In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as the causative agent for coronavirus disease 2019 (COVID-19), a clinical syndrome with high incidence of acute respiratory distress syndrome (ARDS). Part of the pathophysiology of COVID-19-induced ARDS is explained by increased dead space ventilation from pulmonary microthrombi; this is supported by the existence of alveolar capillary microthrombi found on autopsies [1, 2]. This has prompted the use of therapeutic anticoagulation and thrombolytics [3–5]. Thus far, no clinical trials have been completed to evaluate the use of recombinant tissue-type plasminogen activator (rt-PA) to treat COVID-19. The objective of this study was to determine whether rt-PA administration decreases dead space ventilation in patients with COVID-19.
Short abstract
#COVID19-induced ARDS is partly explained by the presence of microthrombi, motivating the use of thrombolytics. This study shows that thrombolytics decrease dead space ventilation in COVID-19 ARDS patients. https://bit.ly/2GdM44a
To the Editor:
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as the causative agent for coronavirus disease 2019 (COVID-19), a clinical syndrome with high incidence of acute respiratory distress syndrome (ARDS). Part of the pathophysiology of COVID-19-induced ARDS is explained by increased dead space ventilation from pulmonary microthrombi; this is supported by the existence of alveolar capillary microthrombi found on autopsies [1, 2]. This has prompted the use of therapeutic anticoagulation and thrombolytics [3–5]. Thus far, no clinical trials have been completed to evaluate the use of recombinant tissue-type plasminogen activator (rt-PA) to treat COVID-19. The objective of this study was to determine whether rt-PA administration decreases dead space ventilation in patients with COVID-19.
Charts from COVID-19-positive patients admitted to the intensive care unit at Robert Wood Johnson University Hospital, New Brunswick, NJ, USA, between 24 March 2020 and 24 May 2020 were retrospectively reviewed. The study was approved by the Rutgers institutional review board (IRB #Pro2020000766). Inclusion criteria were a positive COVID-19 PCR test, Berlin criteria for ARDS and treatment with rt-PA [6]. Exclusion criteria included rt-PA administration in a peri-cardiac arrest period or unavailability of minute ventilation (V′E) or arterial blood gas. If a patient received repeat doses of rt-PA separated by >48 h, both data sets were included.
The primary outcome of our study was the change in dead space ventilation after rt-PA. According to Enghoff's method, physiological dead space ventilation is calculated with the equation VD/VT = (PaCO2−PECO2)/PaCO2 (where VD is the dead space volume, VT is the tidal volume, PaCO2 is the arterial carbon dioxide tension and PECO2 is the expired carbon dioxide tension), which in the absence of capnography can be approximated to the ventilatory ratio (PaCO2×V′E)/(predicted body weight×100×37.5) [7, 8]. We approximated dead space ventilation using the equation VD/VT ≈ PaCO2×V′E. Based on previous studies, the pharmacodynamics of rt-PA and haemodynamic improvements 24 and 48 h after administration, we elected to analyse dead space ventilation immediately at the end of the rt-PA infusion, and after 12, 24 and 48 h [9, 10]. Secondary outcome measurements included the ratio of arterial oxygen tension (PaO2) to inspiratory oxygen fraction (FIO2) before rt-PA, immediately after rt-PA and after 12, 24 and 48 h, and correlation between changes in dead space ventilation with timing of rt-PA administration, fibrinogen and D-dimer levels. Changes in dead space ventilation and oxygenation were calculated as the percentage difference between values before and after thrombolytic therapy and analysed using a paired two-tailed t-test. Correlations between variables were determined using Pearson correlation coefficients.
Between 24 March 2020 and 24 May 2020, 29 COVID-19-positive patients were treated with rt-PA. 14 patients were not included (five were administered rt-PA in the peri-arrest period and nine did not have available data). In total, 15 patients and 18 doses of rt-PA were included in the analysis, as three patients had rt-PA re-dosed (>48 h after the first dose, thus classified as a new dose). 13 patients were therapeutically anticoagulated prior to rt-PA administration, the mean first dose of rt-PA was 42 mg administered over a mean time of 136 min, five patients were started on a rt-PA drip after the first dose of rt-PA was given (mean dose 27 mg, mean time 9 h), and all of the patients were started on therapeutic heparin after the rt-PA infusion.
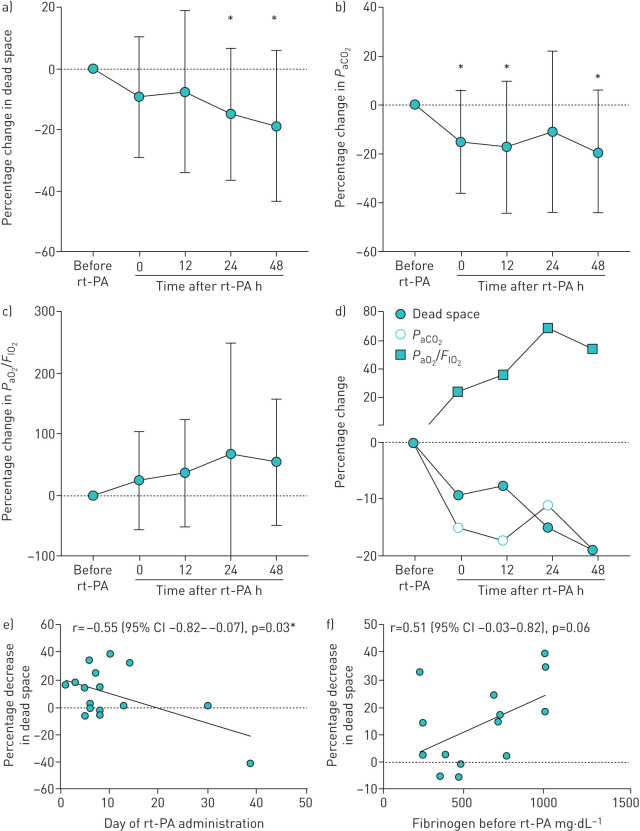
Physiological dead space significantly decreased 24 and 48 h after rt-PA administration, with a change of −14.91% (95% CI −26.45– −3.37%, p=0.015) and −18.87% (95% CI −34.50– −3.23%, p=0.022), respectively. Physiological dead space decreased nonsignificantly at 0 and 12 h after rt-PA administration (−9.23% (95% CI −19.74–1.275%, p=0.08) and −7.57% (95% CI −23.53–8.39%, p=0.32), respectively) (figure 1a). PaCO2 followed the same trend as dead space ventilation, with a significant decrease after rt-PA administration: −15.06% (95% CI −26.25– −3.89%, p=0.01), −17.30% (95% CI −33.46– −1.14%, p=0.04), −11.07% (95% CI −28.65–6.50%, p=0.20) and −19.29% (95% CI −35.26– −3.33%, p=0.02) (figure 1b) at 0, 12, 24 and 48 h after rt-PA, respectively. To account for changes in mechanical ventilation that may have affected dead space, positive end-expiratory pressure (PEEP) and V′E before and after rt-PA were compared and no significant differences were noted. PaO2/FIO2 ratio after rt-PA administration increased nonsignificantly by 24.05% (95% CI −18.77–66.88%, p=0.25), 36.08% (95% CI −14.97–87.13%, p=0.15), 68.59% (95% CI −24.31–161.5%, p=0.14) and 54.49% (95% CI −7.89–116.9%, p=0.23) at 0, 12, 24 and 48 h after rt-PA administration, respectively (figure 1c). Decrease in dead space ventilation was inversely correlated with the timing of rt-PA administration (the earlier in the hospital course the rt-PA was administered, the greater the decrease in dead space) (figure 1e), with r=−0.55 (95% CI −0.82– −0.07, p=0.03), r=−0.16 (95% CI −0.65–0.43, p=0.60), r=−0.13 (95% CI −0.59–0.39, p=0.62) and r=−0.51 (95% CI −0.84–0.08, p=0.09) at 0, 12, 24 and 48 h after rt-PA administration, respectively. The mean fibrinogen level before rt-PA administration was 601 mg·dL−1 and was not significantly decreased after rt-PA (493 mg·dL−1; p=0.07). The decrease in dead space was positively correlated with the level of fibrinogen before rt-PA (figure 1f), with r=0.51 (95% CI −0.03–0.82, p=0.06), r=0.70 (95% CI 0.21–0.91, p=0.01), r=0.54 (95% CI 0.01–0.83, p=0.05) and r=0.42 (95% CI −0.28–0.83, p=0.22) at 0, 12, 24 and 48 h post rt-PA respectively. The mean D-dimer level before rt-PA was 18 059 ng·mL−1 and significantly increased to 47 809 ng·mL−1 after rt-PA (p=0.001). There was an indication that the greater the increase in D-dimer levels after rt-PA, the greater the decrease in dead space; however, none of these analyses were significant. Of the 15 patients who received rt-PA, there were two bleeding events (intra-muscular and intra-cranial haemorrhage). There was no association between the cumulative dose of rt-PA and the incidence of bleeding.
FIGURE 1.
a–d) Evolution of dead space, arterial carbon dioxide tension (PaCO2) and the ratio of arterial oxygen tension (PaO2) to inspiratory oxygen fraction (FIO2), after recombinant tissue-type plasminogen activator (rt-PA) administration. Data are presented as means with standard deviations (dots and whiskers, respectively). a) Dead space (approximated by the equation PaCO2×minute ventilation) was significantly decreased at 24 and 48 h after rt-PA. b) PaCO2 was significantly decreased at 0, 12 and 48 h after rt-PA administration. c) PaO2/FIO2 ratio was not significantly increased after rt-PA administration. d) Dead space, PaCO2 and PaO2/FIO2 ratio after rt-PA administration, means plotted together. e and f) Correlations between dead space decrement immediately after rt-PA administration and e) timing of rt-PA administration (day during hospital stay) and f) fibrinogen level before rt-PA. Earlier administration of rt-PA and higher fibrinogen level before rt-PA correlated with a greater decrement in dead space. *: p<0.05.
The finding of diffuse alveolar microthrombi in COVID-19 patients suggests a potential benefit of anticoagulation and thrombolytics [1, 5, 11]. The use of thrombolytics in ARDS has been previously described [12]. In our study, thrombolytic therapy significantly decreased physiological dead space in our COVID-19-positive patients with ARDS. While we did not find a significant improvement in PaO2/FIO2 ratio, there was a trend toward improved oxygenation after thrombolytic therapy. The greater improvement in ventilation, as opposed to oxygenation, may be explained by treatment of microthrombi without changes to the alveolar epithelial damage observed in COVID-19 [13]. It was noted that patients with higher fibrinogen levels seemed to be the best candidates for thrombolytic therapy. This is not surprising, as high fibrinogen levels are associated with increased annexin-A2 receptors, enabling binding of rt-PA and fibrinolysis [14]. Thrombolytic efficiency was time sensitive, with improved outcomes when delivered earlier. Improvement in dead space ventilation has the potential to decrease the tidal volume and respiratory rates, and therefore the mechanical power, promoting lung protective ventilation strategies [15]. This study is limited by its retrospective nature and small number of subjects, and the potential for treatment bias. To limit treatment bias, we accounted for PEEP and V′E before and after rt-PA and did not note a significant difference. However, unadjusted variables such as paralytic therapy and sedation regimens may have influenced ventilation by improving patient–ventilator synchrony. Lastly, patients received different doses of rt-PA but we did not demonstrate an association between dose of rt-PA and dead space reduction.
In conclusion, the use of rt-PA in a COVID-19 population decreases dead space ventilation. It is unclear if this intervention leads to improved clinical outcomes, but our results support further investigation with randomised controlled trials to assess safety and efficiency of thrombolytic therapy as a treatment for COVID-19 ARDS.
Footnotes
Author contributions: S. Orfanos, I. El Husseini, J. Radbel and S. Hussain designed the study; S. Orfanos, I. El Husseini, J. Radbel, T. Nahass and S. Hussain collected and analysed data; S. Orfanos and I. El Husseini wrote the manuscript; J. Radbel and S. Hussain reviewed the manuscript. All authors approved the final version of the manuscript.
Conflict of interest: S. Orfanos has nothing to disclose.
Conflict of interest: I. El Husseini has nothing to disclose.
Conflict of interest: T. Nahass has nothing to disclose.
Conflict of interest: J. Radbel has nothing to disclose.
Conflict of interest: S. Hussain has nothing to disclose.
References
- 1.Ackermann M, Verleden SE, Kuehnel M, et al. . Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menter T, Haslbauer JD, Nienhold R, et al. . Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020; 77: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whyte CS, Morrow GB, Mitchell JL, et al. . Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemos 2020; 18: 1548–1555. doi: 10.1111/jth.14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Hajizadeh N, Moore EE, et al. . Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost 2020; 18: 1752–1755. doi: 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett CD, Oren-Grinberg A, Chao E, et al. . Rescue therapy for severe COVID-19-associated acute respiratory distress syndrome with tissue plasminogen activator: a case series. J Trauma Acute Care Surg 2020; 89: 453–457. doi: 10.1097/TA.0000000000002786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranieri VM, Rubenfeld GD, Thompson BT, et al. . Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 7.Ferluga M, Lucangelo U, Blanch L. Dead space in acute respiratory distress syndrome. Ann Transl Med 2018; 6: 388. doi: 10.21037/atm.2018.09.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Calfee CS, Beitler JR, et al. . Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med 2019; 199: 333–341. doi: 10.1164/rccm.201804-0692OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhaber SZ, Haire WD, Feldstein ML, et al. . Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341: 507–511. doi: 10.1016/0140-6736(93)90274-K [DOI] [PubMed] [Google Scholar]
- 10.Kucher N, Boekstegers P, Müller OJ, et al. . Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129: 479–486. doi: 10.1161/CIRCULATIONAHA.113.005544 [DOI] [PubMed] [Google Scholar]
- 11.Moore HB, Barrett CD, Moore EE, et al. . Is there a role for tissue plasminogen activator as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome? J Trauma Acute Care Surg 2020; 88: 713–714. doi: 10.1097/TA.0000000000002694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardaway RM, Harke H, Tyroch AH, et al. . Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg 2001; 67: 377–382. [PubMed] [Google Scholar]
- 13.Calkovska A, Mokra D, Calkovsky V. Lung surfactant alterations in pulmonary thromboembolism. Eur J Med Res 2009; 14: Suppl. 4, 38–41. doi: 10.1186/2047-783X-14-S4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev 2015; 29: 17–24. doi: 10.1016/j.blre.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva PL, Ball L, Rocco PRM, et al. . Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med Exp 2019; 7: Suppl. 1, 38. doi: 10.1186/s40635-019-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]