Abstract
Background
In suckling piglets, transmissible gastroenteritis virus (TGEV) causes lethal diarrhea accompanied by high infection and mortality rates, leading to considerable economic losses. This study explored methods of preventing or inhibiting their production. Bovine antimicrobial peptide-13 (APB-13) has antibacterial, antiviral, and immune functions.
Objectives
This study analyzed the efficacy of APB-13 against TGEV through in vivo and in vitro experiments.
Methods
The effects of APB-13 toxicity and virus inhibition rate on swine testicular (ST) cells were detected using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT). The impact of APB-13 on virus replication was examined through the 50% tissue culture infective dose (TCID50). The mRNA and protein levels were investigated by real-time quantitative polymerase chain reaction and western blot (WB). Tissue sections were used to detect intestinal morphological development.
Results
The safe and effective concentration range of APB-13 on ST cells ranged from 0 to 62.5 µg/mL, and the highest viral inhibitory rate of APB-13 was 74.1%. The log10TCID50 of 62.5 µg/mL APB-13 was 3.63 lower than that of the virus control. The mRNA and protein expression at 62.5 µg/mL APB-13 was significantly lower than that of the virus control at 24 hpi. Piglets in the APB-13 group showed significantly lower viral shedding than that in the virus control group, and the pathological tissue sections of the jejunum morphology revealed significant differences between the groups.
Conclusions
APB-13 exhibited good antiviral effects on TGEV in vivo and in vitro.
Keywords: Transmissible gastroenteritis virus, infections, viruses, RNA messenger
INTRODUCTION
The porcine transmissible gastroenteritis virus (TGEV) is a coronavirus that causes severe diarrhea, vomiting, and dehydration in piglets less than 2 weeks old, and it has high morbidity and mortality rates [1]. TGEV infections usually occur in winter and early spring [2] and cause substantial economic losses to the swine industry. TGEV is a positive single-stranded RNA virus of the Coronavirus family that has 4 structural proteins, including the spike (S), membrane (M), nucleocapsid (N), and envelope (E) proteins, as well as 5 non-structural proteins [3,4]. The N protein plays a vital role in the replication and transcription of viral RNA [5]. Several experiments have shown that many substances inhibit the replication of TGEV by impeding the expression of its related proteins and mRNA [6,7]. Currently, there are no specific drugs for the prevention and control of TGEV infections. Although a vaccine is used to prevent and control the TGEV, the effects of the vaccine are unsatisfactory. The TEGV causes an intestinal disease primarily in piglets, and maternal antibodies provide insufficient protection for piglets. Therefore, it is particularly important to find drugs or reagents that can treat TGEV infections effectively.
Indolicidin is a cationic 13-residue antimicrobial peptide (ILPWKWPWWPWRR-NH(2)) that is unusually rich in tryptophan and proline. Previous studies reported that antimicrobial peptides have antibacterial effects [8], antiviral effects [9,10], and anti-parasitic actions [11]. Bovine antimicrobial peptides-13 (APB-13) also have antibacterial [12], antiviral [13] and anticancer [14] activity. Indolicidin from the bovine cathelicidins reduces the herpes simplex virus (HSV)-1 and HSV-2 yields by 99% and decreases the infectivity of a Junin virus (JV) suspension by 50% compared to the untreated control sample [15]. Moreover, indolicidin can directly inactivate the virus particles for human immunodeficiency virus (HIV)-1 [16]. On the other hand, the effects of APB-13 on TGEV replication have not been reported. Antibacterial peptides are popular because of their non-toxic, non-residual, safe, and effective characteristics, and they are ideal substitutes for antibiotics in antibiotic-free breeding. The purpose of this research was to determine if APB-13 has an antiviral effect on the TGEV and, if so, to elucidate its mechanism of action. This study will be of great significance as a reference for the control of porcine transmissible gastroenteritis.
MATERIALS AND METHODS
Ethics statement
The research protocols for the experiments on live pigs in this study were approved by the Animal Care and Use Committee of Henan Agricultural University (Zhengzhou, China) (No. HAU20181120-6). All surgeries were performed under anesthesia using isoflurane, and all attempts were made to minimize animal suffering.
APB-13, cells, and virus
The sequence for APB-13 is ILPWKWPWWPWRR-NH2, and the peptide was derived from cattle. The peptides used in this study were synthesized by GL Biochemistry (China). The working solutions of APB-13 were prepared in fetal bovine serum (FBS)-free Dulbecco's modified Eagle's medium (DMEM) and 1% penicillin/streptomycin (Biochrom, Germany). APB-13 comes in liquid and lyophilized powder forms, and the activity of both is equivalent.
Swine testicular (ST) cells were grown in DMEM supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. Professor Zhanyong Wei of Henan Agricultural University isolated and identified the TGEV HN-2012 strain, which was grown in ST cells in the current study. Monolayers of ST cells in 75 cm2 cell culture flasks were infected with TGEV at a multiplicity of infection (MOI) of 5. When an 80% cytopathic effect emerged at 24 h post-infection (hpi) at 37°C, the flask was stored at −80°C and freeze-thawed 3 times; the supernatant was then harvested. All in vitro infection experiments were performed at an MOI of 5. All experiments related to the TGEV were performed in a P2 biosafety laboratory and carried out in strict accordance with the Laboratory Biosafety Manual of the authors' laboratory.
Cellular toxicity assays
The viability and growth of ST cells were tested in vitro using a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay, as reported previously [17,18]. Different concentrations of APB-13 dissolved in serum-free DMEM were added to 90% confluent ST cells in 9 6-well plates for 24 h at 37°C with 5% CO2. Subsequently, 15 µL of MTT reagent (5 mg/mL) was added to each well for 4 h of incubation at 37°C. After the medium was discarded, 200 µL of a dimethyl sulfoxide (DMSO) solution was added, and the plates were oscillated for 10 min. The mean optical density (OD) at 570 nm was measured. The relative cell viability was counted as a percentage of that of the control based on the mean OD. The MTT assays were performed in triplicate, and 8 wells were repeated per plate.
Viral titer assay
The ST cells were seeded in 96-well cell culture plates and incubated until they reached 90–100% confluence. The monolayers were washed twice with D-Hank's solution and then infected with 100 µL of 10-fold serial dilutions of the virus samples. The dilution range of TGEV was 10−1 to 10−12, with 8 replicates per dilution. After incubation for 72 h, the viral titers in the wells were calculated as the 50% tissue culture infective dose (TCID50)/mL using the Reed-Muench method [19].
Antiviral activity effect of APB-13 on TGEV in vitro
The cells were cultured in a 96-well plate for 24 h and grown into a monolayer. The cells were washed and infected with TGEV for 1 h. The diluted virus was discarded, and then the cells were washed twice. Four different safe and effective concentrations of APB-13 (150 µL) were added to the cells (8 replicates per concentration) and incubated for 24 h. The virus controls and cell controls were established at the same time. Subsequently, 10 µL MTT reagent (5 mg/mL) was added and incubated for another 4 h. The medium was then discarded, and 200 µL of DMSO was added and incubated for 10 min with oscillation. The mean OD at 570 nm was measured. The viral inhibition rate was calculated using the following formula:
ST Monolayers in 6-well plates were infected with the TGEV for 1 h. After washing twice, various concentrations of APB-13 were added to a 6-well plate, and the cells were incubated for 23 h. The plates were freeze-thawed 3 times at −80°C, and the viral titer was determined using a TCID50 assay. Each sample was measured in triplicate from 3 independent experiments.
Inhibitory effect of APB-13 on TGEV proliferation in vitro
Pretreatment effect of APB-13 on TGEV
Monolayer ST cells (90% confluent) were washed twice. Different concentrations of APB-13 and FBS-free DMEM were added to 6-well plates containing cells for pretreatment for 3 h or 6 h. The ST cells were then infected with the TGEV and incubated for 1 h at 37°C. After the cells were washed and the diluted virus was removed, FBS-free DMEM was added to the 6-well plates, and the plates were then incubated at 37°C with 5% CO2. The cell control and virus control samples were established at the same time (3 replicates). After incubation for 23 h, the plates were freeze-thawed 3 times at −80°C. The mixtures were harvested, and the viral titer was tested using a TCID50 assay.
Attachment assay
Equal volumes of TGEV and APB-13 in a total volume of 1 mL was added to the monolayer cells and incubated for 1 h at 4°C. The unabsorbed viruses were removed by washing twice. The attachment was stopped, and 2 mL of FBS-free DMEM was added to the 6-well plates. The plates were placed at 37°C with 5% CO2 for 23 h. The cell control and virus control samples were established at the same time (3 replicates). The next steps were the same as those described above [20].
Penetration assay
Confluent monolayers of ST cells were infected with the TGEV for 1 h at 4°C. At this temperature, the virus can only attach to the cell surface but not penetrate the cells. The non-adsorbed virus was removed by washing twice with D-Hank's solution. Different concentrations of APB-13 or FBS-DMEM were added to each 6-well plate, and the plate was incubated at 37°C with 5% CO2 for 3 h. The cell control and virus control samples were established at the same time (3 replicates). The remaining steps were similar to those in the prevention assay [21].
TGEV replication and release assay
Confluent monolayers of ST cells were infected with TGEV for 3 h or 6 h. After washing twice, different concentrations of APB-13 and FBS-free DMEM were added to a 6-well plate at 37°C with 5% CO2 for 21 h and 18 h. The remaining steps were similar to those in the prevention assay [22].
Direct effect of APB-13 on TGEV particles
Equal quantities of different concentrations of APB-13 were mixed thoroughly with the TGEV in a total volume of 1 mL. The mixtures were incubated for 2 h at 37°C with 5% CO2. FBS-free DMEM control and virus control samples were established at the same time (3 replicates). The viral titers of the mixtures were determined using a TCID50 assay [20].
Influence of APB-13 on TGEV N protein
mRNA level
ST cells were seeded in 6-well plates, cultivated, and infected with the TGEV. Different concentrations of APB-13 were added, and ST cells were collected from each well after infection for the indicated period. The total RNA was extracted from the TGEV-infected ST cells with TRIzol Reagent (Invitrogen, USA) according to the manufacturer's protocol. The RNA was then reverse-transcribed with PrimeScript™ RT Master Mix (Takara, Japan) according to the manufacturer's instructions. Real-time quantitative polymerase chain reaction (RT-qPCR) analysis was carried out to amplify the N protein gene using cDNA as the template, and the β-actin gene was used as the internal standard. The thermal cycling parameters were as follows: 5 min at 95°C, 10 sec at 95°C, and 40 cycles of 15 sec at 58°C and 20 sec at 72°C followed by 10 sec at 95°Cand 5 sec at 65°C. The data analysis was based on measurements of the cycle threshold (Ct).
Protein level
The ST cells infected with and without the TGEV were washed twice with cold D-Hank's solution, harvested with a cell scraper, and centrifuged at 4°C for 10 min. The pellets were suspended in cold lysis buffer (Beyotime, China) containing the protease inhibitor, phenylmethylsulfonyl fluoride (PMSF; 0.5 µm). The protein concentration of the lysates was determined by BCA protein assay kit (CW Biotech, China) and equalized with phosphate-buffered saline. Equivalent amounts of proteins were suspended in a sodium dodecyl sulfate (SDS) sample buffer containing 100 mM 4-dithiothreitol (Sigma-Aldrich) and boiled at 100°C for 10 min. They were then separated on 12% SDS-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes (ISEQ00010; Millipore, USA). The membranes were blocked for 2 h in Tris-buffered saline containing 5% non-fat dry milk at room temperature and incubated overnight with the rabbit anti-TGEV-N polyclonal antibody (Sangon Biotech, China) as the primary antibodies (1:3,000) and a GAPDH antibody (Sangon Biotech) as the internal control antibody (1:2,000) at 4°C. After thorough washing with Tris-buffered saline with Tween-20, the membranes were reacted with the horseradish peroxidase-conjugated goat anti-rabbit IgG (Sangon Biotech) at 4°C for 1.5 h, and the proteins were determined with 3,3-diaminobenzidine and detected by enhanced chemiluminescence (Genshare Biological, China) and autoradiography.
Antiviral effect of antibacterial peptides on piglets
Pigs and feed
Newborn 4-day-old crossbred pigs (Duroc × Changbai × Big White) were purchased from a sow farm in Anyang, Henan Province, and confirmed to be healthy piglets. Artificial milk substitute was purchased from Beijing Aidi Technology Co., Ltd. (China).
In vivo test design of the pigs
The 27 healthy piglets used in the experiment had uniform body weights. The piglets were divided randomly into 3 groups with 3 replicates per group and 3 pigs per replicate: the mock infection group (MOCK), the virus-only group (TGEV), and the APB-13-treated group (TGEV+APB-13). APB-13 (10 g/kg) was fed to the piglets from the date of purchase. On the night of the sixth day, the piglets in all 3 groups were orally inoculated with 10 mL of TGEV (1×108 TCID50/mL) or 10 mL of DMEM per head. The 3 groups were kept in different animal test rooms and managed by different breeders. The pre-test period was 2.5 days, and the test period was 4 days.
Animal sample collection and processing
During the test period, fecal samples were taken from the rectum and placed quickly at −80°C every day, the fecal samples were used to detect the amount of TGEV mRNA by RT-qPCR. At the end of the experiment, the piglets were euthanized. The duodenum, jejunum, and ileum sections were collected and stored in a 12% formalin solution to prepare the pathology sections.
Intestinal tissue morphology detection
The fixed duodenal, jejunal and ileal tissue samples were subjected to a series of treatments, such as dicing, flushing, dehydration, clearing, waxing, embedding, sectioning, mounting, dewaxing, and hematoxylin & eosin (H&E) staining, and the small intestinal morphology was observed under an optical microscope (DMi8; Leica, Germany). A typical field of view was selected for imaging, and a Leica Q-Win image analysis system was used to measure the villus height (VH, the vertical height of the villus from the root) and crypt depth (CD, the distance from the root to the basal layer) and to calculate the villus/crypt ratio (VH/CD).
Statistics
Data were expressed as the mean ± SD. Statistical analyses were performed using GraphPad Prism 6.01. The significance was determined by an analysis of the variance with thresholds of p < 0.05 and p < 0.01.
RESULTS
Fifty percent cytotoxicity value (CC50) of APB-13 on ST cells
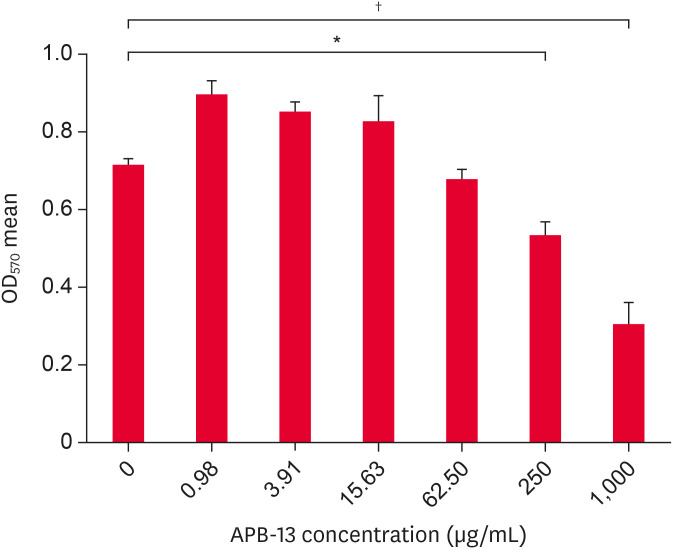
The cytotoxicity of APB-13 was detected using an MTT assay. APB-13 was diluted by 4-fold serial dilutions of FBS-free DMEM, with concentrations of 1,000 µg/mL (C1), 250 µg/mL (C2), 62.50 µg/mL (C3), 15.63 µg/mL (C4), 3.91 µg/mL (C5), and 0.98 µg/mL (C6). The results showed that the APB-13 concentrations from 0 to 62.5 µg/mL were relatively non-toxic to the cells, and the cell states and mean OD values of the ST cells treated with APB-13 for these concentrations were similar to those of the control cells. The concentrations of C4, C5, and C6 of APB-13 could promote ST cell growth (Fig. 1). The CC50 of APB-13 was 603 µg/mL on ST cells.
Fig. 1. APB-13 toxicity in ST cells. ST cells were cultured in 96-well plates and incubated with different concentrations (µg/mL) of APB-13 for 24 h. The mean cell OD was detected using an MTT assay. The concentration of APB-13 1,000 and 250 µg/mL had significantly greater cytotoxicity than the control (p < 0.01). The concentration of APB-13 62.50 to 0.98 µg/mL was almost non-toxic and even had growth-promoting effects on cells. The data shown are the mean ± SD of 8 replicates per concentration.
ST, swine testicular; APB-13, antimicrobial peptides-13; OD, optical density; MTT, 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide.
*p < 0.05; †p < 0.01.
In vitro inhibitory effect of APB-13 on TGEV
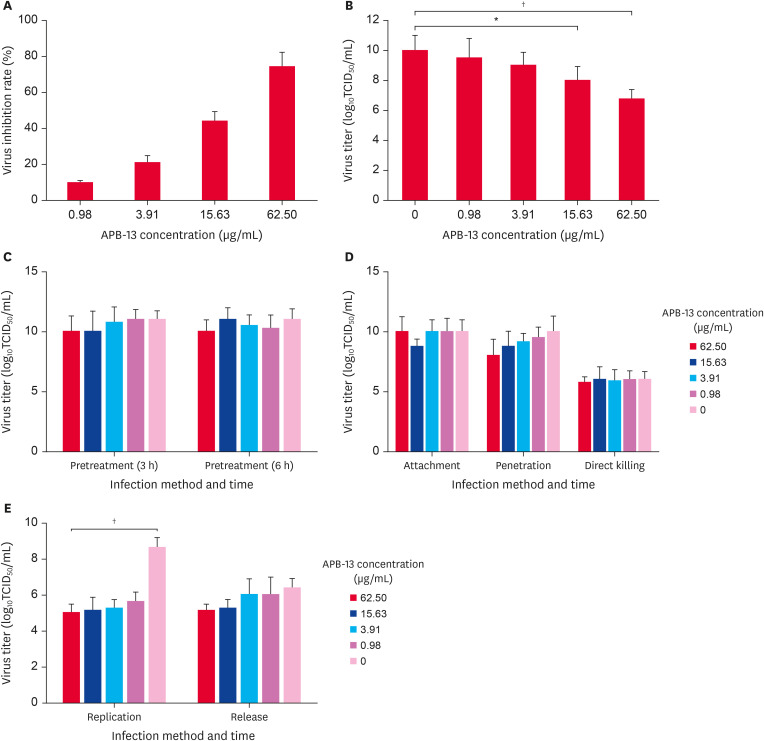
APB-13 had a significant inhibitory effect on the TGEV, and the viral inhibitory rates of APB-13 at different concentrations were 9.6%, 20.8%, 43.9%, and 74.1% (Fig. 2A). The log10TCID50 of APB-13 62.50 (p < 0.01) and 15.63 µg/mL (p < 0.05) were reduced significantly by 3.25 and 2, respectively, compared to the virus control (Fig. 2B).
Fig. 2. Antivirus activity of APB-13 on TGEV. (A) Viral inhibition rate of APB-13 on TGEV. ST cells were cultured in 96-well plates, and 100 µL APB-13 (62.50, 15.63, 3.91, and 0.98 µg/mL) was added after infection with the TGEV (MOI = 5) for 1 h. After 24 hpi, the mean cell OD was detected using an MTT assay. The data are shown as the mean ± SD of 8 replicates per concentration. (B) ST cell monolayers were infected with TGEV (MOI = 5) for 1 h, and then various concentrations of APB-13 were added to a 6-well plate for incubation. After 23 hpi, the mixtures of supernatant and cells were harvested for estimating TCID50 assay. The data shown are the mean ± SD of 3 replicates per concentration. (C-E) TGEV-infected (MOI = 5) ST cells were cultured in 6-well plates. APB-13 was added at -3, -6, 0, 1, 3, and 6 h after infection. The effects of the APB-13 treatment on the pretreatment (3 h), pretreatment (6 h), attachment, penetration, replication, and release of TGEV were examined. The mixtures of APB-13 and TGEV in a cell-free plate were used to detect the direct killing effect. At the indicated time, the 6-well plates were transferred to − 80°C and freeze-thawed 3 times. The cell and supernatant mixture in each well was collected to measure the viral titer using TCID50 assay. The difference between the APB-13 62.50 and 0 µg/mL during the replication phase was extremely significant (p < 0.01). The data shown are the mean ± SD of 3 independent experiments.
APB-13, antimicrobial peptides-13; TGEV, transmissible gastroenteritis virus; ST, swine testicular; MOI, multiplicity of infection; hpi, h post-infection; TCID50, 50% tissue culture infective dose.
*p < 0.05; †p < 0.01.
The stage of the TGEV proliferation process that was inhibited by APB-13 could not be determined. Therefore, further experiments were conducted to determine the exact stage that APB-13 affected TGEV replication. As shown in Fig. 2C-E, the APB-13 treatment had little or no effect on the pretreatment, attachment, penetration, replication, and release of TGEV, nor did it have a direct killing effect. On the other hand, the APB-13 treatment inhibited the TGEV replication phase significantly. The log10TCID50 of APB-13 62.50 µg/mL was 3.63 lower than that of the virus control. In other words, the antiviral activity of APB-13 62.50 µg/mL was 4,266 times higher than that of the virus-positive control.
APB-13 decreases the mRNA and protein levels of the TGEV N protein
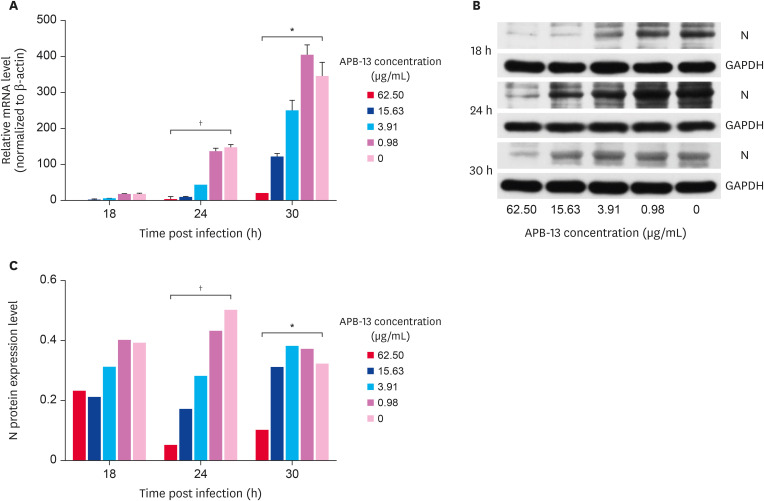
To study the inhibitory effects of APB-13 on the TGEV replication process further, ST cell monolayers were infected with TGEV for 3 h, and different concentrations of APB-13 were added to the cells in 6-well plates. The cell pellets were collected at 18, 24, and 30 hpi. The mRNA expression levels of the TGEV N gene were detected by RT-qPCR. As shown in Fig. 3A, within a specific time range, the expression of TGEV mRNA increased gradually over time, and the mRNA expression of APB-13 62.50 and 0 µg/mL at 24 hpi showed the greatest differences from that at 18 h and 30 hpi.
Fig. 3. Inhibitory effects of APB-13 on TGEV N protein. (A) Relative mRNA expression during TGEV replication. The ST cells were infected with TGEV (MOI = 5) for 3 h, and APB-13 was added to the cell medium. The cells were collected at 18, 24, and 30 hpi. The total RNA was extracted and reverse transcribed, and RT-qPCR was performed. The data are representative of 3 independent experiments. (B) TGEV N protein expression. The ST cells were infected with TGEV (MOI = 5) for 3 h, and different concentrations of APB-13 were added. The infected ST cells were collected at 18, 24, and 30 hpi. The TGEV N protein expression was determined by averaging the densitometric intensities from the WBs. (C) Statistical analysis of the optical densities of target protein bands from Fig. 3B.
APB-13, antimicrobial peptides-13; TGEV, transmissible gastroenteritis virus; ST, swine testicular; MOI, multiplicity of infection; hpi, h post-infection; RT-qPCR, real-time quantitative polymerase chain reaction; N, nucleocapsid; WB, western blot; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
*p < 0.05; †p < 0.01.
In addition, the collected cells were tested for TGEV protein expression by western blot (WB) analysis. The expression of the TGEV N protein at 18, 24, and 30 hpi increased gradually with decreasing APB-13 concentration. The differences in expression between the APB-13 62.50 µg/mL and 0 µg/mL were significant at 24 hpi (p < 0.01) and 30 hpi (p < 0.05). (Fig. 3B and C).
The results indicated that APB-13 is not toxic to ST cells within a specific range and even promotes cell growth. Furthermore, APB-13 had a potent antiviral effect on the TGEV. An in vivo test was performed to verify the effect of APB-13 on piglets.
Effect of APB-13 on the morphological development of the small intestine in piglets
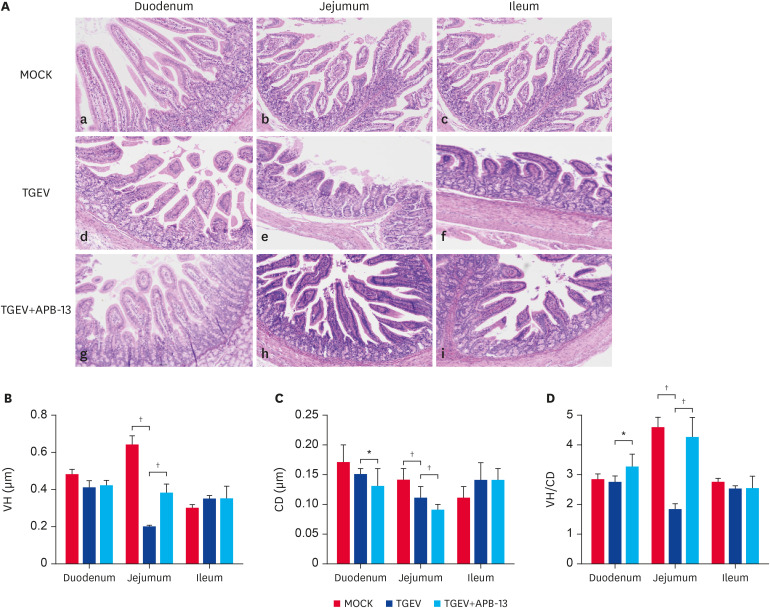
To examine the effects of APB-13 on the morphology of the piglet small intestine, 3 representative piglets per group were euthanized, and the duodenal, jejunum, and ileum samples were collected and stained with H&E. The results indicated that APB-13 had the greatest effect in the jejunum. Moreover, the differences in the jejunum VH, CD, and VH/CD between the TGEV and TGEV+APB-13 groups were significant (p < 0.01) (Fig. 4). The differences in the duodenal CD and VH/CD between the TGEV and TGEV+APB-13 groups were significant (P<0.05). The differences between the MOCK and TGEV groups were significant in the jejunum VH, CD, and VH/CD (P<0.05). The jejunum villi of the TGEV+APB-13 group were long, slender, and intact compared to those of the TGEV group. The duodena of the TGEV+APB-13 group exhibited a slightly better morphology than those of the TGEV group, and the small intestinal villi of the TGEV group were atrophied, shed, and ruptured compared to the MOCK and TGEV+APB-13 groups.
Fig. 4. Histological and morphometrical analyses of the piglet small intestines. (A) Morphometric tissue sections of the duodenum, jejunum, and ileum were stained with H&E (100×). (B-D) VH, CD, and VH/CD values of the small intestine (scale bars: 50 µm). Differences were recorded. The piglets were divided randomly into 3 groups with 3 replicates per group and 3 pigs per replicate: the mock infection group (MOCK), the virus-only group (TGEV), and the APB-13-treated group (TGEV+APB-13).
H&E, hematoxylin & eosin; VH, villus height; CD, crypt depth.
*p < 0.05; †p < 0.01.
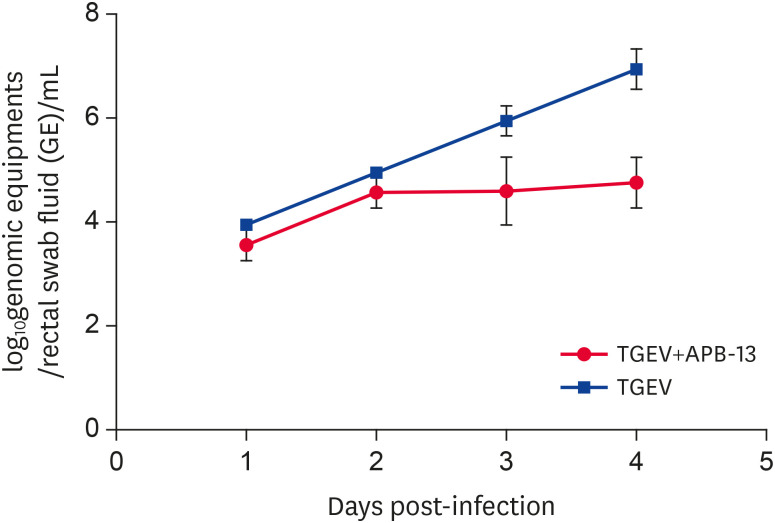
Viral shedding in the rectum of the TGEV-infected piglets
The piglets were infected, and in vivo TGEV shedding was detected (Fig. 5) on the first, second, third, and 4th days post-infection. Viral shedding of piglet rectum in both groups increased with increasing infection time. On the third and fourth days, however, viral shedding of the TGEV+APB-13 group was significantly lower than that of the TGEV group. These results suggest that APB-13 reduces the production of TGEV particles, i.e., APB-13 has an in vivo antiviral effect on TGEV.
Fig. 5. Viral shedding in the rectum of the TGEV-infected piglets. On the sixth day of birth, the piglets were administered 10 mL/head TGEV (1 × 108 TCID50/mL) orally, and the fecal samples were collected from the rectum on the 1st, 2nd, 3rd, and 4th day after challenge. The amount of viral shedding was detected by absolute quantitative PCR.
TGEV, transmissible gastroenteritis virus; TCID50, 50% tissue culture infective dose; PCR, polymerase chain reaction; APB-13, antimicrobial peptides-13.
DISCUSSION
Antibacterial peptides are used widely in animal breeding because of their broad-spectrum antiviral effects. This study examined the antiviral effect of APB-13 on TGEV. The results showed that APB-13 has an inhibitory effect on TGEV production in vitro and in vivo.
ST and PK-15 cells are used widely for TGEV isolation and propagation [23]. Therefore, it is necessary to determine a safe APB-13 concentration range in ST cells. In the current study, high concentrations of APB-13 were harmful to ST cell growth, while low concentrations of APB-13 promoted ST cell growth. Concentrations of APB-13 at 0 to 62.50 µg/mL were almost non-toxic to ST cells and were considered suitable for ST cell growth. Within this range, the antiviral effect was dependent on the concentration of APB-13. The results indicated that 3.91 µg/mL is the most suitable concentration for ST cell growth but not necessarily the best concentration for anti-TGEV activity. The lack of protection is probably related to the active concentration of this antimicrobial peptide [24]. These results are consistent with those reported by Yao and Duan, who reported that high concentrations inhibited growth, but low concentrations promoted cell growth [25,26]. Therefore, a suitable concentration range must be determined.
Based on the safe concentration range of APB-13, the effects of APB-13 on the TGEV were examined using an MTT assay. The highest inhibition rate was 74.1% at 62.50 µg/mL APB-13. Subsequent research confirmed that the difference was most obvious (i.e., APB-13 had the greatest inhibitory effect on TGEV production) at 3 hpi. In addition, the viral titer for the TGEV-only groups used in the experiment was originally 11, but this was reduced to approximately 6 at 3 and 6 hpi. Therefore, APB-13 plays a vital role in inhibiting TGEV production.
The N protein is an essential component of TGEV replication that acts by binding to the RNA genome and forming a helical nucleocapsid [27]. The TGEV N protein facilitates template switching and is required for efficient transcription [28]. RT-qPCR and WB experiments were performed to determine if APB-13 affects the N protein and its mRNA. The results showed that APB-13 inhibited TGEV replication, and within a certain range, the mRNA and protein expression levels of the N protein increased gradually as the APB-13 concentration decreased. Similarly, EIF4A2 inhibited TGEV replication by reducing mRNA and protein expression [29]. The probiotic Enterococcus faecium exerts antiviral activity against TGEV by reducing the expression of the viral structural proteins [30]. The PERK arm of the unfolded protein response negatively regulates TGEV replication by suppressing protein translation [31]. On the other hand, the expression of the viral N protein and its mRNA was decreased at 30 hpi in the APB-13 0.98 µg/mL group. A TGEV infection activates autophagy, which further inhibits TGEV replication [32]. Therefore, APB-13 reduces TGEV mRNA and N protein expression.
The APB-13 treatment inhibited the TGEV in vitro, but further confirmation will be needed to determine if it also inhibits TGEV in vivo. The main site at which TGEV harms piglets is the small intestine. Therefore, the development of the small intestinal morphology directly reflects the effects of APB-13 on the TGEV. The in vivo test results indicated that the VH and CD of the treatment group were superior to those of the control group. The TGEV-infected pigs exhibited marked villus shortening, clubbing, and blunting as well as reduced CD [33], which is consistent with the present results. These findings suggest that APB-13 has an antiviral effect on TGEV in vivo and suggests that APB-13 may promote the performance of piglets through changes in the intestinal morphology, as described by Alvarez et al. [34]. The intestinal tract is the site of digestion and nutrient absorption and acts as a barrier to exclude harmful pathogens and toxins [18]. This finding is also consistent with the previous CC50 result, suggesting that APB-13 promotes cell growth. Moreover, the reduction of viral shedding in vivo also directly reflected the effects of APB-13 on the TGEV. Therefore, APB-13 has an anti-TGEV effect both in vivo and in vitro.
In conclusion, this study confirmed that an APB-13 treatment effectively reduces TGEV production in vitro and in vivo. This study provides an important reference for the prevention and control of the TGEV and sustainable ecological breeding. Nevertheless, further studies will be needed to determine if APB-13 regulates the immunity-related pathways of the host to inhibit TGEV.
Footnotes
Funding: This study was supported by funds from the National Key Research and Development Program of China (2017YFD0501003 and 2016YFD0500102).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Zhang W, Liang X.
- Data curation: Zhang X.
- Formal analysis: Lian K.
- Funding acquisition: Liang X.
- Investigation: Tian X.
- Methodology: Liang X, Zhang W.
- Project administration: Zhang M.
- Resources: Wang S.
- Software: Chen C.
- Supervision: Nie C.
- Validation: Pan Y.
- Visualization: Han F.
- Writing - original draft: Liang X.
- Writing - review & editing: Wei Z, Zhang W.
References
- 1.Laude H, Rasschaert D, Delmas B, Godet M, Gelfi J, Charley B. Molecular biology of transmissible gastroenteritis virus. Vet Microbiol. 1990;23(1-4):147–154. doi: 10.1016/0378-1135(90)90144-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piñeyro PE, Lozada MI, Alarcón LV, Sanguinetti R, Cappuccio JA, Pérez EM, et al. First retrospective studies with etiological confirmation of porcine transmissible gastroenteritis virus infection in Argentina. BMC Vet Res. 2018;14(1):292. doi: 10.1186/s12917-018-1615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eleouet JF, Rasschaert D, Lambert P, Levy L, Vende P, Laude H. Complete sequence (20 kilobases) of the polyprotein-encoding gene 1 of transmissible gastroenteritis virus. Virology. 1995;206(2):817–822. doi: 10.1006/viro.1995.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L, Huang Y, Du Q, Dong F, Zhao X, Zhang W, et al. TGEV nucleocapsid protein induces cell cycle arrest and apoptosis through activation of p53 signaling. Biochem Biophys Res Commun. 2014;445(2):497–503. doi: 10.1016/j.bbrc.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Xu Y, Chang R, Tong D, Xu X. Transmissible gastroenteritis virus N protein causes endoplasmic reticulum stress, up-regulates interleukin-8 expression and its subcellular localization in the porcine intestinal epithelial cell. Res Vet Sci. 2018;119:109–115. doi: 10.1016/j.rvsc.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Mou C, Yang X, Lin J, Yang Q. Mitophagy in TGEV infection counteracts oxidative stress and apoptosis. Oncotarget. 2016;7(19):27122–27141. doi: 10.18632/oncotarget.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koczulla AR, Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003;63(4):389–406. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Wang FP, She WM, Yang CQ, Li L, Tu CT, et al. Enhanced high-mobility group box 1 (HMGB1) modulates regulatory T cells (Treg)/T helper 17 (Th17) balance via toll-like receptor (TLR)-4-interleukin (IL)-6 pathway in patients with chronic hepatitis B. J Viral Hepat. 2014;21(2):129–140. doi: 10.1111/jvh.12152. [DOI] [PubMed] [Google Scholar]
- 10.Vilas Boas LC, de Lima LM, Migliolo L, Mendes GD, de Jesus MG, Franco OL, et al. Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Biopolymers. 2017;108(2):e22871. doi: 10.1002/bip.22871. [DOI] [PubMed] [Google Scholar]
- 11.Boman HG, Wade D, Boman IA, Wåhlin B, Merrifield RB. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989;259(1):103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Jeong JH, Cho JH, Lee DH, Kim Y. Antimicrobial activity of antimicrobial peptide LPcin-YK3 derived from bovine lactophoricin. J Microbiol Biotechnol. 2018;28(8):1299–1309. doi: 10.4014/jmb.1805.05001. [DOI] [PubMed] [Google Scholar]
- 13.Albar AH, El-Fakharany EM, Almehdar HA, Uversky VN, Redwan EM. In vitro exploration of the anti-HCV potential of the synthetic spacer peptides derived from human, bovine, and camel lactoferrins. Protein Pept Lett. 2017;24(10):909–921. doi: 10.2174/0929866524666161111111320. [DOI] [PubMed] [Google Scholar]
- 14.Arias M, Hilchie AL, Haney EF, Bolscher JG, Hyndman ME, Hancock RE, et al. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem Cell Biol. 2017;95(1):91–98. doi: 10.1139/bcb-2016-0175. [DOI] [PubMed] [Google Scholar]
- 15.Albiol Matanic VC, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents. 2004;23(4):382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Robinson WE, Jr, McDougall B, Tran D, Selsted ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63(1):94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Peck BC, Shanahan MT, Singh AP, Sethupathy P. Gut microbial influences on the mammalian intestinal stem cell niche. Stem Cells Int. 2017;2017:5604727. doi: 10.1155/2017/5604727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27(20):493–497. [Google Scholar]
- 20.Wei Z, Burwinkel M, Palissa C, Ephraim E, Schmidt MF. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro . Vet Microbiol. 2012;160(3-4):468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen GH, Delmas B, Besnardeau L, Vogel LK, Laude H, Sjöström H, et al. The coronavirus transmissible gastroenteritis virus causes infection after receptor-mediated endocytosis and acid-dependent fusion with an intracellular compartment. J Virol. 1998;72(1):527–534. doi: 10.1128/jvi.72.1.527-534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong W, Xie W, Liu Y, Sui B, Zhang H, Liu L, et al. Receptor tyrosine kinase inhibitors block proliferation of TGEV mainly through p38 mitogen-activated protein kinase pathways. Antiviral Res. 2020;173:104651. doi: 10.1016/j.antiviral.2019.104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Z, An K, Xie L, Wu W, Zhang R, Wang D, et al. Transmissible gastroenteritis virus infection induces NF-κB activation through RLR-mediated signaling. Virology. 2017;507:170–178. doi: 10.1016/j.virol.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang HN, Pan CY, Chen JY. Grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 exhibits antiviral activity against foot-and-mouth disease virus in vitro . Peptides. 2018;106:91–95. doi: 10.1016/j.peptides.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Liu T, Wang X, Zhang D. The contrary effects of Sirt1 on MCF7 cells depend on CD36 expression level. J Surg Res. 2019;238:248–254. doi: 10.1016/j.jss.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 26.Duan L, Chen Q, Duan S. Transcriptional analysis of Chlorella pyrenoidosa exposed to bisphenol A. Int J Environ Res Public Health. 2019;16(8):1374. doi: 10.3390/ijerph16081374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Zhang H, Li Y, Wang G, Tang B, Zhao J, et al. Cathelicidin-derived antimicrobial peptides inhibit zika virus through direct inactivation and interferon pathway. Front Immunol. 2018;9:722. doi: 10.3389/fimmu.2018.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zúñiga S, Cruz JL, Sola I, Mateos-Gómez PA, Palacio L, Enjuanes L. Coronavirus nucleocapsid protein facilitates template switching and is required for efficient transcription. J Virol. 2010;84(4):2169–2175. doi: 10.1128/JVI.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Z, Yang Y, Wang L, Wang K, Ran L, Xie Y, et al. EIF4A2 interacts with the membrane protein of transmissible gastroenteritis coronavirus and plays a role in virus replication. Res Vet Sci. 2019;123:39–46. doi: 10.1016/j.rvsc.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai W, Burwinkel M, Wang Z, Palissa C, Esch B, Twardziok S, et al. Antiviral effects of a probiotic Enterococcus faecium strain against transmissible gastroenteritis coronavirus. Arch Virol. 2013;158(4):799–807. doi: 10.1007/s00705-012-1543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue M, Fu F, Ma Y, Zhang X, Li L, Feng L, et al. The PERK arm of the unfolded protein response negatively regulates transmissible gastroenteritis virus replication by suppressing protein translation and promoting type I interferon production. J Virol. 2018;92(15):e00431–e18. doi: 10.1128/JVI.00431-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Yu H, Gu W, Luo X, Li R, Zhang J, et al. Autophagy negatively regulates transmissible gastroenteritis virus replication. Sci Rep. 2016;6(1):23864. doi: 10.1038/srep23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia L, Yang Y, Wang J, Jing Y, Yang Q. Impact of TGEV infection on the pig small intestine. Virol J. 2018;15(1):102. doi: 10.1186/s12985-018-1012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez J, Sarradell J, Morrison R, Perez A. Impact of porcine epidemic diarrhea on performance of growing pigs. PLoS One. 2015;10(3):e0120532. doi: 10.1371/journal.pone.0120532. [DOI] [PMC free article] [PubMed] [Google Scholar]