Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has developed into a pandemic with significant morbidity and mortality. SARS-CoV-2 has been reported to invade lung epithelium via the angiotensin-converting enzyme 2 (ACE2) receptor using its glycosylated cell surface spike protein [1]. ACE2 expression in the heart and kidney is regulated by the renin–angiotensin system (RAS), especially angiotensin II (A-II), which is catalysed from angiotensin I (A-I) by angiotensin-converting enzyme (ACE) [2]. In a cohort study in the early period of the COVID-19 outbreak in Wuhan in China, hypertension was found in 30% of the patients and was identified as the most common comorbidity [3]. It has recently been reported that RAS inhibitors are not associated with the severity of COVID-19 in a meta-analysis that included nine studies comprising 3936 patients with hypertension and COVID-19 [4]. The most serious concerns for the use of RAS inhibitors may be related to their role in development of or exacerbation of COVID-19, as suggested in a recent review by Ingraham et al. [5]. However, the alteration in ACE2 expression in pulmonary cells has not been studied.
Short abstract
Pulmonary expression of angiotensin-converting enzyme 2, which is a receptor of severe acute respiratory syndrome coronavirus 2, is not regulated by angiotensin II or renin–angiotensin system inhibitors #COVID19 https://bit.ly/3fkopuO
To the Editor:
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has developed into a pandemic with significant morbidity and mortality. SARS-CoV-2 has been reported to invade lung epithelium via the angiotensin-converting enzyme 2 (ACE2) receptor using its glycosylated cell surface spike protein [1]. ACE2 expression in the heart and kidney is regulated by the renin–angiotensin system (RAS), especially angiotensin II (A-II), which is catalysed from angiotensin I (A-I) by angiotensin-converting enzyme (ACE) [2]. In a cohort study in the early period of the COVID-19 outbreak in Wuhan in China, hypertension was found in 30% of the patients and was identified as the most common comorbidity [3]. It has recently been reported that RAS inhibitors are not associated with the severity of COVID-19 in a meta-analysis that included nine studies comprising 3936 patients with hypertension and COVID-19 [4]. The most serious concerns for the use of RAS inhibitors may be related to their role in development of or exacerbation of COVID-19, as suggested in a recent review by Ingraham et al. [5]. However, the alteration in ACE2 expression in pulmonary cells has not been studied.
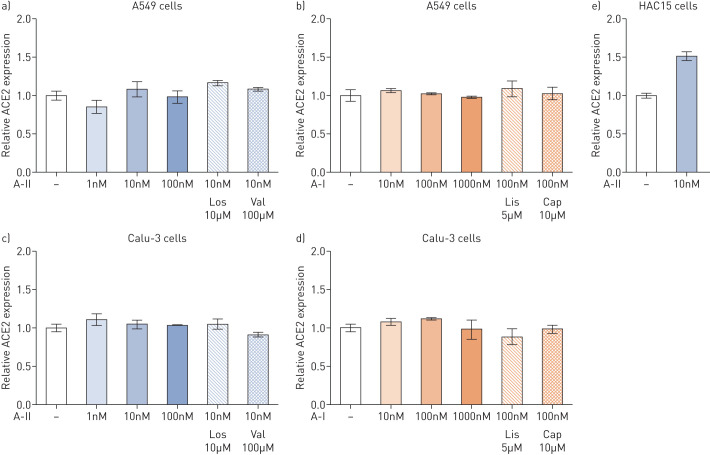
Therefore, we aimed to investigate whether ACE2 expression is regulated by RAS activation or inactivation in pulmonary cells. First, we stimulated a human nonsmall cell lung cancer cell line (Calu-3 cells) and a human alveolar adenocarcinoma cell line (A549 cells) with A-I and A-II. Then the A-I- and A-II-stimulated cells were treated with ACE inhibitors including lisinopril and captopril and A-II receptor Type 1 blockers including losartan and valsartan, respectively. RNA extraction and quantitative PCR (qPCR) assays were performed as previously reported [6]; qPCR primers used to target ACE2 were 5′-CGAAGCCGAAGACCTGTTCTA-3′ and 5′-GGGCAAGTGTGGACTGTTCC-3′. ACE2 expression levels were determined as arbitrary units normalised against GAPDH expression, which was measured as previously described [6], and the results were expressed as fold change relative to unstimulated control cells (figure 1).
FIGURE 1.
Effects of angiotensin I (A-I), angiotensin II (A-II), A-II Type 1 receptor blockers (ARB) and angiotensin-converting enzyme inhibitors (ACEi) on angiotensin-converting enzyme 2 (ACE2) expression in pulmonary and adrenal cells. a, b) A549 and c, d) Calu-3 cells were cultured in Eagle's Minimum Essential Medium and Dulbecco's Modified Eagle Medium (DMEM), respectively, with 10% fetal bovine serum until confluence. e) HAC15 cells were cultured in DMEM:F12 (1:1) supplemented with 10% Cosmic Calf serum until confluence. Cells were then incubated in serum-deprived medium with 0.1% serum for 24 h, after which the medium was replaced with fresh medium containing 0.1% serum with or without ARB or ACEi for 30 min. A-I or A-II were added to the medium, and cells were collected after 3 h of incubation for subsequent quantitative PCR assay. Statistical analyses were performed by one-way ANOVA; no significant differences were observed in A549 cells or Calu-3 cells (p>0.05). The difference was analysed by t-test in HAC15 cells: A-II stimulation significantly increased ACE2 expression (p<0.05). Results were expressed as fold change relative to unstimulated control cells. In each experiment, samples were assayed in triplicate. Three independent experiments were performed. Los: losartan; Val: valsartan; Lis: lisinopril; Cap: captopril.
As shown in figure 1, there was no alteration in ACE2 expression in Calu-3 and A549 cells under all conditions. To address ACE2 expression by A-II in other tissues, we investigated the effects of A-II on ACE2 expression in a human adrenocortical carcinoma (HAC15) cell line. A-II stimulation significantly increased ACE2 expression by 1.5-fold in the HAC15 cell line (p<0.05, figure 1).
Our results showed that activation or inactivation of RAS did not influence ACE2 expression in pulmonary cells, and thus RAS inhibitors are unlikely to alter ACE2 expression in lung epithelium. Even in the acute phase of severe COVID-19, when RAS is activated because of catecholamine release and/or hypovolaemia, ACE2 expression might not be upregulated in pulmonary cells. ACE2 and angiotensin II receptor Type 1 (AGTR1) expression in the human lung is considerably lower than that in the human adrenal gland [7]. Our in vitro study suggests that pulmonary expression of ACE2 is not influenced by A-II or RAS inhibitors.
Angiotensin 1–7, which is generated from A-II by ACE2, shows anti-inflammatory effects [8]. In a meta-analysis of four studies, Gao et al. [9] indicated that RAS inhibitor use tends to have a low risk of mortality in COVID-19. ACE2 upregulation induced by RAS inhibitors may increase the systemic circulation of angiotensin 1–7 and thus may lead to a protective effect against severe COVID-19.
The current study has several limitations. Since this study was performed only in vitro using cancer-derived cell lines, primary lung epithelial cells or airway epithelial cells incubated using an air–liquid interface would be more efficient in clarifying ACE2 expression [10]. Human lung epithelial cells might regulate ACE2 expression differently. Furthermore, chronic effects of A-II or RAS inhibitors were not examined in this study. Further studies are needed to investigate the association between RAS and pulmonary ACE2 expression in vivo and in normal lung epithelial cells. The functional mechanisms involved other than ACE2 expression, such as viral entry and replication, also need to be clarified in the development or exacerbation of COVID-19.
At this point in time, our findings support the recommendation of most medical societies not to withdraw RAS inhibitors to avoid COVID-19. Although we could not show conclusively that RAS inhibitors were not associated with the development or exacerbation of COVID-19, we believe that this study could inform future studies related to COVID-19.
Footnotes
Conflict of interest: R. Baba has nothing to disclose.
Conflict of interest: K. Oki has nothing to disclose.
Conflict of interest: K. Itcho has nothing to disclose.
Conflict of interest: K. Kobuke has nothing to disclose.
Conflict of interest: G. Nagano has nothing to disclose.
Conflict of interest: H. Ohno has nothing to disclose.
Conflict of interest: M. Yoneda has nothing to disclose.
Conflict of interest: N. Hattori has nothing to disclose.
Support statement: This study was financially supported by JSPS KAKENHI Grant Number JP17K09883 (to K. Oki). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002; 417: 822–828. doi: 10.1038/nature00786 [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Zhu Y, Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension 2020; 76: e13–e14. [DOI] [PubMed] [Google Scholar]
- 5.Ingraham NE, Barakat AG, Reilkoff R, et al. Understanding the renin–angiotensin–aldosterone–SARS-CoV axis: a comprehensive review. Eur Respir J 2020; 56: 2000912. doi: 10.1183/13993003.00912-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itcho K, Oki K, Kobuke K, et al. Angiotensin 1-7 suppresses angiotensin II mediated aldosterone production via JAK/STAT signaling inhibition. J Steroid Biochem Mol Biol 2019; 185: 137–141. doi: 10.1016/j.jsbmb.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 7.Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 2014; 13: 397–406. doi: 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasoni D, Italia L, Adamo M, et al. COVID 19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail 2020; 22: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J 2020; 41: 2058–2066. doi: 10.1093/eurheartj/ehaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Rostami MR, Leopold PL, et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med 2020; 202: 219–229. doi: 10.1164/rccm.202003-0541OC [DOI] [PMC free article] [PubMed] [Google Scholar]