Abstract
Mass spectrometry (MS) is an emerging method to determine the accurate concentration of immunogenic gluten peptides. It is of interest to quantify specific peptides within the gluten peptidome due to the role they play in the activation of the celiac immune cascade. Celiac disease is an autoimmune disorder triggered in genetically susceptible individuals by the presence of specific gluten peptides that resist digestion in the gastrointestinal tract. The protocol detailed within this paper can accurately quantify (label-free) the concentration of six immunogenic gluten peptides (including the 33mer) released from a food matrix using the INFOGEST in vitro digestion protocol. This method can be used to monitor small changes in the concentration of these marker peptides in response to exogenous factors such as plant-breeding, fermentation or food processing.
Keywords: Peptidomics, Celiac disease, Bread, In vitro digestion, Wheat
Graphical abstract
Customisation:
-
•
A sample preparation method that allows the user to detect immunogenic gluten peptides within a food matrix digested using the INFOGEST in vitro digestion protocol with minimal matrix effects;
-
•
A targeted, accurate, label-free mass spectrometry quantification method for six marker gluten peptides using an external standard curve that is compatible with the INFOGEST in vitro digestion;
-
•
A methodology that can be expanded to include additional peptide targets such as other immunogenic gluten peptides or celiac peptides from other grains.
Specifications Table
| Subject Area | Biochemistry, Genetics and Molecular Biology |
| More specific subject area | Mass spectrometry of wheat gluten peptides |
| Method name | Targeted mass spectrometry method for gluten peptides |
| Name and reference of original method | van den Broeck, H. C., Cordewener, J. H. G., Nessen, M. A., America, A. H. P. Van der Meer, I. M. Label free targeted detection and quantification of celiac disease immunogenic epitopes by mass spectrometry. Journal of Chromatography A, Volume 1391, 24 April 2015, Pages 60–71. doi:10.1016/j.chroma.2015.02.070 |
| Resource availability |
Background
Celiac disease is an autoimmune disease initiated in genetically susceptible individuals by immunogenic gluten peptides that are resistant to gastrointestinal digestion [1]. Immunogenic gluten peptides range between 9 and 33 amino acids in length and possess epitopes that bind to the human leukocyte antigen (HLA)-DQ2 or 8 receptors [2]. Hundreds of gluten derived peptides have been identified that activate the celiac response. These peptides exhibit a hierarchy of immunodominance [3], meaning that some initiate a stronger immune response than others. Most often, immunogenic peptides are detected and validated within an isolated protein system that was digested using trypsin and/or chymotrypsin. This is notable because the digestion of gluten is altered by the presence of a food matrix [4]. Furthermore in vivo, immunogenic peptides are the product of pepsin, trypsin and chymotrypsin digestion.
To overcome these limitations, the protocol herein describes an accurate label-free mass spectrometry (MS) method to quantify six immunogenic gluten peptides (Table 1) within food matrices that have been digested using the INFOGEST in vitro assay [5], which aims to stimulate digestion within the gastrointestinal tract. This accurate MS method is built on a previous MS method described by van den Broeck 2015 [6] that was developed to determine the relative concentration of peptides P1-P6 within isolated protein systems. All six marker peptides contain at least one celiac epitope, are proline-rich and are unequivocally involved in activation of the celiac immune cascade (Table 1).
Table 1.
The marker immunogenic gluten peptides analysed by the targeted PRM MS method described. All peptides are proline rich and contain at least one epitope that activates celiac pathogenesis.
| Peptide name | Percent proline | Core epitope(s) | Amino acid sequence |
|---|---|---|---|
| P1 | 30.8 | DQ2.5-glia-α1a (x1) | LQLQPFPQPQLPY |
| P2 | 30.8 | DQ2.5-glia-α1a (x1) DQ2.5-glia-α2 (x1) | LQLQPFPQPQLPYPQPQPF |
| P3 | 38.5 | DQ2.5-glia-α1a (x1) DQ2.5-glia-α2 (x1) | LQLQPFPQPQLPYPQPHLPYPQPQPF |
| P4 | 38.5 | DQ2.5-glia-α1a (x1) DQ2.5-glia-α1b (x1) DQ2.5-glia-α2 (x2) | LQLQPFPQPQLPYPQPQLPYPQPQPF |
| 33mer (P5) | 39.4 | DQ2.5-glia-α1a (x1) DQ2.5-glia-α1b (x2) DQ2.5-glia-α2 (x3) | LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF |
| P6 | 38.5 | DQ2.5-glia-α3 (x1) | RPQQPYPQPQPQY |
This manuscript is divided into three sections covering the sample digestion, sample preparation and the quantitative parallel reaction monitoring (PRM) MS method. Each section is further divided into the protocol, and the method development workflow used to develop that protocol. The method development workflow was included to allow the addition of alternative peptides if desired. The peptides investigated herein are α-gliadin derived; however, immunogenic gluten peptides have been identified from all gluten protein classes and therefore future users may want to include a wider range. Overall, this protocol can be applied to determine the accurate concentration of six immunogenic peptides, which are displayed in Table 1, within the digesta of food. When used at different points throughout digestion, the results can create a model of peptide release in response to different experimental and processing treatments.
Materials
Reagents
The following are specialised reagents required to undertake this protocol. In addition to these reagents, the user must obtain those required for the enzyme activity assays as detailed in the references below.
-
•
Marker peptides P1-P6 in lyophilised form, 0.5 mg quantities at >98% purity (New England Peptide (MA, USA)).
-
•
Methanol (AnalaR grade or better)
-
•
Acetonitrile (ACN) (LC-MS grade)
-
•
Formic acid (FA) (LC-MS grade)
-
•
KCl
-
•
KH2PO4
-
•
NaHCO3
-
•
NaCl
-
•
MgCl2.(H2O)6
-
•
(NH4)2CO3
-
•
CaCl2
-
•
Salivary α-amylase (Sigma: A6255)
-
•
Pepsin (Sigma: P7012)
-
•
α-chymotrypsin (Sigma: C4129)
-
•
trypsin (Sigma: T0303)
-
•
Trifluoroacetic acid (TFA) (LC-MS grade)
Equipment
The following specialist equipment is required to undertake this protocol. General lab equipment is also required including pipettes and a centrifuge capable of spinning 1.5 mL microcentrifuge tubes at 14,000 × g.
-
•
Orbitrap Q Exactive™ Plus (Thermo Fisher Scientific) (or equivalent)
-
•
Vanquish ultra-high-performance liquid chromatography (UPLC) system (or equivalent)
-
•
Aeris™ 1.7 µm PEPTIDE XB-C18 100 Å, LC Column 150 × 2.1 mm (Phenomenex) fitted with a C18-Peptide SecurityGuard™ ULTRA Cartridge (Phenomenex)
-
•
Strata™-XL 100 µm Polymeric Reversed Phase, 30 mg/mL tubes (Phenomenex)
-
•
Vacuum manifold
-
•
SINGLE StEP™ filter vial (Thomson Instrument Company™) (or equivalent)
Software
-
•
XcaliburⓇ Suite (Thermo Fisher Scientific) (or equivalent)
-
•
MicrosoftⓇ ExcelⓇ (or equivalent)
Method details
Sample digestion
This section describes the INFOGEST in vitro digestion assay [5] with minor modifications that make it compatible with the PRM MS method described below. The INFOGEST in vitro digestion assay was chosen as it simulates protein digestion in the human gastrointestinal tract [7]. The specific modification herein is the reaction quenching method. As well as the digestion protocol, the workflow employed to optimise the sample quenching method is described. Matrix effects occurred when pancreatin was used as the digestive component in the intestinal phase; therefore, the use of pure trypsin and chymotrypsin is recommended. This method is specifically optimised for the digestion of bread.
Method development workflow
Gluten derived peptides that activate celiac disease are rich in proline and glutamine residues and thus behave unusually in aqueous solution [8]. The method development workflow for the in vitro digestion protocol was centred on identifying an appropriate quenching reagent. Some quenching reagents can cause peptide precipitation and matrix effects—these are peptide-specific and unpredictable meaning they must be experimentally investigated.
During method development, various quenching reagents were selected from literature and their suitability assessed, including the addition of 70% ethanol at 1:1 v/v [9], 0.1% v/v TFA [10], ACN with 0.1% TFA at 1:1 v/v [11], no quenching [12], and an trypsin-chymotrypsin inhibitor from Glycine max (soybean) [5]. The compatibility of each quenching reagent was assessed by determining the immunogenic peptide abundance via PRM MS with and without the reagent. This experiment was undertaken in both a blank matrix and the sample matrix of bread digesta by spiking synthetic marker peptides at known concentrations. Only P1H was used in the sample matrix. If the presence of the quenching reagent decreased the marker peptide abundance it was not selected as the quenching reagent. The most effective quenching reagent for P1-P6 was 0.1% v/v TFA.
Final protocol
The enzymatic activity of α-amylase, pepsin, trypsin and chymotrypsin were defined as described in the supplementary material of the INFOGEST protocol [5]. The activity of α-amylase was determined using a spectrophotometric stop assay adapted from Bernfeld [13] by measuring the concentration of liberated maltose. The activity of pepsin was determined by a spectrophotometric stop assay adapted from Anson [14] using haemoglobin as a substrate. The activity of trypsin was established by a continuous spectrophotometric rate determination assay adapted from Hummel [15] using the substrate p-toluene-sulfonyl-L-arginine methyl ester (TAME). The activity of chymotrypsin was defined by continuous spectrophotometric rate determination assay adapted from Hummel [15] using the substrate N-Benzoyl-L-Tyrosine Ethyl Ester (BTEE). Bread was then digested in biological triplicate using the INFOGEST assay as follows:
-
1.
Bread was freeze-dried.
-
2.
Stock solutions of digestive fluids were formulated at 1.25 times the working concentration (Table 2) and stored at −20 °C for up to one year.
-
3.Simulated digestive fluids (Table 3) were prepared using the stock solutions adding the enzymatic components by their pre-defined activity.
-
i.Fluids were prepared 25 min before required and prewarmed to 37 °C. For example, the intestinal fluid was prepared after 95 min of gastric digestion.
-
ii.Enough fluid for all replicates was prepared in a stock solution to improve the method's reproducibility.
-
i.
-
4.The freeze-dried sample was rehydrated with 1.25:4 v/w with milliQ water.
-
i.For example, 1.25 g of freeze-dried bread was added to 4 mL of water.
-
ii.If feasible (dependent on the sample volume), add an additional isotopically labelled internal standard at this step.
-
i.
-
5.After rehydration, oral fluid was immediately added at a 1:1 v/w ratio with the original bread weight, and the sample was triturated with a fork to simulate chewing for 2 min
-
i.For example, 1.25 mL of oral fluid was added to 1.25 g of bread.
-
i.
-
6.Gastric fluid was added at a 1:1 v/w ratio with the total solution and incubated for 2 h at 37 °C whilst shaking.
-
i.For example, 2.5 mL of gastric fluid was added to 1.25 g of bread.
-
i.
-
7.The intestinal fluid was added at a 1:1 v/w ratio with the total solution and digested for 2 h at 37 °C, whilst shaking.
-
i.For example, 5 mL of intestinal fluid was added to 1.25 g of bread.
-
ii.If samples were removed during the gastric phase, this loss of volume was taken into account when the intestinal fluid was added.
-
i.
-
8.At the desired time points, aliquots of digesta were removed and the reaction quenched with TFA from a 12% v/v stock to achieve 0.1% v/v.
-
i.After TFA addition, samples were vortexed for 5 s, immediately snap-frozen in N2(l), then stored at −20 °C for up to one year.
-
ii.To efficiently quench the reaction, the appropriate volume of TFA was added to all sampling tubes at the start of the experiment.
-
i.
Table 2.
The composition of the stock digestive fluids x1.25 working concentration.
| Constituent | Oral (mmol L−1) | Gastric (mmol L−1) | Intestinal (mmol L−1) |
|---|---|---|---|
| KCl | 15.1 | 6.90 | 6.80 |
| KH2PO4 | 3.70 | 0.90 | 0.80 |
| NaHCO3 | 13.6 | 25.0 | 85.0 |
| NaCl | – | 47.2 | 38.4 |
| MgCl2.(H2O)6 | 0.15 | 0.10 | 0.33 |
| (NH4)2CO3 | 0.06 | 0.50 | – |
| pH | 7.00 | 3.00 | 7.00 |
Table 3.
The composition of the simulated digestion fluids used during the INFOGEST digestion assay. Enzymes were added based on predefined activity.
| Oral | Gastric | Intestinal |
|---|---|---|
| SSF x1 | SGF x1 | SIF x1 |
| α-amylase, 75 U mL−1 | Pepsin, 2000 U mL−1 | α-chymotrpysin, 25 U mL−1 and trypsin 100 U mL−1 |
| CaCl2, 0.75 mmol L−1 | CaCl2, 0.075 mmol L−1 | CaCl2, 0.3 mmol L−1 |
| milliQ water | milliQ water | milliQ water |
| pH 7 | pH 3 | pH 7 |
Sample preparation
Before MS, bread samples digested with the INFOGEST in vitro assay were prepared by solid-phase extraction (SPE) using Strata™-XL 100 µm Polymeric Reversed Phase, 30 mg mL−1 tubes (Phenomenex). Both experimental variability and matrix effects were monitored by addition of an isotopically labelled internal standard (P1H; Table 10) to the sample supernatant at 3 µg mL−1 before SPE.
Table 10.
Transitions detected during PRM target MS. The HCD fragment ions were used to confirm the identity of each precursor ion during data processing. *L-Phenylalanine-13C9, 15N at residue six.
| Peptide name | Amino acid sequence | Molecular weight (kDa) | Precursor m/z (charge state) | Fragment ions m/z | Retention time (min) |
|---|---|---|---|---|---|
| P1 | LQLQPFPQPQLPY | 1.568 | 784.927 (2+) | 279.134 (y2) 483.293 (b4) 470.240 (b6) 1290.719 (b11) |
6.11 |
| P1H | LQLQPF*PQPQLPY | 1.578 | 787.927 (2+) | 279.134 (y2) 483.293 (b4) 476.254 (b6)* 1296.731 (b11) |
6.11 |
| P2 | LQLQPFPQPQLPYPQPQPF | 2.263 | 755.068 (3+) | 488.251 (y4) 952.526 (b8) 1049.544 (b9) |
6.59 |
| P3 | LQLQPFPQPQLPYPQPHLPYPQPQPF | 3.097 | 1029.543 (3+) | 263.139 (y2) 824.429 (b7) 713.357 (y6) 952.527 (b8) 1290.722 (b11) |
7.12 |
| P4 | LQLQPFPQPQLPYPQPQLPYPQPQPF | 3.087 | 1032.543 (3+) | 263.139 (y2) 488.250 (y4) 713.358 (y6) 973.479 (y8) |
6.43 |
| P5 (33mer) | LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF | 3.912 | 978.264 (4+) | 488.252 (y4) 824.429 (b21) 973.480 (y8) 1290.726 (b11) |
7.29 |
| P6 | RPQQPYPQPQPQY | 1.626 | 813.905 (2+) | 407.194 (y3) 967.513 (a8) 995.508 (b9) |
3.48 |
Method development workflow
There is no one-size-fits-all sample preparation method. Development of the SPE sample preparation method was centred on screening the efficiency of elution for various eluates using P1H and the workflow depicted in Fig. 1. SPE was chosen as the sample preparation method due to its predefined ability to reduce matrix effects and reduced sample handling time compared to other techniques [6,10,[16], [17], [18].
Fig. 1.
Workflow to screen the efficiency of the sample preparation method for new gluten derived peptides. Substitute P1H for new peptides.
Briefly, bread was digested using the INFOGEST assay, reaction quenched, then the supernatant obtained by centrifugation. The isotopically labelled peptide P1H was then spiked into the supernatant at 3 µg mL−1 and digesta purified by SPE. The general SPE protocol detailed by the manufacturer (displayed below in Table 4) was employed substituting different eluates in Step 5. The eluates trialled were 50% MeOH, 50% ACN; 100% ACN, 1% formic acid; 100% MeOH; 79.5% ACN, 1% formic acid. Following peptide elution, the abundance of P1H was determined using PRM MS comparing the abundance within the cleaned sample matrix, to P1H dissolved in a blank control matrix. Once the most efficient eluate was identified, its efficacy was confirmed for peptides P2-P6 (non-isotopically labelled) in a blank sample matrix.
Table 4.
SPE purification protocol.
| Step number | Step name | Solution | Volume |
|---|---|---|---|
| 1 | Wash 1 | 100% methanol | 1 mL x3 |
| 2 | Wash 2 | H2O/H+, pH 3 | 1 mL x2 |
| 3 | Load | Sample | 200 µL |
| 4 | Wash 3 | 5% ACN | 1 mL x2 |
| 5 | Elute | 79.5% ACN, 1% FA | 210 µL x2 |
Final protocol
-
1.
Digesta was defrosted on ice, vortexed, then centrifuged at 14,000 × g for 10 min.
-
2.
An aliquot of the supernatant was removed (200 µL) and P1H added from a 50 µg mL−1 stock to a final concentration of 3 µg mL−1.
-
3.Peptides were extracted by SPE from the digesta supernatant as detailed in Table 4.
-
i.The SPE tubes were dried for at least 30 s between each step.
-
i.
-
4.The eluate of Step 5 (Table 4) was collected and filtered using a SINGLE StEP™ filter vial (Thomson Instrument Company™),
-
i.These samples were stored at 4 °C for 2–3 days or −20 °C for extended periods of time.
-
i.
Targeted mass spectrometry
The MS method herein was optimised using an Orbitrap Q Exactive™ Plus in PRM mode for the six marker immunogenic peptides displayed in Table 1, all of which are unequivocally involved in celiac disease.
Method development workflow
Peptides P1-P6 were selected from literature [6] and lyophilised synthetic versions were obtained in 0.5 mg quantitates (98% purity, New England Peptide). A PRM instrument method was assembled in Xcalibur, matrix effects assessed, the methods replicability determined and the limit of detection (LOD) and limit of quantification (LOQ) defined.
Assembling the PRM method
Each peptide was analysed individually in full scan data-dependent analysis (DDA) tandem fragmentation (MS/MS) mode using the ionisation and fragmentation parameters described in Table 5. Each peptide's retention time, dominant charge state and mass-to-charge ratio (m/z) were defined by examining the raw spectra. In silico MS/MS was undertaken using the PROTEOMICS TOOLKIT fragment ion calculator (or equivalent software). Direct sequence fragment ions (b+ and y+) were identified from the raw spectra by matching the in silico MS/MS ions to those experimentally observed. The highest intensity 4–5 direct sequence fragment ions were selected as identifier and quantifier ions for use in data processing. Using this information, a PRM method was assembled in the ‘Thermo Xcalibur Instrument Setup’ window.
Table 5.
Instrument parameters for MS of gluten peptides on an Orbitrap Q ExactiveTM Plus.
| Parameter | Value |
|---|---|
| Spray voltage | 35 kV |
| Spray current | 17 µA |
| Aux gas flow rate | 10 |
| Sheath gas flow rate | 45 |
| Scan range | 108–4015 m/z |
| Resolving power | 70,000 |
| Capillary temperature | 320 °C |
| Normalised collision energy | Stepped 18–27 |
Assessing matrix effects
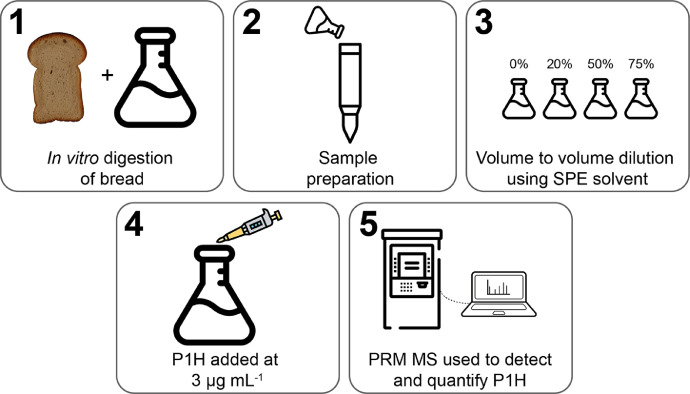
Matrix effects can occur during electrospray ionisation (ESI) due to the intrinsic competition for charge-transfer that occurs between analytes within the same aerosol droplet [19,20]. Matrix effects cause an analyte to be selectively suppressed or enhanced, which may unpredictably alter the peptide abundance and concentration calculated during PRM MS. An in-depth discussion of matrix effects and how to overcome them is detailed in Taylor 2005 [21]. Fig. 2 displays the workflow used to assess matrix effects due to components within the sample. The heavy (H) label indicates the isotopically labelled peptide.
Fig. 2.
Workflow used to assess matrix effects during method development. Step 1 DIGEdigest the bread sample was digested and prepared via SPE (Step 2) without P1H. In step 3, the eluate was diluted to three concentrations, following which P1H was added to each (Step 4). The degree of matrix effects was then determined by comparing the peptide concentration within each matrix dilution by MS with a pure sample of P1H at 3 µg mL−1.
Bread was digested using the INFOGEST assay, then prepared using the SPE as detailed above (Fig. 2). The SPE eluate was diluted serially to 75%, 50% and 20% in pure SPE eluate. The isotopically labelled peptide P1H was then added to each sample at 3 µg mL−1 and the peptide abundance was determined using PRM MS. The control sample contained 100% SPE eluate with P1H at 3 μg mL−1. The difference in P1H abundance between the pure and diluted samples represents the effect of the sample matrix.
Defining the methods replicability
The day-to-day replicability of the PRM and sample preparation method was defined by determining the peptide concentration within the same sample, over multiple days. To do so, in vitro bread digesta was separated into aliquots and frozen. Each day a sample was prepared (using the SPE protocol) and the abundance of P1-P6 determined using PRM MS. The values obtained were compared over subsequent days and coefficient of variation (CV) calculated using GraphPad. A CV of <10% indicates low day-to-day variability.
Defining the methods LOD and LOQ
The limit of detection (LOD) and limit of quantitation (LOQ) must be determined experimentally as they are unpredictable by regression modelling. The LOD is the lowest analyte concentration distinguishable from zero, and LOQ the lowest concentration that can be determined with quantitative accuracy [22]. The LOD and LOQ were defined for peptides P1-P6 using the method described by the International Council for Harmonisation of Technical Requirements1 (ICH) using ‘the Standard Deviation of the Response and the Slope’ .
Peptide P1-P6 were spiked (in triplicate) into a blank sample matrix previously purified by SPE (matrix matched). The sample was then diluted using SPE eluate at decreasing concentrations towards the limit of the assay, specifically to 10, 5, 2, 1, 0.5, 0.3, 0.1, 0.05, 0.03 and 0.01 µg mL−1. Each individual sample was injected and analysed using PRM MS at least five times [22]. The abundance (extracted ion current, defined using ‘Xcalibur Quant’) of each peptide was determined at each decreasing concentration; the LOQ was calculated using the standard deviation of the residuals as detailed in Eqs. (1) and (2) (for help with these calculations see https://www.youtube.com/watch?v=DXiGL72twow), and the LOD calculated using the standard deviation of the response and the slope as detailed in Eqs. (3) and (4) (for help with these calculations see https://www.youtube.com/watch?v=u7LCGkFuUFE).
| (1) |
S = standard deviation of the residuals.
n = number of data points.
γ = residual values.
= mean of the data values.
| (2) |
S = standard deviation of the residuals.
Slope = obtained by linear regression.
| (3) |
S = standard deviation of the residuals.
Slope = obtained by linear regression.
| (4) |
= mean value.
k = multiplication coefficient (10 [22]).
S = standard deviation of the mean.
Protocol for quantitative mass spectrometry
This optimised PRM and sample preparation method can accurately quantify peptides P1-P6 with an LOD between 0.027–0.161 µg mL−1 (peptide dependent) and LOQ between 0.218–0.769 (Table 6). As highlighted in Fig. 3, P1-P6 display a linear relationship between peptide concentration and peptide abundance between 0.5 µg mL−1 and 10 µg mL−1, which extends to the defined LOQ. As recommended by the ICH1, Table 7 displays the typical regression properties of the calibration curves used to extrapolate the peptide abundance to concentration. The PRM method has minimal day-to-day variability, which is highlighted in Table 8 by a CV of <10%. Additionally, P1-P6 do not show matrix effects due to the efficiency of the SPE sample preparation method described above. This accurate MS method builds on the relative MS method developed by van den Broeck 2005 [6]. The optimised protocol is detailed in full:
- 1.
-
2.An external standard curve was prepared using synthetic versions of P1-P6 between 0.5 and 10 µg mL−1.
-
i.The six individual synthetic peptides were pooled into a 50 µg mL−1 stock, then diluted serially with SPE eluate to 0.5, 1, 2, 5 and 10 µg mL−1.
-
i.
-
3.The column was preheated to 40 °C and equilibrated with 97% mo mobile phase A and 3% mobile phase B.
-
i.Mobile phase A composition: 99.9% water, 0.1% formic acid.
-
ii.Mobile phase B composition: 99.9% ACN, 0.1% formic acid.
-
i.
-
4.The 5 µg mL−1 stock of P1-P6 was initially injected into the column (2 µL) and data collected using the PRM MS method.
-
i.This was repeated three times comparing the peak retention times and fragmentation patterns of each sample to ensure instrument and chromatography replicability.
-
i.
-
5.
A blank sample was injected onto the column (2 µL) and analysed in PRM mode to ensure no carryover was occurring.
-
6.All samples digested with the INFOGEST protocol and prepared via SPE were then analysed using the PRM method.
-
i.The injection volume was always 2 µL.
-
ii.The P1-P6 standard curve (0.5–10 ppm) was run every 10–15 samples throughout.
-
i.
Table 6.
The LOD and LOQ for peptides P1-P6 using the quantitative PRM method described herein.
| Peptide | LOQ (µg mL−1) | LOD (µg mL−1) |
|---|---|---|
| P1 | 0.218 | 0.072 |
| P2 | 0.373 | 0.123 |
| P3 | 0.258 | 0.038 |
| P4 | 0.251 | 0.027 |
| 33mer (P5) | 0.312 | 0.029 |
| P6 | 0.769 | 0.161 |
Fig. 3.
Peptides P1-P6 demonstrate a linear relationship between peptide abundance and peptide concentration between 0.5 and 10 µg mL−1 allowing the use of linear regression to predict the concentration of peptides with known abundance. (A) P1, (B) P2, (C) P3, (D) P4, (E) P5, and (F) P6. At least seven replicates were analysed. Error bars display the standard deviation of the mean.
Table 7.
The properties of the linear regression modelling of P1-P6 as determined using the least sum of squares method. Pearson's correlation coefficient is displayed.
| Correlation coefficient | y-intercept | Slope | Residual sum of squares | |
|---|---|---|---|---|
| P1 | 0.99 | 404,809 | 3,405,436 | 1.71 × 1013 |
| P2 | 1.00 | 224,381 | 1,776,389 | 3.49 × 1013 |
| P3 | 1.00 | 63,340 | 1,637,587 | 7.22 × 1012 |
| P4 | 1.00 | −13,216 | 2,063,579 | 2.60 × 1013 |
| P5 | 0.99 | 16,042 | 420,669 | 7.21 × 1011 |
| P6 | 0.99 | −729,751 | 1,140,396 | 1.20 × 1013 |
Table 8.
Day-to-day replicability of the marker peptide concentration in the simulated digesta of bread detected using PRM MS. Samples were analysed each day in triplicate. The mean and CV represent the variation seen between days.
| Peptide | Mean concentration (µg mL−1) | CV (%) |
|---|---|---|
| P1 | 10.69 | 5.32 |
| P2 | 6.23 | 3.77 |
| P3 | 2.93 | 5.52 |
| P4 | 9.42 | 4.82 |
| P5 | 18.38 | 6.24 |
| P6 | 4.56 | 8.64 |
Table 9.
Chromatography schedule used for quantitative mass spectrometry of gluten.
| Elution time (minutes) | Percent of mobile phase B | Flow rate (mL min−1) |
|---|---|---|
| 0 | Inject sample | |
| 1.00 | 7 | 0.30 |
| 3.00 | 25 | 0.30 |
| 3.90 | 40 | 0.30 |
| 8.00 | 95 | 0.40 |
| 9.50 | 95 | 0.40 |
| 12.0 | 95 | 0.30 |
| 12.5 | 7 | 0.30 |
| 15.0 | 7 | End |
The raw PRM files were processed using the Xcalibur ‘Quan Browser’ (Thermo Fisher Scientific). Xcalibur integrates the total extracted ion current from the defined fragment ions for each precursor ion, then uses linear regression to calculate the peptide concentration from the standard curve.
-
1.
An automatic processing method (.pmd) was created in the Xcalibur ‘Processing Setup’ tool using the transitions in Table 10.
-
2.
The .pmd method was assigned as the ‘Proc Method’ in the Xcalibur ‘sequence setup’.
-
3.Data was processed using the automatic processing function in Xcalibur then viewed in the Quan Browser.
-
i.Both the standard curve and peak integration were visually validated.
-
i.
-
4.
Peptide concentrations were exported to Excel where the SPE dilution factor and P1H suppression were taken into account to produce the final concentration values.
Conclusion
The protocol described herein allows the user to simulate the digestion of bread (or gluten-containing food) and model the release profile of six immunogenic gluten peptides from within a food matrix. The use of the international standardised INFOGEST in vitro digestion protocol increases the relevance of the method to human gastrointestinal digestion and improves the inter-laboratory replicability. The detailed digestion-MS method can compare differences in immunogenic peptide release profile such as those induced by food processing or formulation. The digestion, sample preparation and ionisation parameters described within this manuscript can be applied to a discovery proteomics workflow, to identify and model the release profile of unknown immunogenic gluten peptides. The method development steps highlighted within this manuscript allow future users to incorporate further project-specific gluten peptides.
Acknowledgments
Thanks to the Riddet Institute, Plant & Food Research and The University of Auckland for providing funding and resources for this research. Thank you to Nigel Joyce (Plant & Food Research) for providing technical assistance.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Footnotes
International Conference on Harmonisation, Validation of Analytical Procedures: Text and Methodology Q2(R1), 2005.
References
- 1.Tye-Din J.A., Galipeau H.J., Agardh D. Celiac disease: a review of current concepts in pathogenesis, prevention and novel therapies. Front. Pediatr. 2018;6(350) doi: 10.3389/fped.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sollid L.M. Update 2020: nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4(+) T cells. Immunogenetics. 2020;72(1-2):85–88. doi: 10.1007/s00251-019-01141-w. [DOI] [PubMed] [Google Scholar]
- 3.Tye-Din J.A. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2010;2(41):41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 4.Smith F. Digestibility of gluten proteins is reduced by baking and enhanced by starch digestion. Mol. Nutr. Food Res. 2015;59(10):2034–2043. doi: 10.1002/mnfr.201500262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minekus M. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- 6.van den Broeck H.C. Label free targeted detection and quantification of celiac disease immunogenic epitopes by mass spectrometry. J. Chromatogr. A. 2015;1391(1):11. doi: 10.1016/j.chroma.2015.02.070. [DOI] [PubMed] [Google Scholar]
- 7.Egger L. Digestion of milk proteins: comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2019;118:32–39. doi: 10.1016/j.foodres.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Alves T.O. Modern approaches in the identification and quantification of immunogenic peptides in cereals by LC-MS/MS. Front. Plant Sci. 2019;10(1470) doi: 10.3389/fpls.2019.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moron B. Sensitive detection of cereal fractions that are toxic to celiac disease patients by using monoclonal antibodies to a main immunogenic wheat peptide. Am. J. Clin. Nutr. 2008;87(2):405–414. doi: 10.1093/ajcn/87.2.405. [DOI] [PubMed] [Google Scholar]
- 10.Schalk K. Quantitation of the 33-mer peptide from alpha-gliadins in wheat flours by LC-MS/MS. Anal. Res. Rep. 2016;7 doi: 10.1038/srep45092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sealey-Voyksner A.J. Novel aspects of quatitation of immunogenic wheat gluten peptides by liquid chromatography-mass spectrometry/mass spectrometry. J. Chromatogr. A. 2010;1217(1):16. doi: 10.1016/j.chroma.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 12.Prandi B. Qualitative and quantitative determination of peptides related to celiac disease in mixtures derived from different methods of simulated gastrointestinal digestion of wheat products. Anal. Bioanal. Chem. 2014;406(19):10. doi: 10.1007/s00216-014-7858-9. [DOI] [PubMed] [Google Scholar]
- 13.Bernfeld P. Amylases, α and β. Methods Enzymol. 1955;1(1):9. doi: 10.1016/0076-6879(55)01021-5. [DOI] [Google Scholar]
- 14.Anson M.L. The estimation of Pepsin, Trypsin, Papin and Cathepsin with Hemoglobin. J. Gen. Physiol. 1938;22(1):10. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel B.C.W. A modified spectrophotometric determination of chymotrypsin, trpysin and thrombin Canadian. J. Biochem. Physiol. 1959;37(12):6. doi: 10.1139/y59-157. [DOI] [PubMed] [Google Scholar]
- 16.Schalk K., Koehler P., Scherf K.A. Targeted liquid chromatography tandem mass spectrometry to quantitate wheat gluten using well-defined reference proteins. PLOS One. 2018;13(2) doi: 10.1371/journal.pone.0192804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamone G. Tracking the fate of pasta (T. Durum Semolina) immunogenic proteins by in vitro simulated digestion. J. Agric. Food Chem. 2015;63(10):7. doi: 10.1021/jf505461x. [DOI] [PubMed] [Google Scholar]
- 18.Picariello G. Proteomics, peptidomics, and immunogenic potential of wheat beer (Weissbier) J. Agric. Food Chem. 2015;63(13):3579–3586. doi: 10.1021/acs.jafc.5b00631. [DOI] [PubMed] [Google Scholar]
- 19.Annesley T.M. Ion suppression in mass spectrometry. Clin. Chem. 2003;49(7):1041–1044. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee S., Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2011;2012:40. doi: 10.1155/2012/282574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P.J. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry. Clin. Biochem. 2005;38(4):328–334. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Evard H. University of Tartu; Estonia: 2016. Estimating Limit of Detection For Mass Spectrometric Analysis methods, in Faculty of Science and Technology. [Google Scholar]