Abstract
The present study aimed to investigate the effects of sufentanil on sepsis-induced acute lung injury (ALI), and identify the potential molecular mechanisms underlying its effect. In order to achieve this, a rat sepsis model was established. Following treatment with sufentanil, the lung wet/dry (W/D) weight ratio was calculated. Histopathological analysis was performed via hematoxylin and eosin staining. Levels of inflammatory factors in bronchoalveolar lavage fluid were determined via ELISA. Furthermore, malondialdehyde (MDA) content and the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) in tissue homogenates were assessed using commercial kits. Western blot analysis was performed to determine kininogen-1 (KNG1) protein expression. In addition, alveolar epithelial type II cells (AEC II) were stimulated with lipopolysaccharide (LPS) to mimic ALI. The levels of inflammation and oxidative stress were evaluated following overexpression of KNG1. Protein expression levels of nuclear factor-κB (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling were determined via western blot analysis. The results of the present study demonstrated that sufentanil alleviated histopathological injury and the W/D ratio in lung tissue. Following treatment with sufentanil, levels of inflammatory factors also decreased, accompanied by decreased concentrations of MDA, and increased activities of SOD, CAT and GSH-Px. Notably, KNG1 was decreased in lung tissues following treatment with sufentanil. Furthermore, overexpression of KNG1 attenuated the inhibitory effects of sufentanil on LPS-induced inflammation and oxidative stress in AEC II. Sufentanil markedly downregulated NF-κB expression, while upregulating Nrf2 and HO-1 expression levels, which was reversed following overexpression of KNG1. Taken together, the results of the present study suggested that sufentanil may alleviate inflammation and oxidative stress in sepsis-induced ALI by downregulating KNG1 expression.
Keywords: sepsis, acute lung injury, inflammation, oxidative stress, kininogen-1
Introduction
Sepsis is a systemic inflammatory response syndrome caused by infection, which leads to an increased risk of multiple organ injuries and mortality (1). Acute lung injury (ALI) is one of the most common side effects of sepsis due to pulmonary susceptibility, and is associated with high morbidity and mortality worldwide (2,3). Despite progression in efforts to understand the pathogenesis of sepsis-induced ALI, current treatment strategies remain ineffective (4). Thus, it remains critical to develop novel and effective therapeutic agents for the treatment of ALI.
Sepsis-induced ALI is generally characterized by an overwhelming response of innate inflammation, which stimulates excessive production of inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β and monocyte chemoattractant protein-1 (MCP-1) (5). This overactivation of the inflammatory response results in pathological damage of the alveolar epithelium and vascular endothelium, eventually resulting in ALI (6). Furthermore, oxidative stress plays a crucial role in the development of ALI. The excessive production of reactive oxygen species results in severe intracellular oxidative damage (7). Since inflammation and oxidative stress function are key for ALI development, identification of therapeutic agents with anti-inflammatory and antioxidative characteristics are required for direct and effective treatment of sepsis-induced ALI. Increasing evidence has demonstrated that anesthetic agents can protect against sepsis (8). A recent study reported that dexmedetomidine mitigates ALI in rats by suppressing caveolin-1 downstream signaling (9). Sufentanil, a derivative of fentanyl, is considered an opioid, with a high affinity to opioid receptors. A previous study demonstrated that sufentanil inhibited the inflammatory response and oxidative stress in hepatic ischemia-reperfusion injury (10). Previous studies reported that sufentanil could alleviate ALI induced by hemorrhagic shock in rabbits (11,12). The STITCH database (http://stitch.embl.de) predicted that the κ-type opioid receptor (OPRK1) can combine with sufentanil, and the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org) predicted that kininogen-1 (KNG1) can interact with OPRK1. KNG1 can encode high-molecular weight kininogen proteins, and increasing evidence has demonstrated that KNG1 plays a significant role in inflammation and coagulation (13). A previous study reported that KNG1 serves as a pro-inflammatory cytokine, which accelerates the progress of inflammation (14). However, the role of sufentanil in sepsis-induced ALI via regulation of KNG1 remains unclear.
Thus, the present study investigated the therapeutic effects of sufentanil on sepsis-induced ALI. A rat sepsis model was used to determine the underlying molecular mechanisms of sufentanil on sepsis-induced ALI, in order to evaluate its potential as a candidate therapeutic agent against ALI.
Materials and methods
Animal study
A total of 40 specific-pathogen-free-grade male Sprague-Dawley rats (age, 6–8 weeks; weight, 180–200 g) were provided by the Model Animal Research Center of Nanjing University (Nanjing, China). All animals were housed in individually ventilated cages (n=2 in each cage) under a controlled temperature (20–25°C), humidity (20–30%) and 12-h light/dark cycle conditions with free access to food and water. Rats were allowed to adapt to the environment for one week before the experiments. Animal experimental procedures were performed according to the Guide for Care and Use of Laboratory Animals (15,16) and protocols were approved by the Animal Experiment Ethics Committee of The First People's Hospital (Wuhan, China).
Establishment of septic ALI model
The rat sepsis model was established using the cecal ligation and puncture (CLP) method, as previously described (17). Briefly, all rats were deprived of from food 12 h before surgery, but had free access to water during this time. Rats were anesthetized with an intraperitoneal injection of 50 mg/kg sodium pentobarbital. Following anesthesia, a 2-cm incision was made along the middle of the abdomen to expose the cecum. Subsequently, the cecum was ligated with the No. 4 suture below the ileocecal valve and double-punctured using a sterile 18-gauge needle, which released a small amount of feces. The intestinal contents within the cecum were squeezed through the puncture wound and the cecum was restored, followed by suturing of the abdominal incision, layer by layer. Rats in the sham groups underwent identical laparotomy and resuscitation procedures; however, ligation and perforation were not performed. All rats were allowed free access to food and water following CLP. The behavior of the rats was observed every 2 h. There were no abnormal deaths during the experiment. All animals were euthanized with an intraperitoneal injection of 200 mg/kg sodium pentobarbital 24 h after CLP surgery, which was in accordance with previous studies (18,19). Death was determined when the animals' hearts stopped completely and pupils dilated. Lung tissues were collected for further experimentation. If the rats went into shock, or showed decreased activity, lethargy or dyspnea, the animals were euthanized prior to the experimental endpoint.
Grouping and drug administration
All rats were randomly allocated into four groups (10 rats in each group) as follows: i) Sham group; ii) sham + sufentanil group; iii) CLP group; and iv) CLP + sufentanil group. Sufentanil was purchased from Yichang Humanwell Pharmaceutical Co., Ltd. Rats in the sham + sufentanil and CLP + sufentanil groups were intravenously injected with sufentanil (3 µg/kg; 1 ml) 30 min prior to surgery, whereas rats in the sham only and CLP only groups were intravenously injected with normal saline at the same time.
Test for lung wet/dry (W/D) weight ratio
In order to assess the magnitude of lung tissue edema, the upper right lobe of lung tissues was immediately excised and the surface fluid and blood was absorbed using filter paper. After weighing, the tissue samples were placed in an incubator at 80°C for 48 h to acquire the dry weight, and the W/D ratio was subsequently calculated using the following calculation: Lung W/D weight ratios=wet weight/dry weight.
Histopathological examination
Lung tissues were fixed in 10% buffered formalin for 24 h at 4°C, conventionally dehydrated, cleared and waxed. The tissue samples were subsequently embedded in paraffin and cut into 5-µm-thick sections. The tissue sections were deparaffinized in xylene and rehydrated in a descending ethanol series. The sections were stained with hematoxylin (5 min, room temperature) and eosin (3 min, room temperature), prior to dehydration in a graded ethanol series and xylene. The stained slides were observed under a light microscope (Olympus Corporation; magnification, ×400).
Cell culture and treatment
Alveolar epithelial type II cells (AEC II) A549 cells (cat. no. SCSP-503) were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences and maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5% CO2.
In order to mimic the process of ALI, AEC II were treated with 50 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) for 24 h at 37°C. Cells in the control group were not manipulated, while cells in the treatment groups were stimulated with sufentanil (5, 10, 20 and 40 µM) for 2 h prior to treatment with LPS at 37°C in 5% CO2.
Cell transfection
Cells were seeded into 6-well plates (1×106 cells/well) and cultivated in a humidified chamber until they reached 70% confluence. AEC II were transfected with KNG1 overexpression pcDNA3.1 plasmid (Oe-KNG1-1 or Oe-KNG1-2; 5 µl; 50 ng) or empty vector plasmid (Oe-NC; both from Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Cells were harvested and analyzed 48 h post-transfection. Transfection efficiency was determined via reverse transcription-quantitative (RT-q) PCR analysis.
Cell viability
Cell viability was detected using Cell Count Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.) according to the manufacturer's instructions. After transfection, cells were seeded in 96-well plates (1×104 cells/well). Cells were incubated with sufentanil for 2 h followed by treatment with 50 µg/ml LPS for 24 h at room temperature. A total of 10 µl CCK-8 solution was added to each well. Following incubation for 1 h at room temperature, the absorbance was detected at 450 nm.
Detection of cytokine in bronchoalveolar lavage fluid (BALF)
The rats were euthanized 24 h after CLP and the left lungs were washed three times with 0.5 ml saline. Subsequently, BALF was collected and centrifuged at 850 × g for 10 min at 4°C. The supernatant was collected and preserved at −80°C. The concentrations of the inflammatory factors TNF-α (cat. no. F16960), IL-1β (cat. no. F15810), IL-6 (cat. no. F15870) and MCP-1 (cat. no. F16120) in BALF were assessed using ELISA kits (Shanghai Xitang Biotechnology Co., Ltd.), according to the manufacturer's protocol.
Determination of oxidative stress
The partial lung tissue specimens were collected and homogenized (10%, w/v) in cold saline. Malondialdehyde (MDA; cat. no. A003-1-2) content and the activity levels of superoxide dismutase (SOD; cat. no. A001-1-2), catalase (CAT; cat. no. A007-1-1) and glutathione peroxidase (GSH-Px; cat. no. A005-1-2) were determined in the tissue homogenates or AEC II using colorimetric commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocols.
RT-qPCR
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was synthesized using the PrimeScript RT kit (Takara Bio, Inc.) at 37°C for 15 min and 85°C for 5 sec. The cDNA solution was stored at −80°C. Subsequently, qPCR was performed using the iTaq™ Universal SYBR-Green Supermix (Bio-Rad Laboratories, Inc.) on an ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) at 95°C for 3 min, 95°C for 30 sec and 58°C for 30 sec. Relative expression levels were calculated using the 2−ΔΔCq method and normalized to the internal reference gene GAPDH (20). The sequences of all primers were as follows: GAPDH: Forward, 5′-CGCCTGGAGAAAGCTGCTA-3′ and reverse, 5′-ACGACCTGGTCCTCGGTGTA-3′; and KNG1: Forward, 5′-TAATACGACTCACTATAGGG-3′ and reverse, 5′-TAGAAGGCACAGTCGAGG−3′.
Western blot analysis
Total proteins were extracted from lung tissue samples or AEC II using RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). An equal amount of protein sample (40 µg per lane) was separated via SDS-PAGE on a 10% gel and subsequently transferred onto nitrocellulose membranes. Membranes were blocked with 5% non-fat milk at room temperature for 2 h, prior to incubation with primary antibodies (overnight at 4°C) for the target proteins. Following incubation with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h (1:10,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.), the protein bands were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) and detected using ImageJ software (version 1.8.0; National Institutes of Health). Anti-KNG1 antibody (1:1,000; cat. no. ab175386) was obtained from Abcam. Anti-nuclear factor erythroid 2-related factor 2 (Nrf2; 1:1,000; cat. no. 12721T), anti-phospho-nuclear factor-κB (p-NF-κB p65; 1:1,000; cat. no. 3033T), anti-NF-κB p65 (1:1,000; cat. no. 8242T); anti-heme oxygenase-1 (HO-1; 1:1,000; cat. no. 43966S), anti-Lamin B1 (1:1,000; cat. no. 13435S) and anti-GAPDH (1:1,000; cat. no. 5174T) antibodies were purchased from Cell Signaling Technology, Inc. Protein expression was normalized to the internal reference gene GAPDH or Lamin B1.
Statistical analysis
All results were obtained from at least three independent experiments. Data are presented as the mean ± standard deviation. All statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, Inc.). A Student's t-test was used to compare differences between two groups, while one-way ANOVA, followed by Tukey's post hoc test was used to compare differences between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Sufentanil improves lung tissue pathobiology and edema in CLP-induced ALI rats
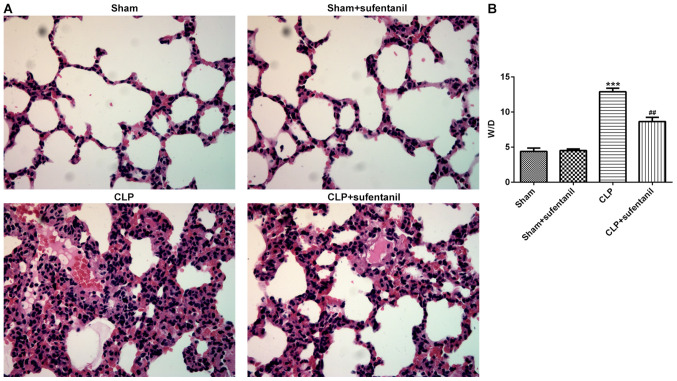
H&E staining of lung tissue sections was performed to determine the pathological changes in each group in order to investigate the effects of sufentanil in sepsis-induced ALI. Normal pulmonary alveoli structure was observed in sham-operated rats, whereas no significant difference in lung tissue pathology was observed between rats in the sham only and sham + sufentanil groups (Fig. 1A). However, animals presented with destructive alveolar structure, thickened alveolar septal walls, visible vascular congestion and inflammatory cell infiltration following CLP. Notably, the morphological changes observed in rat lung tissues following CLP were attenuated after treatment with sufentanil. Furthermore, the degree of lung tissue edema was calculated using the W/D ratio. The results demonstrated that the W/D ratio significantly increased following CLP, whereas treatment with sufentanil notably attenuated this (Fig. 1B). Taken together, these results suggested that sufentanil may effectively alleviate lung injury and edema in sepsis-induced ALI rats.
Figure 1.
Sufentanil improves lung tissue pathobiology and edema in CLP-induced acute lung injury rats. (A) H&E staining of lung tissue. Magnification, ×400. (B) W/D ratio of lung tissue was calculated. ***P<0.001 vs. sham; ##P<0.01 vs. CLP. CLP, cecal ligation and puncture; H&E, hematoxylin & eosin; W/D, wet/dry.
Sufentanil decreases inflammation and oxidative stress in CLP-induced ALI rats
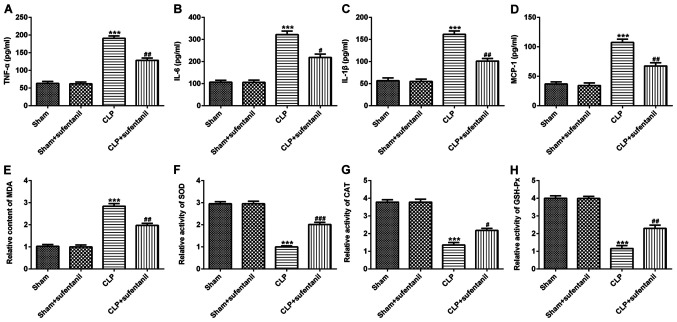
The effect of sufentanil on inflammation was assessed by detecting the concentrations of inflammatory cytokines, TNF-α, IL-6, IL-1β and MCP-1, in the BALF. The results demonstrated that the levels of inflammatory cytokines significantly increased in the CLP group compared with the sham group, whereas treatment with sufentanil significantly decreased the levels of cytokines in the CLP group (Fig. 2A-D). Furthermore, commercial kits were used to assess the levels of oxidative stress-associated markers. Treatment with sufentanil significantly decreased MDA content, whereas the activity levels of the antioxidant enzymes SOD, CAT and GSH-Px increased compared with the CLP group (Fig. 2E-H). Collectively, these results suggested that sufentanil may relieve inflammation and oxidative stress in CLP-induced ALI rats.
Figure 2.
Sufentanil alleviates inflammation and oxidative stress in CLP-induced acute lung injury rats. Levels of (A) TNF-α, (B) IL-6, (C) IL-1β and (D) MCP-1 were determined via ELISA. (E) MDA content and the activities of (F) SOD, (G) CAT and (H) GSH-Px were assessed using commercial kits. ***P<0.001 vs. sham; #P<0.05, ##P<0.01, ###P<0.001 vs. CLP. CLP, cecal ligation and puncture; TNF-α, tumor necrosis factor-α; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase.
KNG1 expression is notably downregulated following treatment with sufentanil
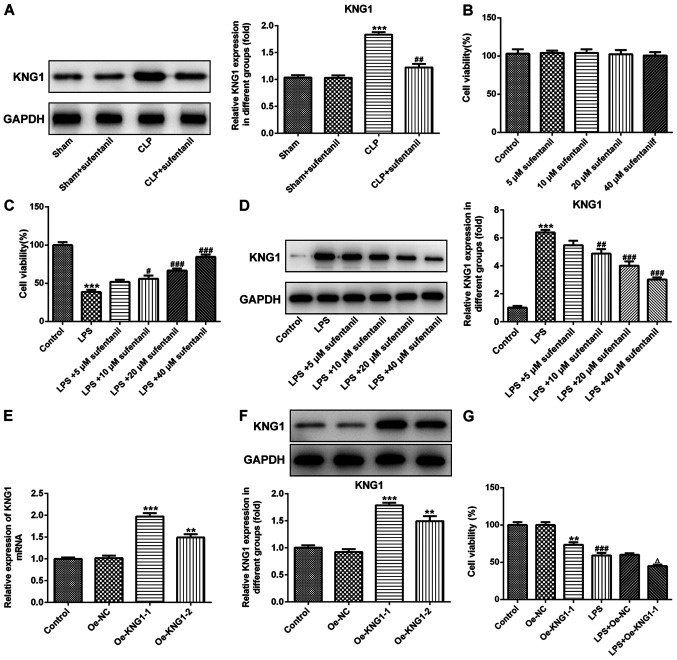
In order to investigate the potential molecular mechanisms of sufentanil function during ALI, KNG1 expression was determined in lung tissues via western blotting. No significant difference in KNG1 expression was observed between the sham and sham + sufentanil groups (Fig. 3A). However, KNG1 expression was significantly upregulated in the CLP group, which was reversed following treatment with sufentanil.
Figure 3.
KNG1 expression is significantly downregulated following sufentanil treatment. (A) Western blot analysis was performed to determine KNG1 protein expression in lung tissues of acute lung injury rats. ***P<0.001 vs. sham; ##P<0.01 vs. CLP. (B) CCK-8 assay was performed to assess cell viability following treatment with different concentrations of sufentanil. (C) CCK-8 assay was performed to assess cell viability and (D) expression of KNG1 was assessed using western blot analysis in alveolar epithelial type II cells induced by LPS, following treatment with sufentanil. ***P<0.001 vs. control; #P<0.05, ##P<0.01, ###P<0.001 vs. LPS. (E) Reverse transcription-quantitative PCR and (F) western blotting were performed to determine KNG1 expression following cell transfection. **P<0.01, ***P<0.001 vs. Oe-NC. (G) Cell viability was measured using a CCK-8 assay after KNG1 overexpression with and without LPS. **P<0.01 vs. Oe-NC; ###P<0.001 vs. control; ΔP<0.05 vs. LPS + Oe-NC. KNG1, kininogen-1; LPS, lipopolysaccharide; CLP, cecal ligation and puncture; Oe, overexpression; NC, negative control; CCK-8, Cell Counting Kit-8.
AEC II were subsequently treated with different concentrations of sufentanil (5, 10, 20 and 40 µM) and cell viability was assessed via CCK-8 assays. The results indicated that treatment with a series of concentrations of sufentanil had little effect on the cell viability of AEC II (Fig. 3B). It was also found that cell viability significantly decreased following treatment of AEC II with LPS, compared with the control group, while sufentanil dose-dependently enhanced cell viability (Fig. 3C).
Subsequently, the expression of KNG1 was examined by western blot analysis. As exhibited in Fig. 3D, KNG1 was significantly upregulated in AEC II treated with LPS, whereas sufentanil downregulated KNG1 expression in a dose-dependent manner. Next, KNG1 was successfully overexpressed by transfection with an overexpression plasmid (Fig. 3E and F). Cells transfected with Oe-KNG1-1 were used for all subsequent experiments. Additionally, cell viability was assessed using a CCK-8 assay after KNG1 overexpression with and without LPS. As shown in Fig. 3G, overexpression of KNG1 and/or LPS treatment significantly reduced the viability of AEC II, and the lowest viability was observed in LPS + Oe-KNG1-1 group. Taken together, these results indicated that sufentanil downregulated KNG1 expression in CLP-induced ALI rats and AEC II.
Overexpression of KNG1 significantly reverses the inhibitory effects of sufentanil on inflammation and oxidative stress
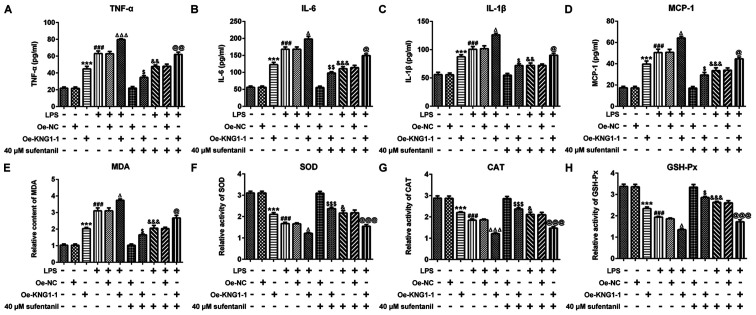
In order to determine whether the inhibitory effects of sufentanil on inflammation and oxidative stress in ALI were achieved by regulating KNG1 expression, the levels of inflammatory- and oxidative stress-associated markers were assessed in AEC II treated with LPS. As demonstrated in Fig. 4A-D, KNG1 overexpression or LPS treatment significantly increased the levels of TNF-α, IL-6, IL-1β and MCP-1, and the highest levels of the aforementioned factors were found in the LPS + Oe-KNG1-1 group. Sufentanil significantly decreased the levels of TNF-α, IL-6, IL-1β and MCP-1 in LPS-treated cells, which was consistent with the results of the CLP-induced ALI rat model. Overexpression of KNG1 significantly alleviated the inhibitory effects of sufentanil on the levels of inflammatory factors. Furthermore, overexpression of KNG1 in LPS-induced AEC II significantly elevated MDA content, which was accompanied by a decline in the activity levels of SOD, CAT and GSH-Px, compared with the control group (Fig. 4E-H). Taken together, these results indicated that overexpression of KNG1 significantly attenuated the inhibitory effects of sufentanil on inflammation and oxidative stress.
Figure 4.
Overexpression of KNG1 significantly reverses the inhibitory effects of sufentanil on inflammation and oxidative stress. Levels of (A) TNF-α, (B) IL-6, (C) IL-1β and (D) MCP-1 were determined via ELISA. (E) MDA content and the activities of (F) SOD, (G) CAT and (H) GSH-Px were assessed using commercial kits. ***P<0.001 vs. Oe-NC; ###P<0.001 vs. control; ΔP<0.05, ΔΔΔP<0.001 vs. LPS + Oe-NC; $P<0.05, $$P<0.01, $$$P<0.001 vs. 40 µM sufentanil + Oe-NC; &P<0.05, &&P<0.01, &&&P<0.001 vs. LPS; @P<0.05, @@P<0.01, @@@P<0.001 vs. LPS + 40 µM sufentanil + Oe-NC. KNG1, kininogen-1; TNF-α, tumor necrosis factor-α; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; Oe, overexpression; NC, negative control.
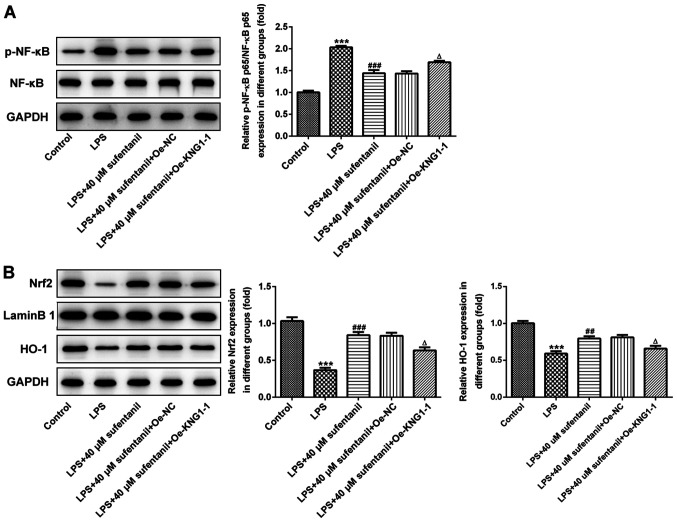
Overexpression of KNG1 markedly reverses the effects of sufentanil on NF-κB and Nrf2/HO-1 signaling
In order to further investigate the molecular mechanisms underlying inflammation and oxidative stress regulated by sufentanil in ALI, the expression levels of NF-κB and Nrf2/HO-1 signaling pathway proteins were measured via western blot analysis, following overexpression of KNG1 in LPS-induced AEC II. The expression levels of p-NF-κB p65 significantly increased in the LPS group, which was decreased following treatment with sufentanil. The effects of sufentanil treatment were reversed following overexpression of KNG1 (Fig. 5A). In addition, expression levels of Nrf2 and HO-1 were significantly elevated following treatment with LPS + sufentanil compared with the LPS group (Fig. 5B). Overexpression of KNG1 significantly reversed the regulatory effects of sufentanil on the Nrf2/HO-1 signaling pathway. Taken together, these results suggested that sufentanil relieved inflammation and oxidative stress in ALI by downregulating KNG1 expression.
Figure 5.
Overexpression of KNG1 notably restores the regulatory effects of sufentanil on NF-κB and Nrf2/HO-1 signaling. Western blot analysis was performed to detect the protein expression levels of (A) p-NF-κB and NF-κB, and (B) Nrf2 and HO-1 in LPS-stimulated alveolar epithelial type II cells. ***P<0.001 vs. control; ##P<0.01, ###P<0.001 vs. LPS; ΔP<0.05 vs. LPS + 40 µM sufentanil + Oe-NC. KNG1, kininogen-1; NF-κB, nuclear factor-κB; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; LPS, lipopolysaccharide; Oe, overexpression; NC, negative control; p-, phosphorylated.
Discussion
Sepsis can result in multiple organ failure and is considered a leading cause of mortality in intensive care unit patients (21). The lung serves as the most susceptible target organ in sepsis that can further develop into life-threating acute respiratory distress syndrome (ARDS), which is the principle risk factor for mortality (22,23). The present study demonstrated that sufentanil protected against sepsis-induced ALI by regulating KNG1-mediated NF-κB and Nrf2/HO-1 signaling.
Increasing evidence has demonstrated that anesthesia possesses indirect functions to regulate the progression of human disease. For example, a previous study found that ropivacaine suppressed lung endothelial hyperpermeability, induced by pressure in an acute hypertension model (24). Furthermore, etomidate restrained the expression of NF-κB by downregulating expression of the glucocorticoid receptor in septic rats (8), whereas desflurane was found to alleviate ventilator-induced lung injury in rats with ARDS (25). Sufentanil is an opioid, with high affinity to opioid receptors (26). It has been reported that µ-opioid receptor signaling could ameliorate LPS-induced acute ARDS, and AEC was demonstrated to express opioid receptors (27,28). To the best of our knowledge, the effects of sufentanil on sepsis-induced ALI were investigated for the first time in the present study. H&E staining analysis and the W/D ratio demonstrated that sufentanil notably improved lung tissue pathobiology and CLP-induced edema, suggesting the protective effects of sufentanil on ALI.
The early phase of sepsis is characterized by excessive inflammation, which is mediated by the sustained secretion of inflammatory cytokines, including TNF-α, IL-6, IL-1β and MCP-1 (29). Increasing evidence has demonstrated that persistently increased concentrations of the aforementioned inflammatory cytokines in plasma are highly predicative of mortality in patients with ALI (30). Thus, inhibiting inflammation is critical to effectively treat sepsis-induced ALI. A previous study reported that sufentanil preconditioning can specifically protect against myocardial ischemic-reperfusion injury in rabbits (31). Furthermore, sufentanil has been demonstrated to inhibit inflammatory response and oxidative stress in hepatic ischemia-reperfusion injury (10). In the present study, sufentanil notably decreased the levels of inflammatory factors and MDA content, whereas the activities of the antioxidant enzymes (SOD, CAT and GSH-Px) increased. These findings suggested that sufentanil attenuated inflammation and oxidative stress in sepsis-induced ALI.
In order to determine the underlying regulatory mechanisms of sufentanil on ALI, KNG1 expression was detected in lung tissues following CLP. KNG1 encodes high-molecular weight kininogen proteins, and increasing evidence has demonstrated that KNG1 plays a significant role in inflammation and coagulation (12,32). A previous study reported that the absence of KNG1 protein decreased thrombosis and inflammation in ischemic mice (33). Furthermore, as a pro-inflammatory cytokine, KNG1 has been demonstrated to accelerate the progress of inflammation (14). An increasing trend of KNG1 expression was found in lung tissues of chronic obstructive pulmonary disease models and inhibition of KNG1 could relieve cellular inflammation (34). The results of the present study demonstrated that KNG1 expression was significantly upregulated in lung tissues following CLP, and treatment with sufentanil restored the expression, suggesting an underlying regulatory association between sufentanil and KNG1. In order to determine the biological basis of this association, KNG1 was overexpressed in LPS-induced AEC II. The results indicated that the inhibitory effects of sufentanil on inflammation and oxidative stress were reversed following overexpression of KNG1. Thus, the present study demonstrated that sufentanil attenuated sepsis-induced ALI by downregulating KNG1 expression.
NF-κB signaling is essential for the regulation of the inflammatory response in sepsis-induced ALI (35), and activation of the Nrf2/HO-1 signaling pathway may relieve sepsis-induced ALI by suppressing inflammation and oxidative stress (36,37). Increasing evidence has demonstrated that regulation of the Nrf2/HO-1 and NF-κB signaling pathways alleviates lung injury induced by ventilator, by suppressing inflammation and oxidative stress (38). In order to further investigate the molecular mechanisms underlying sufentanil in ALI, the expression levels of NF-κB and Nrf2/HO-1 signaling pathway proteins were measured via western blot analysis, following overexpression of KNG1 in LPS-stimulated AEC II. The results demonstrated that overexpression of KNG1 partially reversed the regulatory effects of sufentanil on the NF-κB and Nrf2/HO-1 signaling pathways. Taken together, these results suggested that sufentanil modulated NF-κB and Nrf2/HO-1 signaling in ALI by downregulating KNG1 expression.
In conclusion, the results of the present study indicated that sufentanil may protect lung tissues against sepsis-induced inflammation and oxidative stress damage by regulating the KNG1-mediated NF-κB and Nrf2/HO-1 signaling pathways. Thus, sufentanil could be a potential novel therapeutic agent for effective clinical treatment of sepsis-induced ALI. However, it remains unknown if AEC II express opioid receptors and future studies on human lung injury samples are required to further verify the results of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QH and YY designed the study and performed the experiments. YY wrote the manuscript. QW and CH performed the statistical analysis and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols were approved by the Animal Experiment Ethics Committee of The First People's Hospital (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Peng LY, Yuan M, Shi HT, Li JH, Song K, Huang JN, Yi PF, Fu BD, Shen HQ. Protective effect of piceatannol against acute lung injury through protecting the integrity of air-blood barrier and modulating the TLR4/NF-κB signaling pathway activation. Front Pharmacol. 2019;10:1613. doi: 10.3389/fphar.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: Incidence and mortality, has it changed? Current Opin Crit Care. 2014;20:3–9. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Zhang L, Jiang Y, Dai J, Tang L, Liu G. Emodin reactivated autophagy and alleviated inflammatory lung injury in mice with lethal endotoxemia. Exp Anim. 2019;68:559–568. doi: 10.1538/expanim.19-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu B, Miao X, Ye J, Pu X. The protective effects of protease inhibitor MG-132 on sepsis-induced acute lung rats and its possible mechanisms. Med Sci Monit. 2019;25:5690–5699. doi: 10.12659/MSM.915743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Lin X, Xiao J, Tian Y, Zheng B, Teng H. Sonchus oleraceus Linn protects against LPS-induced sepsis and inhibits inflammatory responses in RAW264.7 cells. J Ethnopharmacol. 2019;236:63–69. doi: 10.1016/j.jep.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 7.Guo K, Jin F. Dipeptidyl peptidase-4 (DPP-4) inhibitor saxagliptin alleviates lipopolysaccharide-induced acute lung injury via regulating the Nrf-2/HO-1 and NF-κB pathways. J Invest Surg. 2019:1–8. doi: 10.1080/08941939.2019.1680777. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Li RM, Wang C, Liu N, Lv S, Xiong JY. Etomidate inhibits nuclear factor-kappaB through decreased expression of glucocorticoid receptor in septic rats. Mol Med Rep. 2016;14:5760–5766. doi: 10.3892/mmr.2016.5947. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Huang X, Hu S, He H, Meng Z. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury in rats by inhibition of caveolin-1 downstream signaling. Biomed Pharmacother. 2019;118:109314. doi: 10.1016/j.biopha.2019.109314. [DOI] [PubMed] [Google Scholar]
- 10.Lian YH, Fang J, Zhou HD, Jiang HF, Xie KJ. Sufentanil preconditioning protects against hepatic ischemia-reperfusion injury by suppressing inflammation. Med Sci Monit. 2019;25:2265–2273. doi: 10.12659/MSM.913145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang CM, Yu JL, Zhang Y, Chen LX, Cao HJ, Wang YY. The effect of sufentanil on acute lung injury in rabbits. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:108–110. (In Chinese) [PubMed] [Google Scholar]
- 12.Reupke V, Walliser K, Perl T, Kimmina S, Schraepler A, Quintel M, Kunze-Szikszay N. Total intravenous anaesthesia using propofol and sufentanil allows controlled long-term ventilation in rabbits without neuromuscular blocking agents. Lab Anim. 2017;51:284–291. doi: 10.1177/0023677216660337. [DOI] [PubMed] [Google Scholar]
- 13.Furniss SK, Yao R, Gonzalez G. Automatic gene prioritization in support of the inflammatory contribution to Alzheimer's disease. AMIA Jt Summits Transl Sci Proc. 2014;2014:42–47. [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D, Huo M, Chen X, Zhang Y, Qiao Y. Mechanism of tanshinones and phenolic acids from Danshen in the treatment of coronary heart disease based on co-expression network. BMC Complement Med Ther. 2020;20:28. doi: 10.1186/s12906-019-2712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin S, Wang H, Liu G, Mei H, Chen M. miR215p ameliorates hyperoxic acute lung injury and decreases apoptosis of AEC II cells via PTEN/AKT signaling in rats. Mol Med Rep. 2019;20:4953–4962. doi: 10.3892/mmr.2019.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kata D, Foldesi I, Feher LZ, Hackler L, Jr, Puskas LG, Gulya K. A novel pleiotropic effect of aspirin: Beneficial regulation of pro- and anti-inflammatory mechanisms in microglial cells. Brain Res Bull. 2017;132:61–74. doi: 10.1016/j.brainresbull.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Ren T, Zeng J. Mitochondrial coenzyme Q protects sepsis-induced acute lung injury by activating PI3K/Akt/GSK-3β/mTOR pathway in rats. BioMed Res Int. 2019;2019:5240898. doi: 10.1155/2019/5240898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu HY, Shi D, Hu CL, Yuan X, Zhang J, Sun H. Dexmedetomidine mitigates CLP-stimulated acute lung injury via restraining the RAGE pathway. Am J Transl Res. 2017;9:5245–5258. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu B, Miao X, Ye J, Pu X. The protective effects of protease inhibitor MG-132 on sepsis-induced acute lung rats and its possible mechanisms. Med Sci Monitor. 2019;25:5690–5699. doi: 10.12659/MSM.915743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Guo Z, Zhao C, Zhang X, Wang Z. Potential mechanism and drug candidates for sepsis-induced acute lung injury. Exp Ther Med. 2018;15:4689–4696. doi: 10.3892/etm.2018.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen N, Cheng A, Qiu M, Zang G. Allicin improves lung injury induced by sepsis via regulation of the toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MYD88)/nuclear factor kappa B (NF-κB) pathway. Med Sci Monit. 2019;25:2567–2576. doi: 10.12659/MSM.914114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Fu Y, Liu K, Hou L, Zhang W. miR-206 regulates alveolar type II epithelial cell Cx43 expression in sepsis-induced acute lung injury. Exp Ther Med. 2019;18:296–304. doi: 10.3892/etm.2019.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel M, Chignalia AZ, Isbatan A, Bommakanti N, Dull RO. Ropivacaine inhibits pressure-induced lung endothelial hyperpermeability in models of acute hypertension. Life Sci. 2019;222:22–28. doi: 10.1016/j.lfs.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 25.Strosing KM, Faller S, Gyllenram V, Engelstaedter H, Buerkle H, Spassov S, Hoetzel A. Inhaled anesthetics exert different protective properties in a mouse model of ventilator-induced lung injury. Anesth Analg. 2016;123:143–151. doi: 10.1213/ANE.0000000000001296. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, Ju YN, Gao W, Li DM, Guo CC. Desflurane attenuates ventilator-induced lung injury in rats with acute respiratory distress syndrome. Biomed Res Int. 2018;2018:7507314. doi: 10.1155/2018/7507314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji S, Wang L. µ-Opioid receptor signalling via PI3K/Akt pathway ameliorates lipopolysaccharide-induced acute respiratory distress syndrome. Exp Physiol. 2019;104:1555–1561. doi: 10.1113/EP087783. [DOI] [PubMed] [Google Scholar]
- 28.Dong S, Liu J, Li L, Wang H, Ma H, Zhao Y, Zhao J. The HECT ubiquitin E3 ligase Smurf2 degrades µ-opioid receptor 1 in the ubiquitin-proteasome system in lung epithelial cells. Am J Physiol Cell Physiol. 2019;316:C632–C640. doi: 10.1152/ajpcell.00443.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Liu J, Wang W, Liu S, Yang X, Chen M, Cheng L, Lu J, Guo T, Huang F. Sini decoction ameliorates sepsis-induced acute lung injury via regulating ACE2-Ang (1–7)-Mas axis and inhibiting the MAPK signaling pathway. Biomed Pharmacother. 2019;115:108971. doi: 10.1016/j.biopha.2019.108971. [DOI] [PubMed] [Google Scholar]
- 30.Qiu N, Xu X, He Y. LncRNA TUG1 alleviates sepsis-induced acute lung injury by targeting miR-34b-5p/GAB1. BMC Pulm Med. 2020;20:49. doi: 10.1186/s12890-020-1084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XH, Zeng JF, Lin C, Chen SB. Effects of morphine and sufentanil preconditioning against myocardial ischemic-reperfusion injury in rabbits. Int J Clin Exp Med. 2015;8:15692–15699. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Tan X, Xu J, Li H, Wang M, Chen S, Yang X, Liu Y, Wang F. Negative correlation between CSF lactate levels and MoCA scores in male Chinese subjects. Psychiatry Res. 2017;255:49–51. doi: 10.1016/j.psychres.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Langhauser F, Göb E, Kraft P, Geis C, Schmitt J, Brede M, Göbel K, Helluy X, Pham M, Bendszus M, et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120:4082–4092. doi: 10.1182/blood.V120.21.104.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi K, Chen X, Xie B, Yang SS, Liu D, Dai G, Chen Q. Celastrol alleviates chronic obstructive pulmonary disease by inhibiting cellular inflammation induced by cigarette smoke via the Ednrb/Kng1 signaling pathway. Front Pharmacol. 2018;9:1276. doi: 10.3389/fphar.2018.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M, Cao FL, Zhang YF, Shan L, Jiang XL, An XJ, Xu W, Liu XZ, Wang XY. Tanshinone IIA therapeutically reduces LPS-induced acute lung injury by inhibiting inflammation and apoptosis in mice. Acta Pharmacol Sin. 2015;36:179–187. doi: 10.1038/aps.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qing R, Huang Z, Tang Y, Xiang Q, Yang F. Cordycepin alleviates lipopolysaccharide-induced acute lung injury via Nrf(2)/HO-1 pathway. Int Immunopharmacol. 2018;60:18–25. doi: 10.1016/j.intimp.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Zhao B, Gao W, Gao X, Leng Y, Liu M, Hou J, Wu Y. Sulforaphane attenuates acute lung injury by inhibiting oxidative stress via Nrf2/HO-1 pathway in a rat sepsis model. Int J Clin Exp Pathol. 2017;10:9021–9028. [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Li HB, Chen L, Wang YX, Lu S, Li SN, Cui SN, Xiao HR, Qin L, Hu H, et al. BML-111 accelerates the resolution of inflammation by modulating the Nrf2/HO-1 and NF-κB pathways in rats with ventilator-induced lung injury. Int Immunopharmacol. 2019;69:289–298. doi: 10.1016/j.intimp.2019.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.