Abstract
Typically, tumor-associated macrophages (TAMs), an abundant population of leukocytes in lung cancer, are affected by tumor microenvironment (TME) and shift towards either a pro-tumor (M2-like) or an anti-tumor phenotype (M1-like). M2-polarized macrophages, are one of the primary tumor-infiltrating immune cells and were reported to be associated with the promotion of cancer cell growth, invasion, metastasis, and angiogenesis. TAMs are considered a potential target for adjuvant anticancer therapies, and recent therapeutic approaches targeting the M2 polarization of TAMs have shown encouraging results. The present review discusses recent developments in the role of TAMs in cancer, in particular TAMs functions, clinical implication and prospective therapeutic strategies in lung cancer.
Keywords: tumor-associated macrophages, lung cancer, tumor microenvironment, targeted therapy
1. Introduction
Lung cancer is one of the leading causes of cancer-associated mortalities worldwide, with a 5-year survival rate of <20% (1). Approximately 1.8 million new cases are diagnosed annually, of which 80% present with an advanced stage disease. Furthermore, ~50% of the patients are aged >65 years, while 30–40% are aged >70 years and are ineligible for surgery (2). In clinical practice, chemotherapy is the primary treatment modality for lung cancer. However, the majority of patients acquire chemoresistance and metastatic progression, which leads toward the failure of cancer-targeted therapies.
Several advances in tumor immunology in the past decade have aided the body's natural immune system in combating cancer. The tumor microenvironment (TME), characterized by the lack of nutrients, acidic and hypoxic environment, consists of cancerous and non-cancerous cells supporting tumor growth, invasion and metastasis (3). Furthermore, immune cells lose their anti-tumorigenic ability and antagonize antitumour activity. The mutual conversion of tumor-associated macrophages (TAMs), an abundant population of leukocytes in lung cancer, are determined by the TME (4). The TAM phenotypes dynamically alter during tumor progression. The M1-like macrophages are initially activated, and they produce chemokines and cytokines to recruit the cytotoxic CD8+ T and NK cells, which express high levels of IFN-γ and other cytokines to destroy the tumor cells (4). However, during tumor progression, the M2-like TAMs protect the cancer cells from anti-tumor immune responses, and promote their proliferation, angiogenesis, and metastasis. These M2-like TAMs secrete TGF-β to impede the cytotoxicity of NK cells, and express high levels of programmed cell death ligand 1 (PD-L1) to restrict the anti-tumor activity of T cells (5,6).
Clinical studies have suggested that increased TAM density correlates with a poor prognosis in solid tumors (5,7,8) Several animal model experiments have validated this observation by demonstrating that increased TAM density is associated with tumor progression and metastasis, and overexpression of macrophage growth factors or chemokines (9,10). The deletion or re-differentiation of TAMs enhances immune cell-mediated anti-tumor responses and benefits from chemotherapy (11–13). Therefore, targeting TAMs may be at the forefront of lung cancer research and a novel strategy for lung cancer therapy. The present review provides an overview of TAM biology and proposes a therapeutic strategy for targeting TAMs in lung cancer.
2. Macrophage plasticity in lung cancer development
Origin of TAMs in lung cancer
Accumulating evidence has suggested that TAMs originate from blood monocytes, and are recruited at tumor sites by tumor-derived chemotactic signals, including monocyte chemo-attractant protein-1 (MCP-1), which is also known as CCL2 (11–13). Furthermore, a small wave develops from in situ monocyte-macrophage proliferation and splenic monocytes. However, lung cancer exhibits a high proportion of tissue-resident macrophages, named alveolar macrophages (AMs), which are different to other solid tumors. The AMs are also derived from peripheral blood monocytes, but di-erentiated in response to interferon-γ (IFN-γ) and lipopolysaccharide (LPS) (14). The peripheral monocytes and resident mature monocytes significantly contribute toward the origin of TAMs in lung cancer. Furthermore, the functional diversity of TAMs is affected by local TME, and macrophage polarization occurs at any point in the tumorigenic process.
Opposite properties of M1 and M2 macrophages
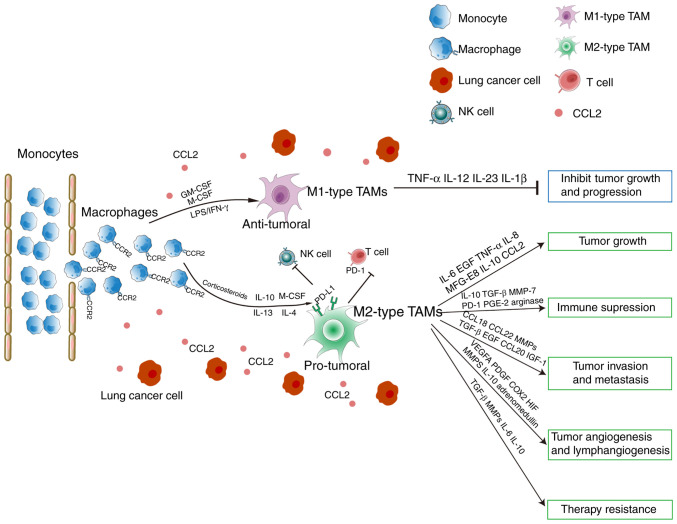
Similarly to two polarized sets of T helper 1/2 (Th1/Th2) cells, the TAMs are divided by dichotomy as classically activated M1 macrophages and alternatively activated M2 macrophages. The classical or M1 macrophages are activated by microbial products or interferon-γ (IFN-γ), conferring pro-inflammatory and microbicidal functions, and the capacity to facilitate tumor cell destruction (15). The microbial products or IFN-γ activate signal transducer and activator of transcription 1 (STAT1), interferon regulatory factor (IRF) 3, IRF5, and NF-κB, enable M1 macrophages to generate additional pro-inflammatory mediators (16). These are characterized by high production of nitric oxide (NO) and reactive oxygen intermediates (ROI), secretion of pro-inflammatory cytokines, including TNF-α, IL-1, IL-12 and IL-23, and high levels of MHC molecules (15,17) (Fig. 1).
Figure 1.
Macrophage polarization and function of TAMs in lung cancer. TAM, tumor-associated macrophage.
Additionally, Th2 cytokines, including IL-4 and IL-13, stimulate monocytes or macrophages to transform into the M2 phenotype (15). This macrophage subset triggers allergic reactions, promotes inflammation resolution and wound healing, and favors angiogenesis and tissue remodeling in cancer (Fig. 1). Apart from IL-4 and IL-13, other stimuli and signaling pathways, including IL-10, glucocorticoid hormones and IL-1R may also induce M2 macrophage polarization. There are central transcription regulators that activate the M2 phenotype, including STAT1, STAT3, STAT6, peroxisome proliferator-activated receptor (PPAR-γ), cAMP response element binding protein (CREB)-CCAAT/enhancer binding protein (C/EBP), hypoxia-inducible factor (HIF), IRF4 and PI3K/Akt (18–21).
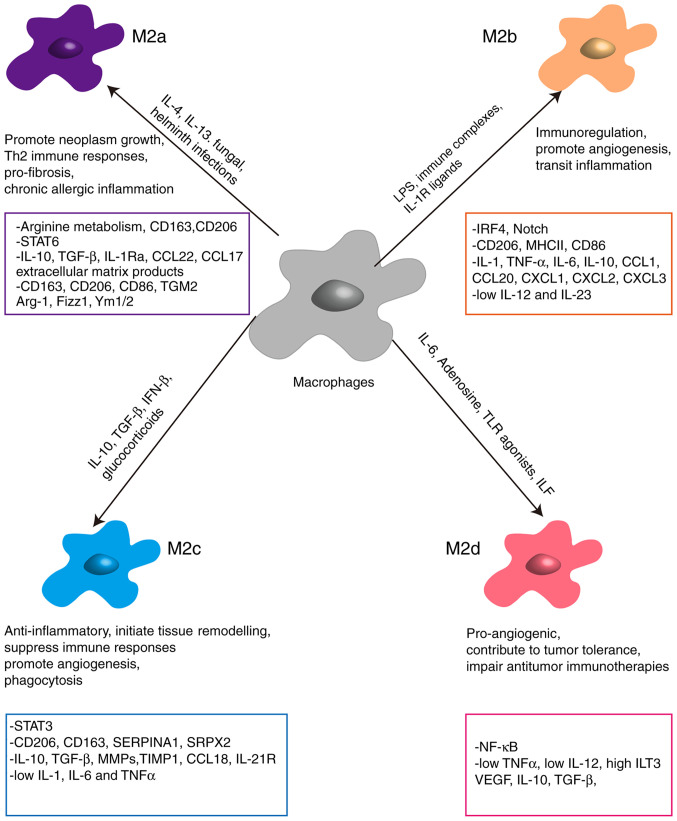
Based on their functions, M2 macrophages are further classified into M2a, M2b, M2c and M2d (Fig. 2). M2a, induced by IL-4 or IL-13, as well as fungal and helminth infections, express high levels of mannose receptor (CD206), CD209, IL-4R and FcεR, and secrete large amounts of TGF-β and insulin-like growth factor, which contribute toward wound healing and tissue repair (22). M2b, stimulated in response to immune complexes, IL-1β and bacterial LPS, are high producers of IL-10, IL-1β, IL-6 and TNF-α, which exert anti-inflammatory effects (23). M2c, induced by IL-10, TGF-β and glucocorticoids, are considered to be involved in immunosuppression, tissue repair and matrix remodeling (24). These macrophages exhibit increased expression of RAGE, CD163 and CD206, and secrete large amounts of IL-10 and TGF-β (25). Finally, M2d, activated by leukocyte inhibitory factor, TLR ligands and adenosine, express low levels of CD206, but produce significant amounts of IL-10, TGF-β and VEGF to promote tumor progression by facilitating immunosuppression and angiogenesis (26).
Figure 2.
Different types of M2-like macrophages in lung cancer. Arg-1, arginase-1; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; Fizz1, found in inflammatory zone 1; VEGF, vascular endothelial growth factor.
TAMs display pro-tumor M2 type macrophages
Compared to M1 macrophages, TAMs produce fewer ROIs and inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-12, CCL3 and CCL4) (27). While the NF-κB pathway is a key regulator of inflammation, TAMs display defective NF-κB activation, indicating low expression of NF-κB-dependent cytotoxic mediators and inflammatory cytokines (16). By contrast, typical M2 markers, including the scavenger receptor-A (SR-A), mannose receptor (MR), arginase-I (Arg-I), YM1 and FIZZ1, and MGL2 showed higher expression in TAMs (16). Previous studies have suggested that TAMs present M2-associated function by secreting pro-angiogenic and tumor-inducing chemokines, including epidermal growth factor (EGF), VEGF and TGF-β (28,29). Therefore, the notion that TAMs resemble M2 macrophages has been supported in vitro and in vivo (30).
The M2-type macrophages may be reversed to M1-type under certain conditions. Macrophages are highly plastic cells that may be differentiated into several phenotypes. Polarization is dynamic and affected by the TME. The dichotomy of M1 and M2 subtypes is over-generalized and only partially represents the continuity of polarization. For example, 5% of the AMs from lung cancer express M1 and M2 markers (31), and mixed polarization phenotypes (displaying M1 and M2 characteristics, HLA-DR, IL-1β, TNF-α, CD163 and IL10) have been described (32). Therefore, M1 and M2 markers may be used to distinguish macrophage populations to a certain extent.
3. Functional aspects of macrophages in lung cancer
TAMs in lung cancer initiation and progression
TAMs provide a suitable microenvironment to support growth, immunosuppression, invasion and therapeutic resistance in lung cancer, primarily by secreting TGF-β, IL-10, CCL18, matrix metalloproteases (MMPs), VEGF, COX2 and PDGF-B (Fig. 1).
IL-10
In vitro, the TAMs derived from THP-1 cells co-cultured with A549 and H1299 cells promoted epithelial-to-mesenchymal transition (EMT) and invasion in lung cancer cells (33,34). Furthermore, TAMs may activate and protect cancer stem cells (CSCs) to promote tumor progression by secreting IL-10 (35). When tumor cell proliferation is uncontrolled, oxygen and nutrition are limited, leading to hypoxia. Hypoxia skews macrophages to the M2-like phenotype with increased expression of IL-10, HIF1α and VEGF (36). Hypoxia then drives macrophage diversity to facilitate lung cancer cell metastasis, angiogenesis, and immune evasion in vitro and in vivo (36,37). Clinical data have demonstrated that increased gene expression of macrophage-derived IL-10 in tumor tissues was significantly correlated with stage, tumor size, lymph node metastasis, lymphovascular invasion, or histologically poor differentiation (38).
IL-6
The macrophages derived from THP-1 exhibit high expression of IL-6 when co-cultured with human non-small cell lung cancer (NSCLC) A549 or H1299 cells, and enhances the invasive ability of lung cancer cells by regulating EMT (34). Additionally, IL-6 may stimulate macrophages to express higher levels of IL-10, and together, IL-6 and IL-10 induce M2 macrophage differentiation in an IL-4-dependent manner via STAT3 activation (39); while, IL-6-induced macrophage infiltration proceeds via the CCL2/CCL5 pathway in NSCLC. Abrogation or suppression of IL-6 expression may inhibit TAM-induced invasion and angiogenesis in lung cancer cells (34,40).
TGF-β
TGF-β, together with its co-receptor endoglin, serves a vital role in tissue repair, and angiogenesis and lymphangiogenesis. A previous study reported an increase in the levels of endoglin during the process of monocyte transition to macrophages (41). Furthermore, macrophages and pro-inflammatory cytokines are significantly downregulated in Eng+/− mice (42). The TGF-β, released by tumor cells and M2 type macrophages, may suppress M1 polarized macrophages, and stimulate mature macrophages to polarize to the pro-tumor M2 type. Maeda et al (43) reported that IL-10 expression in macrophages is positively associated with TGF-β expression, and that TGF-β enhances Mφ to secrete IL-10, promoting tumor progression in tumor-bearing mice (43). A previous study has shown that TGF-β secreted by TAMs promotes EMT, and upregulates the expression of SOX9, which enhances tumor cell proliferation, migration and invasion (44). Furthermore, suppressing the expression of TGF-β may inhibit TGF-β1-induced EMT in A549 lung cancer cells (45).
MMPs
Furthermore, TAMs induce lung cancer cell invasion by producing MMPs, including MMP-9 and MMP-2, and degrading the extracellular matrix. MMP-9 expression is associated with lymph node metastases, tumor progression and prognosis (46). IL-10-induced macrophages enhance MMP-9 and MMP-2 expression and promote cancer cell invasion and migration (47). Therefore, inhibition of MMP production may reverse macrophage-mediated cancer cell invasion and migration activity (46–48).
Chemokines
Chemokines are a family of soluble and chemotactic cytokines that are secreted by and mediate the chemotaxis and migration of immune or tumor cells. Recent advances have indicated that chemokines originating from TAMs, including CCL18, MIP-3α, CCL5, CXCL8, and CCL22, serve critical roles in cancer progression by binding to their cognate receptors in carcinoma cells (49–51). Early evidence has suggested that CCL22 is highly expressed in lung cancer, and is a predictive marker for disease-free survival duration and tumor recurrence (49–52). CCL22 may promote the bone metastasis of lung cancer cells that express CCR4 (53). CXCL8, an M2-related chemokine secreted by TAMs, also serves a role in lung cancer. Previous studies have suggested that CXCL8 may induce EMT, and accelerate invasion and migration via the MAPK/NF-κB and JAK2/STAT3 signaling pathways (54,55). Therefore, therapies or drugs targeting CXCL8 may attenuate cell proliferation, invasion, and migration in lung cancer (55,56).
Angiogenesis
TAMs serve a key role in facilitating angiogenesis by producing pro-angiogenic factors, including IL-8, VEGF, urokinase plasminogen activator (uPA), and MMPs, (Fig. 1). TAM density is associated with intra-tumoral microvessel counts in NSCLC (57). Chen et al (58) reported that the THP-1-derived M2-type macrophages may promote angiogenesis in NSCLC, by producing proangiogenic factors, including IL-8, and supporting the generation of blood vessels (58). Hypoxia is a local attractant for TAMs in the TME, which induces the expression of HIF-1 and HIF-2; and HIF-2 may upregulate VEGF expression (59,60). Additionally, VEGF is also a chemoattractant for TAMs, which forms a positive feedback loop to promote tumor angiogenesis (61).
Immunosuppression
In TME, macrophages not only lose their anti-cancer properties, but also impede the immunoregulatory functions of other immune cells. The TAMs upregulate the expression of PD-L1 to suppress T-cell toxicity and inhibit phagocytosis (5,62). The CD8+ T cells are excluded by TAMs, and thus cannot act near the cancer cells (63). Furthermore, TAMs produce cytokines and other proteins to maintain immunosuppression, including CCL-22, CCL-17, TGF-β, arginase 1 and galectin-3 (28,29). The AMs stimulated by the Th2 cells produce immunosuppressive cytokines, including IL-10 and TGF-β in the lung TME to reduce the number of tumor-infiltrating lung dendritic cells (DCs) and block their maturation (64,65). Furthermore, IL-10 triggers the immunosuppression of T cells by upregulating PD-L1 expression in tumor macrophages (38,66). The blockade or deficiency of IL-10 may induce CD8+ T cell cytotoxicity and promote tumor-resident CD8+ T cell expansion (66). Additionally, the macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment by recruiting Tregs (67). Furthermore, Young et al (68) indicated that NK cell cytotoxicity is also suppressed and facilitates pulmonary metastases (68). Depletion of the AM or reversal of M2 polarization may relieve immunosuppression imposed by the macrophages, and strengthen local Th1 anticancer activity (64).
Chemotherapy resistance
Resistance to chemotherapy increases the difficulty of therapeutic efficacy, and drives tumor progression, recurrence, and distant, bone and lymph node metastasis. A strong correlation has been demonstrated between TAMs and chemotherapy resistance (13,69). A previous study reported that abundant CD68+ and CD163+ macrophages accumulate inside or adjacent to tumors following chemotherapy (69). In a mouse Lewis lung carcinoma model (LLC1s), treatment with chemotherapeutic agents induces neoplastic cells that release CXCL12, which enhances the infiltration of CD206+ TAMs, inhibits tumor cell death, and assists in tumor relapse (13). Additionally, treatment with cisplatin or carboplatin induces tumor cells to secrete IL-6 and/or prostaglandin E2 (PGE2), which mediates M2 macrophage polarization via activation of the STAT3, STAT1 and STAT6 signaling pathways, and resists cytotoxic chemotherapy (70,71). Furthermore, DeNardo et al (72) illustrated that paclitaxel treatment boosts the infiltration of macrophages, which limits the recruitment and efficacy of CD8+ cytotoxic T cells, and inhibits the antitumor activity of paclitaxel (72). Recent large cohort clinical studies have reported a close correlation between the infiltration of M2-macrophages, poor response to chemotherapy, and poor clinical outcomes (73,74). The elimination of TAMs by anti-CSF-1 or anti-CCL2 antibodies, preventing M2-differentiation by COX inhibitors, and/or anti-IL-6R antibodies may enhance the cytotoxic effects of chemotherapeutic agents, including taxol, cisplatin, and doxorubicin (75,76). Therefore, concomitant therapy with an intervention strategy that reduces macrophage population or inhibits M2 polarization may amplify the antitumor activity of chemotherapeutic agents.
4. Clinical implications of TAMs in lung cancer
Clinical studies have suggested that the density of macrophages, particularly M2 type, is associated with a poor prognosis in almost all human cancer types (7,8). However, there are conflicting data with regards to lung cancer. CD68, a common monocyte/macrophage marker, when used to label TAMs, indicated it to act as an independent prognostic factor, and a higher percentage of tumor islets were found to be correlated with improved outcomes (77,78). However, other studies observed no association between CD68+ macrophage densities and tumor islets or stroma with patients' survival duration (79,80). This is possibly due to involvement of the margin or central macrophages.
Usually, the CD68+CD163+ or CD68+CD206+ markers are used to identify M2 macrophages. Zhang et al (81) indicated that levels of M2-type (CD68+CD206+) were positively associated with peritumoral lymphatic microvessel density, but negatively associated with patients' prognoses (81). In line with this, emerging research has suggested that the accumulation of CD163+ macrophages is closely correlated with a poor prognosis in lung cancer. Furthermore, an increased density of CD68+CD163+ macrophages in tumor nests and stroma was associated with lymph node metastases (81), but no such association was observed with recurrence-free survival (RFS), overall survival (OS), and TNM stages (80,82). However, Cao et al (7) found that levels of CD68+CD163+M2 were correlated with OS and DFS in NSCLC (7). Furthermore, increased infiltration of macrophages was observed in patients with lung squamous cell carcinoma (LUSC), wild-type EGFR, and smoking habits (7).
Additionally, M2-TAMs labeled with CD204+ serve a role in prognosis. High infiltration of CD204+TAMs in the stroma may be correlated with TNM stages, presence of vascular and pleural invasion, and OS and RFS in patients with stage II LUSC. However, no association was observed between the levels of CD204+ macrophages and poor patient outcomes (83).
Taken together, the different data or contradictory results of previous studies may be explained by the tumor histological type and origin in patients, methodologies applied in counting TAMs, and definition of islet and stroma. Furthermore, a recent meta-analysis reported that M2-type TAMs or M1/M2 polarization in the lung cancer islets or stroma are associated with tumor progression. Therefore, targeting TAMs may be considered as a newer anti-tumor strategy in lung cancer.
5. TAM-targeted therapeutics
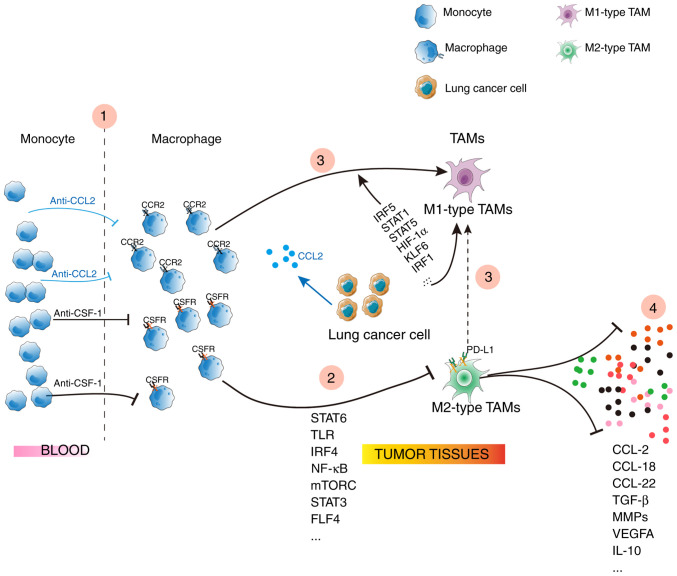
TAMs, the major component of leukocyte infiltration in tumors, serve an important role in tumor behavior, and thus therapies targeting TAMs are employed. To begin with, inhibition of macrophages infiltrating the tumor; CSF1-CSF1R and CCL2-CCR2 may induce macrophage recruitment, and blockade of CCL2-CCR2 or CSF1-CSF1R may decrease TAM infiltration, reversing the immunosuppressive status (84) (Fig. 3), but anti-CCL2 therapy may aggravate metastasis (85). A second strategy is that blockade of TAMs repolarize into the M2-type: Few signaling components regulate M2 macrophage polarization, including the Toll-like receptors (TLR), STAT6 and NK-κB. When these signals are intervened, TAMs lose their ‘alternative’ activated phenotype. A third strategy would involve reeducating TAMs to M1-type or switching M2 to M1: Several drugs, including BTH1677 (a yeast β-glucan immunomodulator), hydroxychloroquine, and celecoxib, switch M2-like TAMs to an antitumor phenotype, or M1-like TAMs (86–88). A final strategy is based on the fact that decreasing the levels of critical TAM-secreted cytokines involved in tumor biology: For example, CCL18, CCL22, and MIP-3α, mainly produced by the M2-type macrophages, confer malignant behaviors (9,10,49). Blockade of CCL18, CCL22, or MIP-3α weakens the TAM-mediated pro-tumor ability (9,10,89).
Figure 3.
Anti-tumor therapies targeted TAMs in lung cancer. IL, interleukin; TGF-β, transforming growth factor-beta; MMPs, matrix metalloproteinases; VEGFA, vascular endothelial growth factor A; TAM, tumor-associated macrophage.
The aforementioned strategies provide enhanced and promising therapeutic effects, although there are a few major issues or side effects that require attention, including the efficiency of specific drug delivery and nontargeting TAMs. Evidence has indicated that nanoparticles or nanoparticle-based drug delivery are more reliable and effective in regulating the macrophage phenotype by ensuring that the drug reaches the cancer site without off-target activity. Several studies have demonstrated that nanodrugs offer superiority in mediating the polarization of macrophages with increased drug uptake. For instance, curcumin (Cur), baicalin (Bai), and ginseng-derived nanoparticles have been reported to alter TAM polarization without discernible toxicity (90–92). Compared to the drugs themselves, their nanoparticle derivatives showed improved pharmacokinetics and bioavailability in systemic circulation, and thus contributed toward excellent antitumor responses (90–92). Furthermore, few materials used in nanoparticle production, including TiO2 and Ag, may preferentially polarize TAMs towards an M1 phenotype (93,94).
We hypothesize that every immune cell serves an equal role in the body, and macrophages have dual property; therefore, eliminating or decreasing macrophages is not a rational approach and has other disadvantages. By contrast, ‘reeducating’ the macrophages or targeting the tumorigenic cytokines or chemokines secreted by the macrophages should be studied as a preferred strategy for combating cancer.
6. Conclusions
Several experimental and clinical studies have demonstrated that TAMs serve a seminal role in the growth, angiogenesis, metastasis, and invasion in lung cancer. Furthermore, TAMs confer chemotherapy resistance and immunosuppression. Therefore, TAMs are now considered a promising target in the treatment of lung cancer. However, no appropriate drugs have been administered in the patients, and newer treatment approaches may ascertain improved clinical outcomes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Development Project of Shanghai Peak Disciplines-Integrative Medicine and Western Medicine (grant no. 20150407), National Natural Science Program of China (grant nos. 81673916 and 81403148) and the Development Plan of Shandong Medical and Health Technology (grant no. 2019WS581).
Availability of data and materials
Not applicable.
Authors' contributions
FX and YW wrote the manuscript; ZT made contributions to the figures; BL and JD contributed toward the literature review and revised the manuscript. All authors read and appvoed the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no competing interests.
References
- 1.Brenman JE, Temple BR. Opinion: Alternative views of AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:321–331. doi: 10.1007/s12013-007-0005-x. [DOI] [PubMed] [Google Scholar]
- 2.Gridelli C, Perrone F, Monfardini S. Lung cancer in the elderly. Eur J Cancer. 1997;33:2313–2314. doi: 10.1016/S0959-8049(97)10050-8. [DOI] [PubMed] [Google Scholar]
- 3.Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. doi: 10.1016/j.cellimm.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer. 2019;136:136–144. doi: 10.1016/j.lungcan.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao L, Che X, Qiu X, Li Z, Yang B, Wang S, Hou K, Fan Y, Qu X, Liu Y. M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag Res. 2019;11:6125–6138. doi: 10.2147/CMAR.S199832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp Ther Med. 2019;18:4490–4498. doi: 10.3892/etm.2019.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Peng Y, Wu X, Meng S, Yu W, Zhao J, Zhang H, Wang J, Li W. CCL18 secreted from M2 macrophages promotes migration and invasion via the PI3K/Akt pathway in gallbladder cancer. Cell Oncol (Dordr) 2019;42:81–92. doi: 10.1007/s13402-018-0410-8. [DOI] [PubMed] [Google Scholar]
- 10.Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, Ng KT, Forbes SJ, Guan XY, Poon RT, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Sarode P, Zheng X, Giotopoulou GA, Weigert A, Kuenne C, Günther S, Friedrich A, Gattenlöhner S, Stiewe T, Brüne B, et al. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: A potential treatment of lung cancer. SCI ADV. 2020;6:eaaz6105. doi: 10.1126/sciadv.aaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura Y, Sumiyoshi M, Baba K. Antitumor and antimetastatic activity of synthetic hydroxystilbenes through inhibition of lymphangiogenesis and M2 macrophage differentiation of tumor-associated macrophages. Anticancer Res. 2016;36:137–148. [PubMed] [Google Scholar]
- 13.Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 2015;75:3479–3491. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty DM, Monick MM, Hinde SL. Human alveolar macrophages are deficient in PTEN. The role of endogenous oxidants. J Biol Chem. 2006;281:5058–5064. doi: 10.1074/jbc.M508997200. [DOI] [PubMed] [Google Scholar]
- 15.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 16.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 17.Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res. 2001;61:7305–7309. [PubMed] [Google Scholar]
- 18.Nam S, Lim J. Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch Pharm Res. 2016;39:1548–1555. doi: 10.1007/s12272-016-0854-1. [DOI] [PubMed] [Google Scholar]
- 19.Gong M, Zhuo X, Ma A. STAT6 Upregulation promotes M2 macrophage polarization to suppress atherosclerosis. Med Sci Monit Basic Res. 2017;23:240–249. doi: 10.12659/MSMBR.904014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198:1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 21.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MP, Christmann BS, Dunaway CW, Morris A, Steele C. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am J Physiol Lung Cell Mol Physiol. 2012;303:L469–L475. doi: 10.1152/ajplung.00158.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol. 2010;184:6465–6478. doi: 10.4049/jimmunol.0904016. [DOI] [PubMed] [Google Scholar]
- 24.Koscsó B, Csóka B, Kókai E, Németh ZH, Pacher P, Virág L, Leibovich SJ, Haskó G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olmes G, Büttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C. CD163+ M2c-like macrophages predominate in renal biopsies from patients with lupus nephritis. Arthritis Res Ther. 2016;18:90. doi: 10.1186/s13075-016-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Ni H, Lan L, Wei X, Xiang R, Wang Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res. 2010;20:701–712. doi: 10.1038/cr.2010.52. [DOI] [PubMed] [Google Scholar]
- 27.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–1362. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 28.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003;63:527–533. [PubMed] [Google Scholar]
- 30.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 31.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 32.Helm O, Held-Feindt J, Grage-Griebenow E, Reiling N, Ungefroren H, Vogel I, Krüger U, Becker T, Ebsen M, Röcken C, et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135:843–861. doi: 10.1002/ijc.28736. [DOI] [PubMed] [Google Scholar]
- 33.Che D, Zhang S, Jing Z, Shang L, Jin S, Liu F, Shen J, Li Y, Hu J, Meng Q, Yu Y. Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE(2)/β-catenin signalling pathway. Mol Immunol. 2017;90:197–210. doi: 10.1016/j.molimm.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Dehai C, Bo P, Qiang T, Lihua S, Fang L, Shi J, Jingyan C, Yan Y, Guangbin W, Zhenjun Y. Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6. Immunol Lett. 2014;160:1–10. doi: 10.1016/j.imlet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Dong Y, Li Y, Wang D, Liu S, Wang D, Gao Q, Ji S, Chen X, Lei Q, et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1 pathway in non-small cell lung cancer. Int J Cancer. 2019;145:1099–1110. doi: 10.1002/ijc.32151. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Cao J, Ma S, Dong R, Meng W, Ying M, Weng Q, Chen Z, Ma J, Fang Q, et al. Tumor hypoxia enhances Non-Small Cell Lung Cancer metastasis by selectively promoting macrophage M2 polarization through the activation of ERK signaling. Oncotarget. 2014;5:9664–9677. doi: 10.18632/oncotarget.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 38.Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, Chen H. Increased IL-10 mRNA expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res. 2011;30:62. doi: 10.1186/1756-9966-30-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng YS, Yu PW, Zhuang Y, Zhao YL. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;66:1597–1608. doi: 10.1007/s00262-017-2052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Yang X, Tsai Y, Yang L, Chuang KH, Keng PC, Lee SO, Chen Y. IL-6 mediates macrophage infiltration after irradiation via up-regulation of CCL2/CCL5 in non-small cell lung cancer. Radiat Res. 2017;187:50–59. doi: 10.1667/RR14503.1. [DOI] [PubMed] [Google Scholar]
- 41.de Cortie K, Russell NS, Coppes RP, Stewart FA, Scharpfenecker M. Bone marrow-derived macrophages incorporate into the endothelium and influence vascular and renal function after irradiation. Int J Radiat Biol. 2014;90:769–777. doi: 10.3109/09553002.2014.920967. [DOI] [PubMed] [Google Scholar]
- 42.Russell NS, Floot B, van Werkhoven E, Schriemer M, de Jong-Korlaar R, Woerdeman LA, Stewart FA, Scharpfenecker M. Blood and lymphatic microvessel damage in irradiated human skin: The role of TGF-β, endoglin and macrophages. Radiother Oncol. 2015;116:455–461. doi: 10.1016/j.radonc.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Maeda H, Kuwahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- 44.Zhang S, Che D, Yang F, Chi C, Meng H, Shen J, Qi L, Liu F, Lv L, Li Y, et al. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. Oncotarget. 2017;8:99801–99815. doi: 10.18632/oncotarget.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HJ, Park MK, Lee EJ, Lee CH. Resolvin D1 inhibits TGF-β1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int J Biochem Cell Biol. 2013;45:2801–2807. doi: 10.1016/j.biocel.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, Liu S, Qin Y, Chen H. Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer. 2011;74:188–196. doi: 10.1016/j.lungcan.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso AP, Pinto ML, Pinto AT, Pinto MT, Monteiro C, Oliveira MI, Santos SG, Relvas JB, Seruca R, Mantovani A, et al. Matrix metalloproteases as maestros for the dual role of LPS- and IL-10-stimulated macrophages in cancer cell behaviour. BMC Cancer. 2015;15:456. doi: 10.1186/s12885-015-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem. 2012;287:38957–38969. doi: 10.1074/jbc.M112.357863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang B, Shi L, Sun X, Wang L, Wang X, Chen C. Production of CCL20 from lung cancer cells induces the cell migration and proliferation through PI3K pathway. J Cell Mol Med. 2016;20:920–929. doi: 10.1111/jcmm.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L, Zhang B, Sun X, Zhang X, Lv S, Li H, Wang X, Zhao C, Zhang H, Xie X, et al. CC chemokine ligand 18(CCL18) promotes migration and invasion of lung cancer cells by binding to Nir1 through Nir1-ELMO1/DOC180 signaling pathway. Mol Carcinog. 2016;55:2051–2062. doi: 10.1002/mc.22450. [DOI] [PubMed] [Google Scholar]
- 51.Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, Wang X, Zhang J, Guo L, Wang S, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234. doi: 10.1038/s41419-020-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakanishi T, Imaizumi K, Hasegawa Y, Kawabe T, Hashimoto N, Okamoto M, Shimokata K. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol Immunother. 2006;55:1320–1329. doi: 10.1007/s00262-006-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura ES, Koizumi K, Kobayashi M, Saitoh Y, Arita Y, Nakayama T, Sakurai H, Yoshie O, Saiki I. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 54.Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H, Zheng P, Zhao S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10:918. doi: 10.1038/s41419-019-2131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao H, Huang Y, Wang L, Wang H, Pang X, Li K, Dang W, Tang H, Wei L, Su M, et al. Leptin promotes migration and invasion of breast cancer cells by stimulating IL-8 production in M2 macrophages. Oncotarget. 2016;7:65441–65453. doi: 10.18632/oncotarget.11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q, Li A, Yu S, Qin S, Han N, Pestell RG, Han X, Wu K. DACH1 antagonizes CXCL8 to repress tumorigenesis of lung adenocarcinoma and improve prognosis. J Hematol Oncol. 2018;11:53. doi: 10.1186/s13045-018-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tataroğlu C, Kargi A, Ozkal S, Eşrefoğlu N, Akkoçlu A. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC) Lung Cancer. 2004;43:47–54. doi: 10.1016/j.lungcan.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 59.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 60.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/A:1020304003704. [DOI] [PubMed] [Google Scholar]
- 61.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guerin M, Biton J, Ouakrim H, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci USA. 2018;115:E4041–E4050. doi: 10.1073/pnas.1720948115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 65.Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A. The chemokine receptor switch paradigm and dendritic cell migration: Its significance in tumor tissues. Immunol Rev. 2000;177:141–149. doi: 10.1034/j.1600-065X.2000.17714.x. [DOI] [PubMed] [Google Scholar]
- 66.Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, Fu K, Peng J, Wang Y, Ren Z, et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8+ T cell apoptosis. Cancer Cell. 2019;35:901–915.e4. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Wang D, Yang L, Yue D, Cao L, Li L, Wang D, Ping Y, Shen Z, Zheng Y, Wang L, Zhang Y. Macrophage-derived CCL22 promotes an immunosuppressive tumor microenvironment via IL-8 in malignant pleural effusion. Cancer Lett. 2019;452:244–253. doi: 10.1016/j.canlet.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 68.Young MR, Endicott RA, Duffie GP, Wepsic HT. Suppressor alveolar macrophages in mice bearing metastatic Lewis lung carcinoma tumors. J Leukoc Biol. 1987;42:682–688. doi: 10.1002/jlb.42.6.682. [DOI] [PubMed] [Google Scholar]
- 69.De Palma M, Lewis CE. Cancer: Macrophages limit chemotherapy. Nature. 2011;472:303–304. doi: 10.1038/472303a. [DOI] [PubMed] [Google Scholar]
- 70.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 71.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang C, Yu X, Gao L, Zhao Y, Lai J, Lu D, Bao R, Jia B, Zhong L, Wang F, Liu Z. Noninvasive imaging of CD206-positive M2 macrophages as an early biomarker for post-chemotherapy tumor relapse and lymph node metastasis. Theranostics. 2017;7:4276–4288. doi: 10.7150/thno.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752–759. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 75.Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349–4356. doi: 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- 76.Salvagno C, Ciampricotti M, Tuit S, Hau CS, van Weverwijk A, Coffelt SB, Kersten K, Vrijland K, Kos K, Ulas T, et al. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol. 2019;21:511–521. doi: 10.1038/s41556-019-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, Ma J, Ma L, You Z. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Welsh TJ, Green RH, Richardson D, Waller DA, O'Byrne KJ, Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol. 2005;23:8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 79.Pei BX, Sun BS, Zhang ZF, Wang AL, Ren P. Interstitial tumor-associated macrophages combined with tumor-derived colony-stimulating factor-1 and interleukin-6, a novel prognostic biomarker in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148:1208–1216.e2. doi: 10.1016/j.jtcvs.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B, Yao G, Zhang Y, Gao J, Yang B, Rao Z, Gao J. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo) 2011;66:1879–1886. doi: 10.1590/S1807-59322011001100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirayama S, Ishii G, Nagai K, Ono S, Kojima M, Yamauchi C, Aokage K, Hishida T, Yoshida J, Suzuki K, Ochiai A. Prognostic impact of CD204-positive macrophages in lung squamous cell carcinoma: Possible contribution of Cd204-positive macrophages to the tumor-promoting microenvironment. J Thorac Oncol. 2012;7:1790–1797. doi: 10.1097/JTO.0b013e3182745968. [DOI] [PubMed] [Google Scholar]
- 84.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 85.Keklikoglou I, De Palma M. Cancer: Metastasis risk after anti-macrophage therapy. Nature. 2014;515:46–47. doi: 10.1038/nature13931. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Cao F, Li M, Li P, Yu Y, Xiang L, Xu T, Lei J, Tai YY, Zhu J, et al. Hydroxychloroquine induced lung cancer suppression by enhancing chemo-sensitization and promoting the transition of M2-TAMs to M1-like macrophages. J Exp Clin Cancer Res. 2018;37:259. doi: 10.1186/s13046-018-0938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Sun Z, Pei J, Luo Q, Zeng X, Li Q, Yang Z, Quan J. Identification of α-mangostin as an agonist of human STING. Chemmedchem. 2018;13:2057–2064. doi: 10.1002/cmdc.201800481. [DOI] [PubMed] [Google Scholar]
- 88.Brandão RD, Veeck J, Van de Vijver KK, Lindsey P, de Vries B, van Elssen CH, Blok MJ, Keymeulen K, Ayoubi T, Smeets HJ, et al. A randomised controlled phase II trial of pre-operative celecoxib treatment reveals anti-tumour transcriptional response in primary breast cancer. Breast Cancer Res. 2013;15:R29. doi: 10.1186/bcr3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu B, Zou L, Cheng X, Lin Z, Duan Y, Wu Y, Zhou F, Chen Z. Administration of MIP-3alpha gene to the tumor following radiation therapy boosts anti-tumor immunity in a murine model of lung carcinoma. Immunol Lett. 2006;103:101–107. doi: 10.1016/j.imlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 90.Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, Tavassoli A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev. 2015;16:3917–3922. doi: 10.7314/APJCP.2015.16.9.3917. [DOI] [PubMed] [Google Scholar]
- 91.Han S, Wang W, Wang S, Wang S, Ju R, Pan Z, Yang T, Zhang G, Wang H, Wang L. Multifunctional biomimetic nanoparticles loading baicalin for polarizing tumor-associated macrophages. Nanoscale. 2019;11:20206–20220. doi: 10.1039/C9NR03353J. [DOI] [PubMed] [Google Scholar]
- 92.Cao M, Yan H, Han X, Weng L, Wei Q, Sun X, Lu W, Wei Q, Ye J, Cai X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J, Song W, Guo J, Zhang J, Sun Z, Li L, Ding F, Gao M. Cytotoxicity of different sized TiO2 nanoparticles in mouse macrophages. Toxicol Ind Health. 2013;29:523–533. doi: 10.1177/0748233712442708. [DOI] [PubMed] [Google Scholar]
- 94.Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, Choi IH, Cheon J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb) 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.