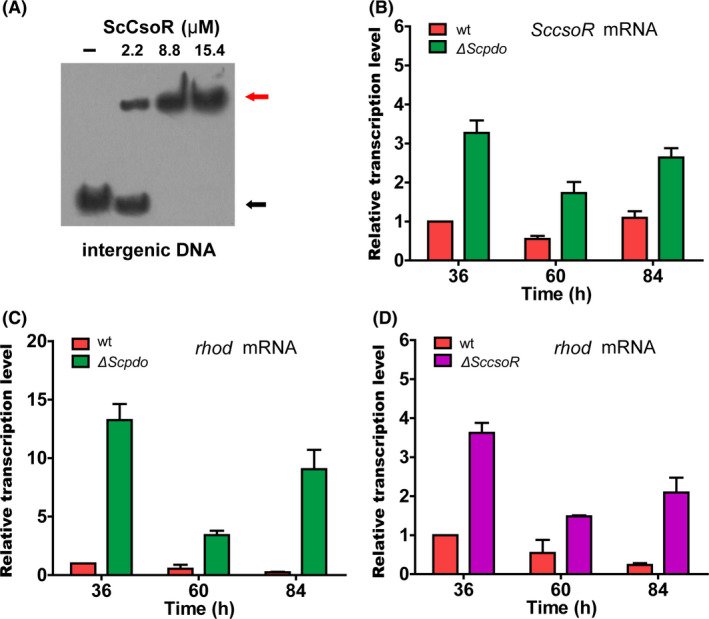
ScCsoR controlled transcription of SccsoR and Scrhod.
A. EMSA analysis of ScCsoR binding affinity to the intergenic DNA located between SccsoR and Scrhod. The intergenic region of SccsoR and Scrhod (261bp) was used as a probe. DNA (1 nM) probe was incubated with different concentrations of ScCsoR (0, 2.2, 8.8, 15.4 µM). Black arrow indicates the free DNA probe, and red arrow indicates the shifted DNA probe.
B. RT‐qPCR analysis of SccsoR mRNA in ΔScpdo strain.
C. RT‐qPCR analysis of Scrhod mRNA in ΔScpdo strain.
D. RT‐qPCR analysis of Scrhod mRNA in ΔSccsoR strain. For B‐D, data are expressed from three independent repeats. For B, C, D, RNA was harvested at 36, 60 and 84 h in YBP agar medium. The hrdB transcription was used as the internal control for normalization. Three independent measurements were carried out; error bars indicate standard deviation
The gene adjacent to Scpdo is sco0619, which encodes a possible membrane protein homologous to the sulfite exporter, TauE (Weinitschke et al., 2007). Hereafter, we refer to this gene as SctauE. RT‐PCR experiments indicated that Scpdo and SctauE were co‐transcribed (Fig. S8A). These analyses suggested that ScCsoR not only controlled the oxidation of cellular sulfane sulfur, but also controlled secretion of the oxidized product, sulfite.
RT‐PCR revealed that Scrhod was not co‐transcribed with sco0622 (Fig. S8A). EMSA experiments demonstrated that ScCsoR could not bind to the promoter of sco0622 (Fig. S8B). RT‐qPCR further demonstrated that sco0622 and sco0623 were co‐transcribed and their expression was not affected by the SccsoR deletion (Fig. S8C), indicating that sco0622 and sco0623 were not controlled by ScCsoR, thereby clarifying the boundary of the ScCsoR controlled gene circuit.
Distribution of sulfane sulfur oxidation gene circuit in other Streptomyces species
To investigate whether a similar gene circuit is present in other species of Streptomyces, we analysed all the sequenced Streptomyces genomes deposited at the website https://patricbrc.org/, which contained 1977 genomes as ascertained on Nov 26, 2019. ScPDO homologous proteins were found in 1248 genomes. Among them, 773 genomes also contained the genes coding for ScCsoR homologous proteins (CsoR‐like TF) close to their pdo genes (within 3‐ORF distance). We further analysed the genomes of 16 strains that were widely studied due to the production of important antibiotics and various polyketide drugs (Table S1), including the neomycin producer S. fradiae, validamycin producer S. hygroscopicus, lincomycin producer S. lincolnensis and natamycin producer S. lydicus. They were all observed to harbour a similar gene circuit containing PDO, TauE, RHOD and CsoR‐like TF as that of ScPDO (Fig. 9). These results suggested that the phenomenon of cellular sulfane sulfur affecting polyketide biosynthesis might be widely present in the Streptomyces species.
Fig. 9.
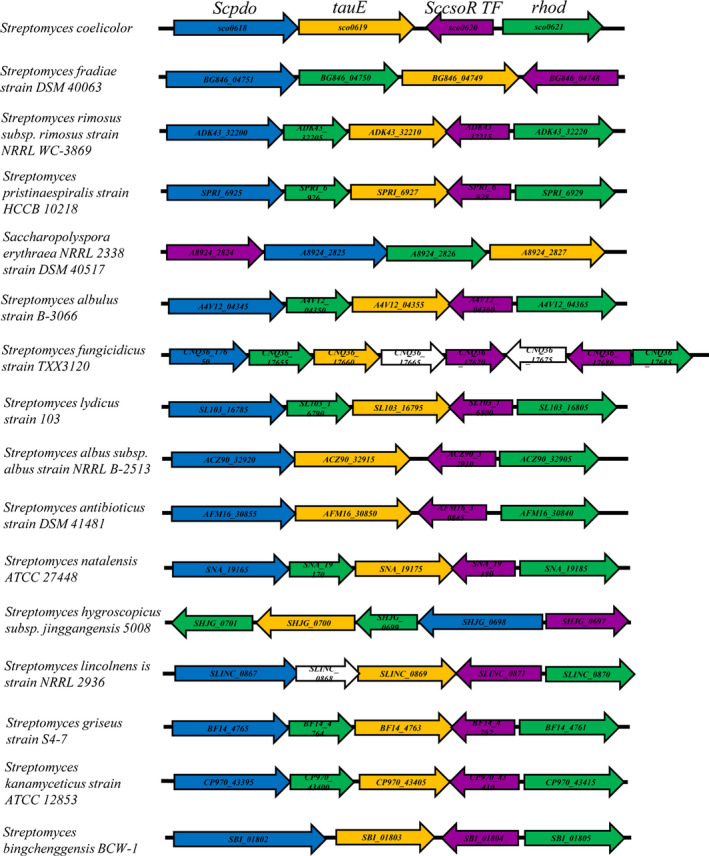
Hypothetical sulfane sulfur oxidation gene circuits found in 16 species of Streptomyces. Details of the strains are provided in Table S1.
Discussion
We identified a gene circuit comprising four genes that specifically oxidizes sulfane sulfur in S. coelicolor M145. On the basis of our results, we have proposed a functioning mechanism for this gene circuit (Fig. 10). ScCsoR is a regulator that represses the expression of all the four genes including it. It exhibits a 46% homology with Staphylococcus aureus CstR is (Fig. S9) and contains two conserved cysteine residues. However, the sensing mechanism of ScCsoR is different from that of CstR. CstR forms intra tri‐ and tetra‐sulfide bonds between its two cysteine residues when reacting with sulfane sulfur (Luebke et al., 2014). However, in the case of ScCsoR, no such bonds are formed and only its Cys37 is persulfidated (Cys37‐SSH), leading to its detachment from the promoters. Interestingly, although Cys66 is not persulfidated by sulfane sulfur, it is also involved in the detachment process, probably via stabilizing Cys37‐SSH, as a role of Cys208 in OxyR (Hou et al., 2019). ScPDO oxidizes sulfane sulfur to sulfite; the latter is exported, probably by TauE. The action of ScPDO and TauE lowers cellular sulfane sulfur. Since the expression of ScCsoR is self‐regulated and is expressed in the presence of high cellular sulfane sulfur, when the sulfane sulfur levels become low, the newly produced ScCsoR do not become persulfidated and quickly bind to the promoters to repress the expression of these genes. This provides a feedback mechanism, representing a new type of regulation, specifically for maintaining the sulfane sulfur homeostasis in bacteria.
Fig. 10.
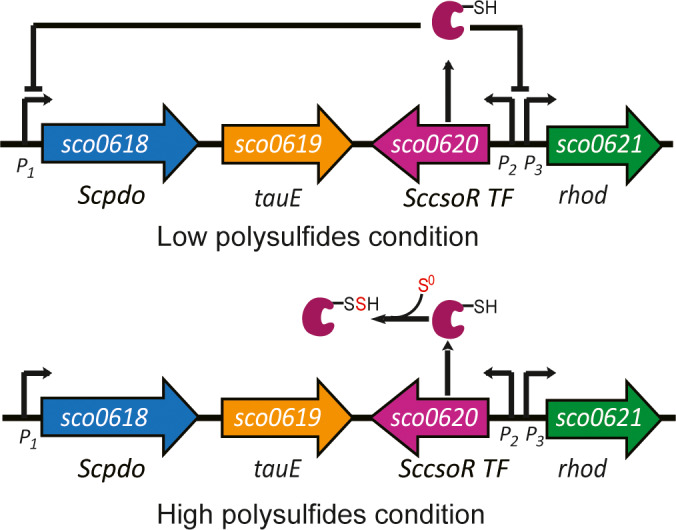
Functioning mechanism of the ScCsoR‐pivoted gene circuit. Reduced ScCsoR (Cys37‐SH) binds to the promoters of Scpdo (P1), SccsoR (P2) and rhod (P3) and inhibits their expression. When the intracellular sulfane sulfur level reaches its threshold, ScCsoR is sulfhydrated (Cys37‐SSH) by sulfane sulfur and dissociates from the promoters, allowing the expression of these genes until the intracellular sulfane sulfur level drops below the threshold.
This gene circuit is different from previously characterized gene circuits responsible for H2S oxidation in bacteria (Luebke et al., 2014; Li et al., 2017b; Shimizu et al., 2017). The H2S‐oxidizing circuits possess both SQR and PDO. SQR oxidizes H2S to sulfane sulfur, which is sensed by the gene regulator to elevate the expression of sqr and pdo. Bioinformatic analyses indicate that both H2S oxidation and sulfane sulfur oxidation gene circuits are widely present in heterotrophic bacteria (Xia et al., 2017; de Lira et al., 2018). Our study indicates that the regulation mechanisms of H2S oxidation and sulfane sulfur oxidation are different. We observed that all the TFs in the H2S‐oxidizing gene circuits are constitutively expressed at low levels and that these circuits are very sensitive even to low levels of H2S (< 10 μM; Xia et al., 2017; Li et al., 2017a), suggesting that bacteria containing H2S‐oxidizing gene circuits cannot tolerate the presence of H2S. However, the expression of TF of the sulfane sulfur oxidation gene circuit is dynamic, which can confer wide amplitude of expression on the genes that it controls – from being completely silenced to be fully turned‐on. This makes the strain tolerant to certain levels of intracellular sulfane sulfur – the gene circuit is turned on by a threshold level.
We found that sulfane sulfur affects ACT production as well as spore formation in S. coelicolor M145. Further investigations are needed to illustrate its exact functioning mechanism. For ACT production, hundreds of genes are involved in this process (Xu et al., 2017; Xu et al., 2019). Among them, ActII‐ORF4 has been identified as a determining regulator and its expression is regulated by at least eight regulatory proteins (Liu et al., 2013). Our preliminary study indicated that sulfane sulfur cannot directly react with ActII‐ORF4, suggesting that sulfane sulfur possibly reacts with a regulatory protein that controls ActII‐ORF4 (data not shown). For the generation of spores, the regulation is more complex than that of ACT production. Spore formation involves more than a dozen regulatory proteins that compose a multiple‐layered regulatory network (Bush et al., 2015). Investigating which of these proteins are affected by sulfane sulfur requires systematic studies, including transcriptomics and/or proteomics approaches. Further, sulfane sulfur levels can also be controlled multiple mechanisms. We observed that sulfane sulfur levels in the ΔScpdo strain varied with the time of cultivation, indicating that the ScCsoR‐pivoted gene cluster is not the only system that acts as an effector. The intracellular redox potential controlled by the ratio of reduced mycothiol/oxidized mycothiol could be another effector (Newton et al., 2008). Thus, the sulfane sulfur metabolism, ACT production and sporulation might be interrelated in S. coelicolor M145.
Our finding is significant with respect to the study of polyketides as well as antibiotics. There are many undeveloped polyketide biosynthetic gene clusters in acetinomycetes of which the regulatory mechanisms are yet unknown. Using genetic engineering methods (most commonly by altering their promoters) to activate these gene clusters usually involves laborious work and are not always effective. Sulfane sulfur may represent a new stimulus that can activate certain polyketide biosynthetic gene clusters. Using exogenous sulfane sulfur to treat acetinomycetes or altering their intracellular sulfane sulfur might help in the discovery of new polyketide drugs from such bacteria.
Experimental procedures
Strains and growth conditions
All the strains and plasmids used in this study are listed in Table S2. S. coelicolor M145 and its derivatives were cultured at 30 °C. Mannitol soya flour (MS) agar medium was used for sporulation and conjugation. YBP (yeast‐beef‐peptone) solid medium was used for determining ACT production, phenotypic observation, growth analysis and RNA extraction. YBP liquid medium was used for quantifying intracellular sulfane sulfur. Antibiotics were added to the media when required. E. coli DH5α was used for plasmid construction, and the E. coli BL21 (DE3) strain was used for protein expression. E. coli ET12567 (pUZ8002) was used to transfer nonmethylated DNA into S. coelicolor M145.
Construction of S. coelicolor ΔScpdo and ΔSccsoR
All the primers used in this study are listed in Table S3.The mutant, ΔScpdo of S. coelicolor, was constructed using the sgRNA‐guided CRISPR‐Cas9 genome editing system (Huang et al., 2015). Upstream (1008 bp) and downstream (1131 bp) regions of Scpdo ORF were amplified from S. coelicolor M145 genomic DNA. A 20‐nt NGG mode sequence (5′‐ACCGGCGGTTCGCTGCTGAT‐3′) was obtained from CRISPy‐web (http://crispy.secondarymetabolites.org/). A special single guide RNA (sgRNA; containing a 20‐nt sequence) was amplified from pKCcas9dO. The linear pKCcas9dO vector was generated by SpeI/HindIII digestion. The upstream and downstream DNA fragments, sgRNA and the linear pKCcas9dO vector were finally assembled to generate the plasmid pKCcas9dO‐sco0618 using the ClonExpress™ II One Step Cloning Kit (TaKaRa). This plasmid was introduced into S. coelicolor M145 by conjugal transfer between E. coli ET12567 (pUZ8002) and M145. The exconjugants were selected and examined following a reported protocol (Li et al., 2017a). After incubation at 37 °C for several rounds of selection, the pKCcas9dO‐Scpdo plasmid was extracted from the mutant and the final construct, ΔScpdo, was obtained. The strain ΔSccsoR was subsequently constructed using a homologous recombination method (Lu et al., 2018).
Construction of the complementary strains
To construct a complementary strain of ΔScpdo, namely, ΔScpdo:: Scpdo, a DNA fragment containing a kasOp* promoter (Bai et al., 2015) and Scpdo ORF was obtained using PCR. This DNA fragment was inserted into the integrative vector pMS82 to make a new plasmid pCom0618 (Table S2), which was then transformed into the ΔScpdo strain using a conjugation method. The ΔSccsoR complementary strain, ΔSccsoR:: SccsoR, was constructed following the same protocol, except that a DNA fragment containing SccsoR ORF and its native promoter (a 507 bp fragment upstream of its ORF) was used. To construct control strains, empty pMS82 vectors were also transformed into ΔScpdo and ΔSccsoR strains.
Examination of the ACT production
For testing the effects of H2S and sulfane sulfur, S. coelicolor M145 spores (2 × 106) were inoculated on YBP agar plates containing different concentrations of NaHS or S8, and the plates were incubated at 30 °C for 6 days. For quantitative analysis of the ACT production, a previously reported method was used (Kieser et al., 2000). Briefly, total ACT was quantified by scraping off all the medium containing mycelia from the plate and mixing it with 1 M KOH. The reaction was conducted overnight at 25 °C, and later, 1 ml aliquot was taken out for quantification. Intracellular ACT was quantified using only the mycelia, and extracellular ACT was quantified using only the medium. ACT in the sample was quantified by measuring absorbance at 640 nm.
Analysis of intracellular sulfane sulfur
To perform the SSP4 test, S. coelicolor M145 and its derivatives (10 ml) were cultured in liquid YBP medium. The mycelia were harvested at different time points, washed with and finally suspended in HEPES buffer (50 mM, pH 7.4). Cells were broken down using a high‐pressure crusher SPCH‐18 (STANSTED). SSP4 (10 μM) was added to each supernatant, and the mixture was incubated at 37 °C for 15 min in the dark with gently shaking (125 rpm). Fluorescence intensity was measured using the SynergyH1 microplate reader. The excitation and emission wavelengths were set at 482 and 515 nm respectively. The final data were converted to fluorescence intensity per 0.5 OD450 of cells.
For HPLC analysis, a method describe previously was used (Ran et al., 2019). Briefly, cells at 1 OD450 were collected and washed with HEPES buffer (50 mM, pH 7.4) and then resuspended in 220 μl of 50 mM Tris‐HCl buffer (pH 9.5) containing 1% (v/v) Triton X‐100, 50 μM DTPA, and 1 mM sulfite. The samples were incubated at 95 °C for 10 min to convert sulfane sulfur to thiosulfate. After centrifugation, 50 μl supernatant was reacted with mBBr (5 μl) in the dark for 30 min, and 100 μl of acetic acid and acetonitrile mixture (v/v, 1:9) was added to stop the reaction. The finally obtained sample was analysed using HPLC equipped with a fluorescence detector.
Protein mutation, expression and purification
The ORFs of Scpdo and SccsoR were amplified from S. coelicolor M145 genomic DNA. ScCsoR mutants were constructed using the revised QuikChange™ method (Xia et al., 2015). The ORFs were ligated into pET15b plasmid (Table S2) for expression. E. coli BL21(DE3) cells containing pET15b derived plasmids were cultured in LB medium at 37 °C until OD600nm reached about 0.5, and then 0.5 mM isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) was added. The temperature was changed to 30 °C, and the cultivation was continued overnight. Cells were harvested by centrifugation and disrupted using a pressure cell homogenizer (SPCH‐18) at 4 °C in buffer I (50 mM NaH2PO4, 250 mM NaCl, 20 mM imidazole, pH 8.0). The His‐tagged protein was purified using Ni‐NTA‐Sefinose column (Sangon) following the manufacturer’s instructions. The purity of the protein was examined using SDS‐PAGE, and its concentration was determined using a bicinchoninic acid assay.
Assay of ScPDO activity
The persulfide dioxygenase activity of ScPDO was determined using a previously reported method (Xin et al., 2016). The reaction system contained 1 μM ScPDO and different concentrations of GSSH (0–0.5 mM) in 3 ml KPi buffer (100 mM, pH 7.4), and the reaction was conducted at 25 °C. O2 consumption was examined using an Orion RDO meter (Thermo Scientific). The kinetic parameters were calculated using the Michaelis–Menten equation as reported previously (Liu et al., 2014). GSSH was prepared as described previously (Xin et al., 2016).
RT‐PCR, RT‐qPCR and 5'‐RACE analysis
RNA from S. coelicolor was prepared by following a previously described protocol (Lu et al., 2018). Mycelia were harvested at different time points (36, 60 and 84 h) and were ground into powder using liquid nitrogen. Total RNA was treated with TRIzol, and DNase Ι was used to obtain chromosomal DNA. RT‐PCR was carried out using a reverse transcriptase kit (Invitrogen). RT‐qPCR was performed using SYBR Premix Ex Taq (Takara). hrdB gene encoding a major sigma factor was used as control to normalize the relative quantities of cDNA. Roche LightCycler 480 thermal cycler was used to determine the melting curve and specificity of the PCR products. Three independent replicates were performed in parallel. 5'‐RACE was performed employing the FirstChoice RLM‐RACE kit (Thermo) following the manufacturer’s instructions.
Electrophoretic mobility shift assay (EMSA)
DNA probes were labelled with 5ˊ‐Biotin, and 1 nM each of the labelled probes was mixed with differing amounts of purified ScCsoR protein in a binding buffer (20 mM Tris‐HCI, 2 mM EDTA, 20 mM KCI, 0.5 mM dithiothreitol (DTT) or 1 mM–3 mM HSnH, 4% (w/v) Ficoll‐400, pH 8.0). HSnH was prepared by mixing H2S with S8 as reported previously (Xin et al., 2016). The reaction was conducted at 25 °C for 15 min, followed by separation on an 8% (w/v) non‐denaturing polyacrylamide gel in an ice bath. Next, the DNA and proteins were transferred from the gel to a positively charged nylon membrane, fixed, blocked, washed and finally stained using solutions of an ECL Western blotting kit (GE Healthcare), and images were captured with a FlourChemQ system (Alpha Innotech).
LC‐MS/MS analysis of ScCsoR
The purified ScCsoR was reacted with 10‐fold (molar ratio) of hydrogen polysulfide (HSnH, n ≥ 2) or DTT for 20 min at room temperature. The reacted protein was treated with a denaturing buffer (0.5 M Tri‐HCl, 2.75 mM EDTA, 6 M guanadine‐HCl, pH 8.0) containing 1 M iodoacetamide (IAM) for 1 h, and the sample was subsequently digested with trypsin (1:25, w/w) at 37 °C for 4 h. LC‐MS/MS analysis was conducted following a previously reported protocol (Li et al., 2017b). The Prominence nano‐LC system (Shimadzu) equipped with a custom‐made silica column (75 μm × 15 cm) packed with 3‐μm Reprosil‐Pur 120 C18‐AQ was used for the analysis. For the elution process, a total gradient of 100 min from 0% to 100% of solvent B (0.1% (v/v) formic acid in 98% (v/v) acetonitrile) at 300 nl min−1 was used; solvent A was 0.1% (v/v) formic acid in 2% (v/v) acetonitrile. The eluent was ionized and electrosprayed via LTQ‐Orbitrap Velos Pro CID mass spectrometer (Thermo Scientific); the run was performed in a data‐dependent acquisition mode with Xcalibur 2.2.0 software (Thermo Scientific). Full‐scan MS spectra (from 400 to 1800 m/z) were detected using the Orbitrap at a resolution of 60,000 at 400 m/z.
Bioinformatics analysis
ScPDO and ScCsoR were used as queries to search homologues from all the sequenced Streptomyces genomes mentioned in the website, https://patricbrc.org. The selection criteria used were E‐value < 1e‐60, identity ≥ 35% and coverage ≥ 70%. ClustalW was used for alignment of these candidates, and a condensed neighbour‐joining tree was further built with bootstrap replications at 1000 by using the p‐distance method, uniform rates and pairwise deletion with a cut‐off at 50% by using the MEGA 7.0 software (Kumar et al., 2016).
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Fig. S1. Amino acid sequence alignment analysis of ScPDO and known type III PDOs. The reported type III PDOs usually contain PDO and rhodanese domains. The representative type III PDOs amino acid sequences of Zunongwangia profunda SM‐A87 (ZpPDOIII, ADF52140.1), Staphylococcus aureus (SaPDOIII, WP_000465474.1) and Bacillus cereus ATCC 10876 (BcPdoIII, EEK49737.1) were downloaded from NCBI and were compared with ScPDO by using ClustalW. The results show that ScPDO is a typical type III PDOs, which is consistent with a previous report (Xia et al., 2017).
Fig. S2. SDS‐PAGE analysis of recombinant ScPDO. Lane 1 is the ladder (kDa), Lanes 2‐4 are ScPDO with different concentrations. The theoretical MW of His‐tag‐ScPDO is about 49 kDa.
Fig. S3. MS2 data of peptide 1, which was from DTT‐treated ScCsoR.
Fig. S4. MS2 data of peptide 2, which was from DTT‐treated ScCsoR.
Fig. S5. MS2 data of peptide 3, which was only detected from polysulfide‐treated ScCsoR.
Fig. S6. EMSA assay of ScCsoR‐C37S, ScCsoR‐C66S and ScCsoR‐C37S‐C66S mutants. The Cys‐to‐Ser mutation did not affect the ScCsoR binding activity to Scpdo promoter; however, it caused the loss of HSnH sensing activity and HSnH could not release ScCsoR mutants from Scpdo promoter. The promoter region of Scpdo (256 bp) was used as the DNA probe. 1 nM DNA probe was incubated with gradient concentration of ScCsoR mutants (0, 0.5, 1.0, 2.0, 4.0, 8.0, 8.0, 8.0, 8.0 μM), different amounts of polysulfides (0, 1, 2, 3 mM) was added to the reaction system (with equal amounts of ScCsoR mutants‐8.0 μM). The black arrow indicates the freedom DNA probe, the red arrow indicates the shifted DNA probe. These results show that the cysteines on ScCsoR play an important role for sulfane sulfur sensing, and the two cysteine are indispensable.
Fig. S7. The intergenic region of SccsoR and Scrhod promoters. The starting codon is shown in bold black, binding site is shown in red font. The number below is the length (bp) between the starting codon and binding site.
Fig. S8. Sco0622 and sco0623 expression was not controlled by ScCsoR. (A) Co‐transcription analysis of Scpdo‐tauE, rhod‐sco0622 and sco0622‐23. Genomic DNA, mRNA, and cDNA as templates for lanes 1, 2, and 3, respectively. (B) EMSA analysis of ScCsoR binding activity to the promoter region of sco0622 (242bp), 1 nM DNA probe was incubated with different amounts of ScCsoR (0, 2.2, 8.8, 15.4, 22.2 μM), the black arrow indicates the unbounded DNA probe. (C) RT‐qPCR analysis of the transcriptional level of sco0622 in wt and ΔSccsoR strains. RNA was harvested at 36 h, 60 h, 84 h from mycelium grown in YBP solid medium. The hrdB gene was used as the internal control to normalize the mRNA level. Three independent measurements were carried out and error bars indicate the standard deviations.
Table S1. The distribution of Scpdo‐rhod in representative Streptomyces genomes.
Table S2. Strains and plasmids used in this study.
Table S3. Primers (5′→3′) used in this study.
Acknowledgements
The work was financially supported by grants from the National Key Research and Development Program of China (2016YFA0601103) and the National Key R&D Program of China (2018YFA0901200).
Microbial Biotechnology (2020) 13(6), 1917–1932
Funding information
The work was financially supported by grants from the National Key Research and Development Program of China (2016YFA0601103) and the National Key R&D Program of China (2018YFA0901200).
References
- Bai, C. , Zhang, Y. , Zhao, X. , Hu, Y. , Xiang, S. , Miao, J. , et al (2015) Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces . Proc Natl Acad Sci USA 112: 12181–12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka, E.A. , Vatsa, P. , Sanchez, L. , Gaveau‐Vaillant, N. , Jacquard, C. , Meier‐Kolthoff, J.P. , et al (2016) Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev 80: 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, S.D. , Chater, K.F. , Cerdeno‐Tarraga, A.M. , Challis, G.L. , Thomson, N.R. , James, K.D. , et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417: 141–147. [DOI] [PubMed] [Google Scholar]
- Bibb, M.J. (2013) Understanding and manipulating antibiotic production in actinomycetes. Biochem Soc Trans 41: 1355–1364. [DOI] [PubMed] [Google Scholar]
- Blin, K. , Shaw, S. , Steinke, K. , Villebro, R. , Ziemert, N. , Lee, S.Y. , et al (2019) antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47: W81–W87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush, M.J. , Tschowri, N. , Schlimpert, S. , Flardh, K. , and Buttner, M.J. (2015) c‐di‐GMP signalling and the regulation of developmental transitions in streptomycetes. Nat Rev Microbiol 13: 749–760. [DOI] [PubMed] [Google Scholar]
- Bystrykh, L.V. , Fernandez‐Moreno, M.A. , Herrema, J.K. , Malpartida, F. , Hopwood, D.A. , and Dijkhuizen, L. (1996) Production of actinorhodin‐related “blue pigments” by Streptomyces coelicolor A3(2). J Bacteriol 178: 2238–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, F.M. , Coyne, H.J. , Cubillas, C. , Vinuesa, P. , Fang, X. , Ma, Z. , et al (2014) Cu(I)‐mediated allosteric switching in a copper‐sensing operon repressor (CsoR). J Biol Chem 289: 19204–19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Liu, C. , Peng, B. , Zhao, Y. , Pacheco, A. , and Xian, M. (2013) New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci 4: 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Zheng, G. , Zhu, H. , He, H. , Chen, L. , Zhang, W. , et al (2016) Roles of two‐component system AfsQ1/Q2 in regulating biosynthesis of the yellow‐pigmented coelimycin P2 in Streptomyces coelicolor . FEMS Microbiol Lett 363: fnw160 10.1093/femsle/fnw160. [DOI] [PubMed] [Google Scholar]
- Coyne, H.J. 3rd , and Giedroc, D.P. (2013) Backbone resonance assignments of the homotetrameric (48 kD) copper sensor CsoR from Geobacillus thermodenitrificans in the apo‐ and Cu(I)‐bound states: insights into copper‐mediated allostery. Biomol NMR Assign 7: 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa, R.A. , Jones, M.B. , Butler‐Wu, S. , Sinsimer, D. , Gerads, R. , Bishai, W.R. , et al (2011) A novel copper‐responsive regulon in Mycobacterium tuberculosis . Mol Microbiol 79: 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto, J.M. , Ignarro, L.J. , Nagy, P. , Wink, D.A. , Kevil, C.G. , Feelisch, M. , et al (2018) Biological hydropersulfides and related polysulfides ‐ a new concept and perspective in redox biology. FEBS Lett 592: 2140–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedroc, D.P. (2017) A new player in bacterial sulfide‐inducible transcriptional regulation. Mol Microbiol 105: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt, T.M. , and Grieshaber, M.K. (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361. [DOI] [PubMed] [Google Scholar]
- Hopwood, D.A. (2007) Streptomyces in nature and medicine: the antibiotic makers. New York, NY, USA: Oxford University Press. [Google Scholar]
- Hou, N. , Yan, Z. , Fan, K. , Li, H. , Zhao, R. , Xia, Y. , et al (2019) OxyR senses sulfane sulfur and activates the genes for its removal in Escherichia coli . Redox Biol 26: 101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Zheng, G. , Jiang, W. , Hu, H. , and Lu, Y. (2015) One‐step high‐efficiency CRISPR/Cas9‐mediated genome editing in Streptomyces . Acta Biochim Biophys Sin (Shanghai) 47: 231–243. [DOI] [PubMed] [Google Scholar]
- Khosla, C. , McDaniel, R. , Ebert‐Khosla, S. , Torres, R. , Sherman, D.H. , Bibb, M.J. , and Hopwood, D.A. (1993) Genetic construction and functional analysis of hybrid polyketide synthases containing heterologous acyl carrier proteins. J Bacteriol 175: 2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser, T. , Bibb, M.J. , Buttner, M.J. , Chater, K.F. , and Hopwood, D.A. (2000) Practical streptomyces genetics. Norwich, UK: John Innes Foundation. [Google Scholar]
- Kumar, S. , Stecher, G. , and Tamura, K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, N. , and Pluth, M.D. (2019) Reactive sulfur species (RSS): persulfides, polysulfides, potential, and problems. Curr Opin Chem Biol 49: 1–8. [DOI] [PubMed] [Google Scholar]
- Li, J.W. , and Vederas, J.C. (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325: 161–165. [DOI] [PubMed] [Google Scholar]
- Li, L. , Jiang, W. , and Lu, Y. (2017a) A novel two‐component system, GluR‐GluK, involved in glutamate sensing and uptake in Streptomyces coelicolor . J Bacteriol 199: e00097‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Li, J. , Lu, C. , Xia, Y. , Xin, Y. , Liu, H. , et al (2017b) FisR activates sigma(54) ‐dependent transcription of sulfide‐oxidizing genes in Cupriavidus pinatubonensis JMP134. Mol Microbiol 105: 373–384. [DOI] [PubMed] [Google Scholar]
- de Lira, N.P.V. , Pauletti, B.A. , Marques, A.C. , Perez, C.A. , Caserta, R. , de Souza, A.A. , et al (2018) BigR is a sulfide sensor that regulates a sulfur transferase/dioxygenase required for aerobic respiration of plant bacteria under sulfide stress. Sci Rep 8: 3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. , Chater, K.F. , Chandra, G. , Niu, G. , and Tan, H. (2013) Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol Mol Biol Rev 77: 112–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Xin, Y. , and Xun, L. (2014) Distribution, diversity, and activities of sulfur dioxygenases in heterotrophic bacteria. Appl Environ Microbiol 80: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, T. , Zhu, Y. , Zhang, P. , Sheng, D. , Cao, G. , and Pang, X. (2018) SCO5351 is a pleiotropic factor that impacts secondary metabolism and morphological development in Streptomyces coelicolor . FEMS Microbiol Lett 365 10.1093/femsle/fny150 [DOI] [PubMed] [Google Scholar]
- Luebke, J.L. , Shen, J. , Bruce, K.E. , Kehl‐Fie, T.E. , Peng, H. , Skaar, E.P. , and Giedroc, D.P. (2014) The CsoR‐like sulfurtransferase repressor (CstR) is a persulfide sensor in Staphylococcus aureus . Mol Microbiol 94: 1343–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, S. , and Nodwell, J.R. (2017) Actinorhodin is a redox‐active antibiotic with a complex mode of action against Gram‐positive cells. Mol Microbiol 106: 597–613. [DOI] [PubMed] [Google Scholar]
- Motl, N. , Skiba, M.A. , Kabil, O. , Smith, J.L. , and Banerjee, R. (2017) Structural and biochemical analyses indicate that a bacterial persulfide dioxygenase‐rhodanese fusion protein functions in sulfur assimilation. J Biol Chem 292: 14026–14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett, M. , Ikeda, H. , and Moore, B.S. (2009) Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep 26: 1362–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton, G.L. , Buchmeier, N. , and Fahey, R.C. (2008) Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol Mol Biol Rev 72: 471–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, H. , Zhang, Y. , Palmer, L.D. , Kehl‐Fie, T.E. , Skaar, E.P. , Trinidad, J.C. , and Giedroc, D.P. (2017) Hydrogen sulfide and reactive sulfur species impact proteome S‐sulfhydration and global virulence regulation in Staphylococcus aureus . ACS Infect Dis 3: 744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran, M. , Wang, T. , Shao, M. , Chen, Z. , Liu, H. , Xia, Y. , and Xun, L. (2019) Sensitive method for reliable quantification of sulfane sulfur in biological samples. Anal Chem 91: 11981–11986. [DOI] [PubMed] [Google Scholar]
- Robertsen, H.L. , and Musiol‐Kroll, E.M. (2019) Actinomycete‐derived polyketides as a source of antibiotics and lead structures for the development of new antimicrobial drugs. Antibiotics 8: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, B. (2015) A new golden age of natural products drug discovery. Cell 163: 1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Keithly, M.E. , Armstrong, R.N. , Higgins, K.A. , Edmonds, K.A. , and Giedroc, D.P. (2015) Staphylococcus aureus CstB is a novel multidomain persulfide dioxygenase‐sulfurtransferase involved in hydrogen sulfide detoxification. Biochemistry 54: 4542–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X. , Festa, R.A. , Ioerger, T.R. , Butler‐Wu, S. , Sacchettini, J.C. , Darwin, K.H. , and Samanovic, M.I. (2014) The copper‐responsive RicR Regulon contributes to Mycobacterium tuberculosis virulence. MBio 5: e00876‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T. , and Masuda, S. (2020) Persulphide‐responsive transcriptional regulation and metabolism in bacteria. J Bio chem 167: 125–132. [DOI] [PubMed] [Google Scholar]
- Shimizu, T. , Shen, J. , Fang, M. , Zhang, Y. , Hori, K. , Trinidad, J.C. , et al (2017) Sulfide‐responsive transcriptional repressor SqrR functions as a master regulator of sulfide‐dependent photosynthesis. Proc Natl Acad Sci USA 114: 2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl, W.R. , and Connors, N.C. (1992) Significance of anthraquinone formation resulting from the cloning of actinorhodin genes in heterologous streptomycetes. Mol Microbiol 6: 147–152. [DOI] [PubMed] [Google Scholar]
- Weinitschke, S. , Denger, K. , Cook, A.M. , and Smits, T.H. (2007) The DUF81 protein TauE in Cupriavidus necator H16, a sulfite exporter in the metabolism of C2 sulfonates. Microbiology 153: 3055–3060. [DOI] [PubMed] [Google Scholar]
- Wright, G.D. (2017) Opportunities for natural products in 21(st) century antibiotic discovery. Nat Prod Rep 34: 694–701. [DOI] [PubMed] [Google Scholar]
- Xia, Y. , Chu, W. , Qi, Q. , and Xun, L. (2015) New insights into the QuikChangeTM process guide the use of Phusion DNA polymerase for site‐directed mutagenesis. Nucleic Acids Res, 43: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y. , Lu, C. , Hou, N. , Xin, Y. , Liu, J. , Liu, H. , and Xun, L. (2017) Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J 11: 2754–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin, Y. , Liu, H. , Cui, F. , Liu, H. , and Xun, L. (2016) Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ Microbiol 18: 5123–5136. [DOI] [PubMed] [Google Scholar]
- Xu, Z. , Wang, Y. , Chater, K.F. , Ou, H.Y. , Xu, H.H. , Deng, Z. , and Tao, M. (2017) Large‐scale transposition mutagenesis of Streptomyces coelicolor identifies hundreds of genes influencing antibiotic biosynthesis. Appl Environ Microbiol 83: e02889‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Li, Y. , Wang, Y. , Deng, Z. , and Tao, M. (2019) Genome‐wide mutagenesis links multiple metabolic pathways with actinorhodin production in Streptomyces coelicolor . Appl Environ Microbiol 85: e03005‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Amino acid sequence alignment analysis of ScPDO and known type III PDOs. The reported type III PDOs usually contain PDO and rhodanese domains. The representative type III PDOs amino acid sequences of Zunongwangia profunda SM‐A87 (ZpPDOIII, ADF52140.1), Staphylococcus aureus (SaPDOIII, WP_000465474.1) and Bacillus cereus ATCC 10876 (BcPdoIII, EEK49737.1) were downloaded from NCBI and were compared with ScPDO by using ClustalW. The results show that ScPDO is a typical type III PDOs, which is consistent with a previous report (Xia et al., 2017).
Fig. S2. SDS‐PAGE analysis of recombinant ScPDO. Lane 1 is the ladder (kDa), Lanes 2‐4 are ScPDO with different concentrations. The theoretical MW of His‐tag‐ScPDO is about 49 kDa.
Fig. S3. MS2 data of peptide 1, which was from DTT‐treated ScCsoR.
Fig. S4. MS2 data of peptide 2, which was from DTT‐treated ScCsoR.
Fig. S5. MS2 data of peptide 3, which was only detected from polysulfide‐treated ScCsoR.
Fig. S6. EMSA assay of ScCsoR‐C37S, ScCsoR‐C66S and ScCsoR‐C37S‐C66S mutants. The Cys‐to‐Ser mutation did not affect the ScCsoR binding activity to Scpdo promoter; however, it caused the loss of HSnH sensing activity and HSnH could not release ScCsoR mutants from Scpdo promoter. The promoter region of Scpdo (256 bp) was used as the DNA probe. 1 nM DNA probe was incubated with gradient concentration of ScCsoR mutants (0, 0.5, 1.0, 2.0, 4.0, 8.0, 8.0, 8.0, 8.0 μM), different amounts of polysulfides (0, 1, 2, 3 mM) was added to the reaction system (with equal amounts of ScCsoR mutants‐8.0 μM). The black arrow indicates the freedom DNA probe, the red arrow indicates the shifted DNA probe. These results show that the cysteines on ScCsoR play an important role for sulfane sulfur sensing, and the two cysteine are indispensable.
Fig. S7. The intergenic region of SccsoR and Scrhod promoters. The starting codon is shown in bold black, binding site is shown in red font. The number below is the length (bp) between the starting codon and binding site.
Fig. S8. Sco0622 and sco0623 expression was not controlled by ScCsoR. (A) Co‐transcription analysis of Scpdo‐tauE, rhod‐sco0622 and sco0622‐23. Genomic DNA, mRNA, and cDNA as templates for lanes 1, 2, and 3, respectively. (B) EMSA analysis of ScCsoR binding activity to the promoter region of sco0622 (242bp), 1 nM DNA probe was incubated with different amounts of ScCsoR (0, 2.2, 8.8, 15.4, 22.2 μM), the black arrow indicates the unbounded DNA probe. (C) RT‐qPCR analysis of the transcriptional level of sco0622 in wt and ΔSccsoR strains. RNA was harvested at 36 h, 60 h, 84 h from mycelium grown in YBP solid medium. The hrdB gene was used as the internal control to normalize the mRNA level. Three independent measurements were carried out and error bars indicate the standard deviations.
Table S1. The distribution of Scpdo‐rhod in representative Streptomyces genomes.
Table S2. Strains and plasmids used in this study.
Table S3. Primers (5′→3′) used in this study.


