Abstract
Renal cell carcinoma (RCC) is a primary malignant kidney cancer subtype. It has been suggested that long non-coding RNAs (lncRNAs) serve important roles in the progression of kidney cancer. In fact, the lncRNA small nucleolar RNA host gene 12 (SNHG12) was discovered to be overexpressed in various types of cancer. However, to the best of our knowledge, the role of SNHG12 in RCC remains unclear. The present study aimed to investigate the function of SNHG12 and its underlying molecular mechanism of action in RCC. In patient samples and datasets from The Cancer Genome Atlas. Reverse transcription-quantitative PCR, demonstrated that SNHG12 expression levels were upregulated in RCC tumor tissues, but not in normal kidney tissues. SNHG12 upregulation was also observed in RCC cell lines. Kaplan-Meier survival analysis indicated a poor prognosis for those patients with RCC who had upregulated SNHG12 expression levels. Following lentivirus transduction, SNHG12 was successfully knocked down (validated by western blot analysis) and cell migration and invasion assays were performed. SNHG12 knockdown markedly inhibited cell viability and invasion, while increasing apoptosis in both A498 and 786O cell lines. The results of the luciferase reporter assay suggested that SNHG12 exerted its role by sponging microRNA (miR)-200c-5p, which led to the upregulation of its target gene, collagen type XI α1 chain (COL11A1). This was further validated, as miR-200c-5p inhibition reduced the effects of SNHG12 downregulation on cell viability and apoptosis, without affecting SNHG12 expression levels. Furthermore, the findings indicated that SNHG12 may partially exert its role through COL11A1, which was also upregulated in RCC. In conclusion, the results of the present study suggested that the SNHG12/miR-200c-5p/COL11A1 axis may be crucial for RCC progression, which provided an insight into potential therapeutic strategies for RCC treatment.
Keywords: long non-coding RNA, small nucleolar RNA host gene 12, renal cell carcinoma, microRNA-200c-5p, collagen type XI α1 chain
Introduction
Kidney cancer is prevalent in both males and females, and renal cell carcinoma (RCC) is the most common form of kidney malignancy (1). Although treatment options for RCC have improved over the last decade from a non-specific immune approach (2), to therapies targeting vascular endothelial growth factor (VEGF) or tyrosine-protein kinase receptor UFO precursor (AXL) (3), the overall survival rate remains low. Although the five-year disease specific survival in patients with stage I–II reaches 80%, it is only 60% in stage III and <10% in stage IV patients (4). Therefore, understanding the molecular mechanisms of RCC development and progression is essential.
Long non-coding RNAs (lncRNAs) are non-protein coding transcripts of >200 nucleotides in length (5). Previous studies have suggested that lncRNAs have a prominent role in the competing endogenous RNA (ceRNA) network, in which they serve as microRNA (miRNA/miR) sponges, thus controlling the expression of genes associated with cancer development (6–8). For instance, the lncRNA HOX transcript antisense RNA (HOTAIR) was discovered to affect the proliferation and tumorigenesis of RCC by inhibiting the miR-217/hypoxia-inducible factor-1 (HIF-1)α/AXL pathway (9). Additionally, lncRNA H19 was suggested to potentially mediate breast cancer cell plasticity by differentially sponging miR-200b/c and let-7b (10). Thus, these findings indicated that lncRNAs may be crucial regulators of cancer progression and metastasis (11–13). Nonetheless, their function in RCC remains poorly understood.
The expression levels of small nucleolar RNA host gene 12 (SNHG12), located on chromosome 1p35.3, were discovered to be upregulated in several types of cancer, including non-small cell lung cancer, glioma, cervical cancer and bladder cancer (14–17). In previous studies, SNHG12 was also reported to function as an miRNA sponge and influence several tumor-related pathways, such as the Wnt/β-catenin signaling pathway and the Jak/STAT3 signaling pathway (15,17–19). In addition, SNHG12 was identified to serve as an oncogene, where it had a crucial role in tumor progression (20,21). SNHG12 has also been associated with the unfavorable prognosis of nasopharyngeal carcinoma and gastric cancer, where it served as a potential biomarker for cancer progression (22,23). However, to the best of our knowledge, the role of SNHG12 in RCC remains unknown.
In the present study, SNHG12 expression levels were investigated in samples obtained from patients with RCC. SNHG12 knockdown significantly inhibited the viability and invasion of RCC cell lines, while increasing apoptosis. Notably, SNHG12 was discovered to directly interact with miR-200c-5p, which indicated that it may serve as a sponge and control collagen type XI α1 chain (COL11A1) expression levels. In conclusion, the findings of the present study suggested that the SNHG12/miR-200c-5p/COL11A1 axis may be involved in RCC progression.
Materials and methods
Clinical samples
A total of 58 patients (36 males and 22 females; age, 31–72 years) diagnosed with RCC by pathological examination after surgery at The Department of Urology, Hainan General Hospital (Haikou, China) (August 2014-August 2017) were enrolled in the present study. Patients who received chemotherapy, radiotherapy or any other therapy were excluded from the present study. Tumor tissues and paired adjacent non-tumor renal tissues were collected and stored at −80°C for subsequent RNA extraction. Patients were classified as RCC according to the World Health Organization criteria (24) and tumor staging was determined using the tumor-node-metastasis (TNM) classification (4). The study was approved by the Medical Ethics Committee of Hainan General Hospital (Haikou, China). Written, informed consent was obtained from all 58 patients.
TCGA database
RNA sequencing and clinical data of patients with RCC were downloaded from Genomic Data Commons Data Portal (portal.gdc.cancer.gov). Patient data were excluded if >75% lncRNA data was missing or if survival information was not complete. Patients >18 years old were included in the study. After screening, a total of 278 patients with RCC and 33 non-tumor kidney samples were used in the present study (25). TCGA data was analyzed using RStudio version 0.99.896 software (RStudio, Inc.). Survival analysis were conducted using Kaplan-Meier plots by SPSS version 22.0 software (IBM, Corp.).
Cell culture
Human renal cell carcinoma cell lines A498, 786O, Caki-1, Caki-2 and ACHN, and the normal renal epithelial cell line HK-2 as well as 293T cell line were purchased from the American Type Culture Collection. Cells were cultured in RPMI-1640 medium, supplemented with 10% FBS and 1% penicillin/streptomycin (all from Gibco; Thermo Fisher Scientific, Inc.). All cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Cell transfection
Small interfering RNAs (siRNAs/si) targeting SNHG12 and negative control (NC; si-NC) were synthesized by Guangzhou RiboBio Co., Ltd. using the following sequences: Si-SNHG12 sense, 5′-GCAGUGUGCUACUGAACUUTT-3′ and antisense, 5′-AAGUUCAGUAGCACACUGCTT-3′; and si-NC sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′. Si-COL11A1 was purchased from Sigma-Aldrich; Merck KGaA. si-NC for si-COL11A1 was the same with si-SNHG12. The miR-200c mimics, miR-NC, miR-200c inhibitor, inhibitor-NC, pcDNA SNHG12 and pcDNA3.1 empty vector were purchased from GeneCopoeia, Inc. The sequences were as follows: Si-COL11A1 sense, 5′-UUCUAAAUUUGAUGGUUUGCGTT-3′ and antisense, 5′-CAAACCAUCAAAUUUAGAAGA-3′; miR-200c-5p mimics, 5′-UAAUACUGCCGGGUAAUGAUGGA-3′; miR-mimics-NC, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′; and miR-200c-5p inhibitor, 5′-UAAUACUGCCGGGUAAUGAUGGA-3′; inhibitor-NC, 5′-CGAACUUAUCUUAGGUACUC-3′. Briefly, 1×106 A498 or 786O cells were seeded into six-well plates and transfected with 50 nM siRNAs, 30 nM mimics, 30 nM inhibitors, 2 µg pcDNA SNHG12 or pcDNA 3.1 empty vector using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. After 48 h of transfection at 37°C, the transfection efficiency was determined by reverse transcription-quantitative PCR (RT-qPCR). Untransfected RCC cells were used as the ‘Blank’ control, and these cells were treated with nothing.
RT-qPCR
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA using PrimeScript Reverse Transcriptase (Takara Biotechnology Co., Ltd.). RT was performed using the following procedure: 30°C for 10 min followed by 42°C for 45 min. qPCR was subsequently performed using the SYBR Premix Taq kit (Takara Biotechnology Co., Ltd.) on a LightCycler Real-Time PCR instrument [Roche Diagnostics (Shanghai) Co., Ltd.]. qPCR was performed using the following conditions: Enzyme activation at 95°C for 2 min, 1 cycle; 40 cycles of denaturation at 95°C for 10 sec and annealing/extension 60°C for 60 sec. U6 was used as the endogenous control for miR-200c-5p and SNHG12, while GAPDH was used as the loading control for COL11A1. The following primer sequences were used for the qPCR: SNHG12 forward, 5′-AGGGCCATGTAACCAGTGAA-3′ and reverse, 5′-GCTGGCCTTAATCTGACTGC-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; miR-200c-5p forward, 5′-TACATCATAATACTGCCGGGTAA-3′ and reverse, 5′-GGATTGGATGTTCTCCACAGTCTC-3′; COL11A1 forward, 5′-AGTGGCATCGGGTAGCAATCA-3′ and reverse, 5′-TGTCCCCCTCAAAAACTTCTTCAT-3′; and GAPDH forward, 5′-CGCTCTCTGCTCCTCCTGTTC-3′ and reverse, 5′-ATCCGTTGACTCCGACCTTCAC-3′. The relative expression levels of each target were quantified using the 2−ΔΔCq method (26).
Western blotting
Cells were washed with PBS and total protein was extracted using RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.) containing a protease inhibitor cocktail (Roche Diagnostics Co., Ltd.). Total protein was quantified using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.) and 40 µg protein/well was separated via 10% SDS-PAGE. The separated proteins were subsequently transferred onto PVDF membranes (EMD Millipore) and blocked with 5% non-fat milk at room temperature for 1 h. The membranes were then incubated with the following primary antibodies overnight at 4°C: Anti-COL11A1 (cat. no. ab64883; Abcam) and anti-β-actin (cat. no. ab6276; Abcam). Antibodies were used at 1:1,000 dilution. Following the primary antibody incubation, the membranes were washed with TBS-0.05% Tween-20 (TBST) 4–6 times for 1 h, then incubated with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature (Goat anti-rabbit and anti-mouse IgG, cat. nos. ab205718 and ab205719, respectively; both Abcam), secondary antibodies were used at 1:5,000 dilution. The membranes were subsequently washed with TBST 4–6 times for 1 h and protein bands were visualized using an ECL substrate kit (Abcam). ImageJ software (version 1.8.0; National Institutes of Health) was used for densitometry.
Transwell invasion assay
Transwell invasion assays were performed using 8-µm pore Matrigel®-precoated (37°C for 3 h and room temperature overnight) polycarbonate membranes (BD Biosciences). Briefly, 3×104 cells were seeded into the upper chambers of Transwell plates in serum-free DMEM (Gibco; Thermo Fisher Scientific, Inc.). The lower chambers contained complete DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. Following incubation at 37°C for 48 h, cells in the upper chamber were removed and the invasive cells on the lower surface of the membranes were fixed in 4% paraformaldehyde at room temperature for 15 min, stained with 0.5% crystal violet at room temperature for 20 min and visualized using a light microscope (magnification, ×100) (Olympus Corporation). ImageJ software (version 1.8.0; National Institutes of Health) was used for data analysis.
Flow cytometric analysis of apoptosis
Early and late (top and lower right corner of the flow dot plot, respectively) stage apoptosis were detected by flow cytometric analysis. Briefly, 0.5×106 A498 or 786O cells were seeded to six-well plates and transfected with the transfectants for 48 h at 37°C. The cells were subsequently harvested (Eppendorf centrifuge 5418R) and washed twice with PBS. The cells were then resuspended in binding buffer (cat. no. BDB556454; BD Biosciences), and stained with Annexin V-FITC and 7-Aminoactinomycin D (AAD) using an Annexin V-FITC/7-AAD Apoptosis Detection kit (BD Biosciences), according to the manufacturer's protocol. Apoptotic cells were subsequently analyzed using a BD FACSVerse flow cytometer (BD Biosciences) and analyzed using FlowJo version 10.0.7 software (FlowJo LLC).
MTT assay
A total of 2×104 cells were seeded/well into a 96-well plate and incubated for 24, 48 or 72 h at 37°C. Cell viability was subsequently analyzed in triplicate using an MTT assay [Roche Diagnostics (Shanghai) Co., Ltd.], according to the manufacturer's protocol. Then, 10 µl MTT solution was added to each well and DMSO was used to dissolve purple formazan. Cell viability was analyzed at an absorbance at 570 nm using an iMark microplate absorbance reader (Bio-Rad Laboratories, Inc.).
Luciferase reporter assay
TargetScan (targetscan.org/mamm_31) and miRcode (mircode.org) were used for the prediction of miRNA targets. The putative miR-200c-5p binding site of the SNHG12 and COL11A1 3′-untranslated region (UTR) sequences were amplified and cloned into the pmirGLO vector (Promega Corporation) to synthesize SNHG12-WT and COL11A1-WT, respectively. Mutant (MUT) 3′-UTR fragments were generated by site-directed mutagenesis (InFusion HD Cloning Plus; Takara Biotechnology Co., Ltd.) and then were cloned into the pmirGLO plasmid (referred to as SNHG12-MUT and COL11A1-MUT, respectively). Then, 2×105 293T cells were seeded into 24-well plates and cultured for 24 h at 37°C. The cells were then co-transfected with 5 µg SNHG12- or COL11A1-WT/MUT luciferase constructs, and miR-200c-5p mimics (Lipofectamine 2000; Thermo Fisher Scientific, Inc.). Following transfection for 48 h at 37°C, cells were collected and luciferase activity was detected using a Dual-Luciferase Reporter Assay system (Promega Corporation). Renilla Luciferase was used for normalization.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 22.0 software (IBM Corp.). Pairwise comparisons were assessed using an unpaired Student's t-test or a paired Student's t-test when the data were paired (Fig. 1B). Multigroup comparisons were performed using two-way ANOVA, followed by a Bonferroni's post hoc test. A Kaplan-Meier curve was used for survival analysis and the P-value was obtained using the log-rank test. A χ2 test was used to determine the association between SNHG12 expression levels and clinicopathological variables, whereas a Spearman's correlation analysis test was used to determine the correlation between SNHG12 and miR-200c-5p expression levels. All experiments were conducted in triplicate. Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference.
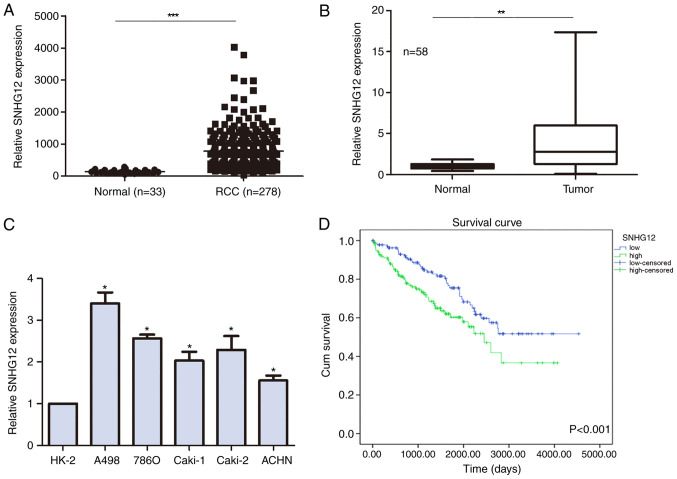
Figure 1.
Relative expression levels of SNHG12 are upregulated in patients with RCC and in RCC cell lines. (A) SNHG12 expression levels in patients with RCC and normal patient samples from TCGA database. (B) SNHG12 expression levels in clinical RCC samples compared with adjacent non-tumor tissues were analyzed using RT-qPCR. Data are expressed in the form of taking logarithm of the counts number to the base 2. (C) SNHG12 expression levels in RCC cell lines and the HK-2 normal kidney cell line were analyzed using RT-qPCR. (D) Kaplan-Meier curve of the survival of patients with RCC from TCGA database according to low and high SNHG12 expression levels. 0, alive; 1, deceased. SNHG12, small nucleolar RNA host gene 12; RCC, renal cell carcinoma; TCGA, The Cancer Genome Atlas; RT-qPCR, reverse transcription-quantitative PCR; Cum, cumulative. *P<0.05, **P<0.01, ***P<0.001.
Results
SNHG12 expression levels are upregulated in RCC patient samples and RCC cell lines
The expression levels of SNHG12 were compared between RCC and non-tumor samples from TCGA database; SNHG12 expression levels were discovered to be significantly upregulated in patients with RCC compared with in non-tumor patient samples (799.9±35.41 vs. 141±8.625 normalized counts, respectively; Fig. 1A). To validate this finding, SNHG12 expression levels were also analyzed in clinical samples and similarly, SNHG12 expression levels were significantly upregulated in the RCC tumor tissues compared with the adjacent non-tumor tissues (Fig. 1B). The expression levels of SNHG12 were also evaluated in RCC cell lines, all of which demonstrated upregulated expression levels of SNHG12 compared with the normal kidney epithelial cell line HK-2 (Fig. 1C).
Association between SNHG12 expression levels and clinical characteristics in patients with RCC
The clinical characteristics analyzed in the present study included age, sex, histological differentiation, TNM stage, tumor size and lymph node and distant metastases (Table I). The median expression levels of SNHG12 were used to categorize patients into ‘high’ and ‘low’ expression groups. High SNHG12 expression levels were significantly associated with a lower histological differentiation advanced TNM stage and further lymph node and distant metastases. TCGA datasets were then used to further investigate whether SNHG12 expression levels influenced the survival of patients with RCC; high SNHG12 expression levels in patients with RCC were associated with a shorter overall survival rate compared with low expression levels (Fig. 1D).
Table I.
Association between SNHG12 expression levels and clinicopathological characteristics in patients with renal cell carcinoma (n=58).
| SNHG12 expression levels | |||
|---|---|---|---|
| Parameter | Low, n | High, n | P-value |
| Age, years | 0.905 | ||
| >60 | 7 | 15 | |
| ≤60 | 12 | 24 | |
| Sex | 0.455 | ||
| Male | 16 | 20 | |
| Female | 12 | 10 | |
| Histological differentiation | <0.001 | ||
| High | 23 | 4 | |
| Low | 9 | 22 | |
| Lymph node metastasis | 0.018 | ||
| N0-N1 | 20 | 9 | |
| N2-NX | 11 | 18 | |
| Distant metastasis | 0.018 | ||
| M0 | 18 | 11 | |
| M1-MX | 9 | 20 | |
| Tumor node metastasis stage | 0.001 | ||
| T1-T2 | 19 | 10 | |
| T3-T4 | 6 | 23 | |
| Tumor size | 0.001 | ||
| ≤4 cm | 20 | 9 | |
| >4 cm | 5 | 24 | |
SNHG12, small nucleolar RNA host gene 12.
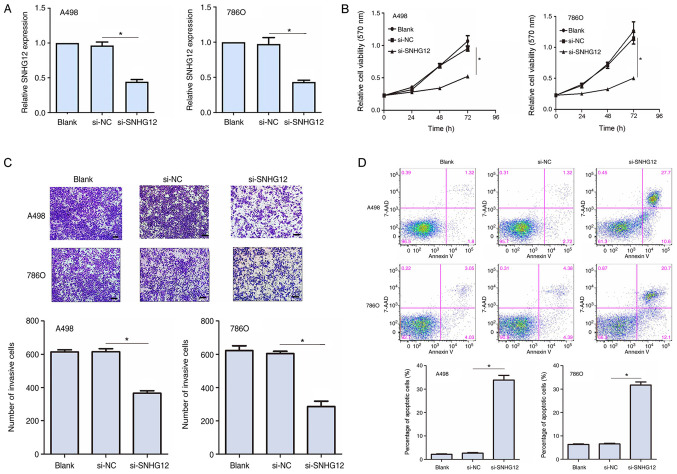
SNHG12 promotes cell viability and invasion and inhibits apoptosis in RCC cell lines
To determine the function of SNHG12 in RCC cells, siRNA was used to knockdown SNHG12 expression levels in the A498 and 786O cell lines. In both cell lines, the transfection with si-SNHG12 significantly downregulated SNHG12 expression levels compared with the si-NC groups (Fig. 2A). An MTT assay was subsequently performed to determine the cell viability in the transfected RCC cells. SNHG12 knockdown significantly reduced cell viability at 48 and 72 h post transfection compared with the si-NC group in both cell lines (Fig. 2B). To investigate the invasive properties of the transfected RCC cells, a Transwell invasion assay was used. The knockdown of SNHG12 expression levels significantly inhibited the invasive ability of A498 and 786O cells (P<0.05; Fig. 2C). Flow cytometric analysis also demonstrated that the knockdown of SNHG12 expression levels significantly increased the percentage of apoptotic cells in both cell lines compared with the si-NC group (Fig. 2D). Altogether, these results indicated that SNHG12 may promote cell viability and invasion, whilst inhibiting cell apoptosis in RCC cell lines.
Figure 2.
Functional role of SNHG12 in RCC cell lines. (A) Transfection efficiency of si-SNHG12 in A498 and 786O cell lines was analyzed using reverse transcription-quantitative PCR. (B) Cell viability was analyzed using an MTT assay following SNHG12 knockdown. (C) Cell invasive ability was detected using a Transwell assay following SNHG12 knockdown. Magnification, ×100; scale bar, 100 µm. (D) Flow cytometric analysis of apoptosis was performed following SNHG12 knockdown. Untransfected RCC cells were used as the ‘blank’ control. *P<0.05. SNHG12, small nucleolar RNA host gene 12; RCC, renal cell carcinoma; si, small interfering RNA; NC, negative control; 7-AAD, 7-Aminoactinomycin D.
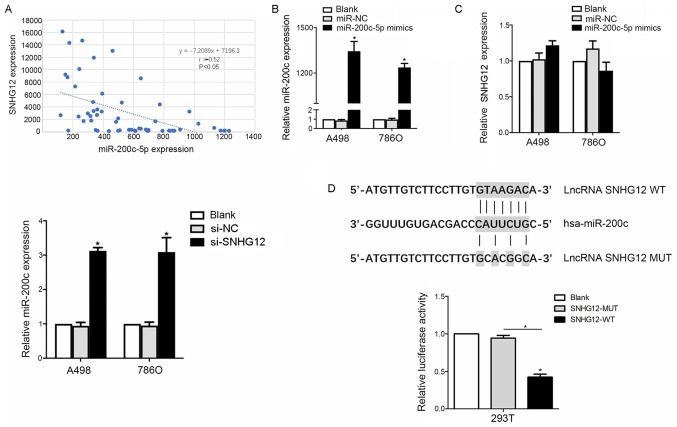
SNHG12 downregulates the expression levels of miR-200c-5p
To further investigate the mechanism of SNHG12 in the development of RCC, miRcode was used to screen for miRNAs predicted to bind to SNHG12, and SNHG12 was discovered to contain putative binding sites for miR-200c-5p. To validate this result, a correlation analysis was conducted between SNHG12 and miR-200c-5p expression levels in clinical RCC patient samples (n=58). The expression levels of miR-200c-5p were revealed to be inversely correlated with SNHG12 expression levels (r=−0.52; P=0.032; Fig. 3A).
Figure 3.
SNHG12 binds to miR-200c-5p and downregulates its expression levels. (A) Inverse correlation was identified between the expression levels of SNHG12 and miR-200c-5p in patient samples. (B) Transfection efficiency of miR-200c-5p mimics in A498 and 786O cell lines was analyzed using reverse transcription-quantitative PCR. (C) Upregulation of miR-200c-5p expression levels in RCC cell lines had no effect on the expression levels of SNHG12. However, si-SNHG12 knockdown significantly upregulated miR-200c-5p expression levels. (D) Luciferase reporter assay demonstrated that the overexpression of miR-200c-5p reduced the intensity of the relative luciferase activity in 293T cells transfected with the SNHG12-WT vector. Untransfected RCC cells were used as the ‘blank’ control. *P<0.05. SNHG12, small nucleolar RNA host gene 12; RCC, renal cell carcinoma; miR, microRNA; NC, negative control; lncRNA, long non-coding RNA; WT, wild-type; MUT, mutant.
Thus, to determine the interaction between miR-200c-5p and SNHG12, RCC cell lines were transfected with miR-200c-5p mimics and si-SNHG12; the transfection efficiency of the miR-200c-5p mimics was successful, as the expression levels were significantly upregulated in the miR-200c-5p mimics group (Fig. 3B). Subsequently, SNHG12 knockdown markedly upregulated miR-200c-5p expression levels, as indicated by RT-qPCR. Notably, no significant differences were observed in the SNHG12 expression levels following the overexpression of miR-200c-5p compared with the two control groups (Fig. 3C). Subsequently, a luciferase reporter assay revealed that the relative luciferase activity was decreased in 293T cells co-transfected with pmirGLO-SNHG12-WT and miR-200c-5p mimics compared with pmirGLO-SNHG12-MUT and miR-200c-5p mimics co-transfected cells (P<0.05; Fig. 3D), which further supported an interaction between SNHG12 and miR-200c-5p.
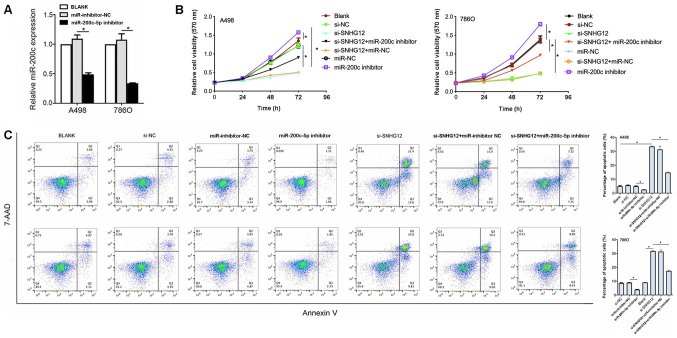
SNHG12 exerts an oncogenic role through the regulation of miR-200c-5p
To determine whether SNGH12 exerted its effects through miR-200c-5p, A498 and 786O cell lines were co-transfected with si-SNHG12 and a miR-200c-5p inhibitor. The transfection of the miR-200c-5p inhibitor into both cell lines was successful, as the expression levels were significantly downregulated compared with inhibitor NC (Fig. 4A). The cell viability was significantly increased in both cell lines co-transfected with si-SNHG12 and the miR-200c-5p inhibitor compared with si-SNHG12 alone (Fig. 4B). In addition, in both cell lines, the percentage of apoptotic cells in the si-SNHG12 + miR-200c-5p inhibitor group was significantly decreased compared with the si-SNHG12 group (P<0.05; Fig. 4C). Altogether, these observations suggested that SNHG12 may exert its oncogenic role through downregulating miR-200c-5p.
Figure 4.
SNHG12 exerts its role by regulating miR-200c-5p. (A) Transfection efficiency of miR-200c-5p inhibitor in A498 and 786O cell lines was determined using reverse transcription-quantitative PCR. (B) MTT assay was used to determine cell viability in RCC cells transfected with si-SNHG12 + miR-200c-5p inhibitor compared with cells transfected with si-SNHG12 alone. (C) Flow cytometric analysis of apoptosis following si-SNHG12 and miR-200c-5p inhibitor co-transfection compared with cells transfected with si-SNHG12 alone. Untransfected RCC cells were used as the ‘blank’ control. *P<0.05. SNHG12, small nucleolar RNA host gene 12; RCC, renal cell carcinoma; miR, microRNA; NC, negative control; si, small interfering RNA; 7-AAD, 7-Aminoactinomycin D.
COL11A1 is a direct target of miR-200c-5p
TargetScan and miRcode were used to determine target genes of miR-200c-5p, which identified COL11A1 as one of the predicted targets. COL11A1 is associated with adhesion and extracellular matrix remodeling (27), which are both important processes in RCC.
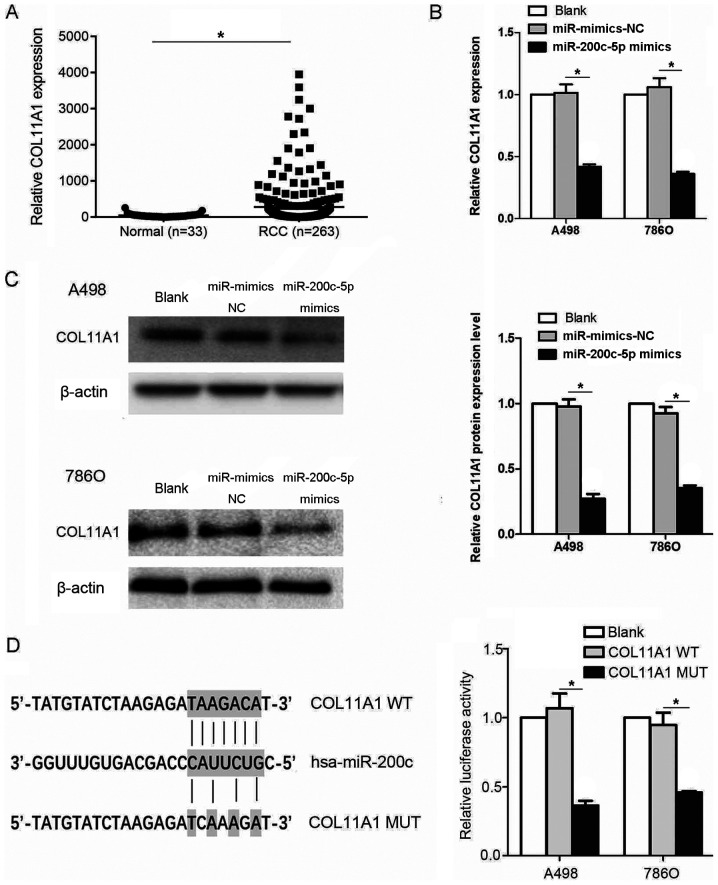
The relative expression levels of COL11A1 were evaluated in datasets from patients with RCC and normal kidney tissues obtained from TCGA database. The expression levels of COL11A1 were significantly upregulated in the tissues from patients with RCC compared with the normal tissues (Fig. 5A). Subsequently, to determine whether COL11A1 was affected by miR-200c-5p, A498 and 786O cell lines were transfected with miR-200c-5p mimics. The mRNA expression levels of COL11A1 were significantly downregulated following the transfection with the miR-200c-5p mimics (Fig. 5B). A similar trend was observed in the protein expression levels of COL11A1 (Fig. 5C). Moreover, the relative luciferase activity was significantly decreased in cells transfected with the COL11A1-WT 3′-UTR construct compared with the COL11A1-MUT 3′-UTR construct, which indicated that COL11A1 was a direct target of miR-200c-5p (Fig. 5D). The binding sites of COL11A1 and miR-200c-5p was discovered using miRanda database.
Figure 5.
COL11A1 is a direct target of miR-200c-5p. (A) Relative COL11A1 expression levels in RCC tissues from The Cancer Genome Atlas database. (P=0.0248) (B) Relative COL11A1 expression levels following the transfection with the miR-200c-5p mimics in RCC cell lines was detected using reverse transcription-quantitative PCR. (C) Western blotting was used to determine the protein expression levels of COL11A1 in the blank, miR-mimics-NC and miR-200c-5p mimics group. (D) Luciferase reporter assay demonstrated a strong binding between the 3′-UTR of COL11A1 and miR-200c-5p. Untransfected RCC cells were used as the ‘blank’ control. *P<0.05. SNHG12, small nucleolar RNA host gene 12; COL11A1, collagen type XI α1 chain; RCC, renal cell carcinoma; miR, microRNA; NC, negative control; WT, wild-type; MUT, mutant; UTR, untranslated region. The binding sites of COL11A1 and miR-200c-5p were discovered using miRanda database.
SNHG12 regulates COL11A1 expression levels through miR-200c-5p
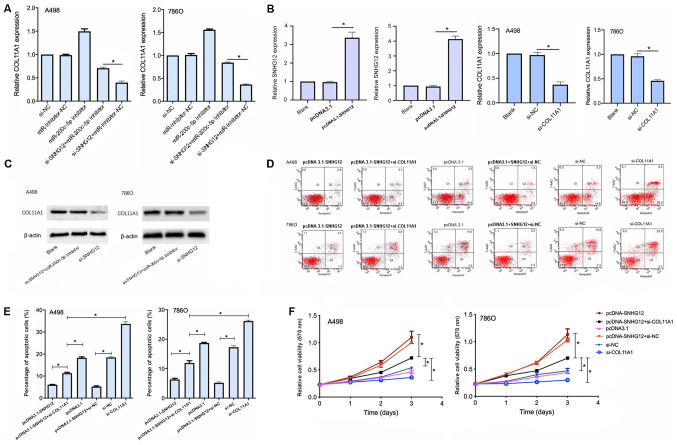
To investigate whether SNHG12 regulated COL11A1 expression levels by interacting with miR-200c-5p, COL11A1 mRNA expression levels were analyzed following the transfection with si-SNHG12 and the miR-200c-5p inhibitor. The transfection with si-SNHG12 significantly downregulated the expression levels of COL11A1 compared with the si-SNHG12 + miR-200c-5p inhibitor transfection (Fig. 6A). Thus, these findings indicated that the inhibition of miR-200c-5p may partially reverse COL11A1 downregulation induced by si-SNHG12, indicating that SNHG12 may regulate COL11A1 expression levels through miR-200c-5p.
Figure 6.
COL11A1 participates in the progression of RCC through the SNHG12/miR-200c-5p/COL11A1 axis. (A) COL11A1 expression levels were discovered to be regulated by SNHG12 in a miR-200c-5p-dependent manner. COL11A1 expression levels were analyzed using RT-qPCR. (B) Expression levels of SNHG12 and COL11A1 were analyzed using RT-qPCR following the transfection with pcDNA3.1-SNHG12 or si-COL11A1, respectively. (C) miR-200c-5p inhibitor rescued COL11A1 expression levels downregulated by si-SNHG12. (D) Flow cytometric analysis of apoptosis was performed in cells co-transfected with pcDNA3.1-SNHG12 and si-COL11A1. (E) Quantification of the apoptosis results were shown as bar plots. (F) Cell viability was determined using an MTT assay following the transfection with pcDNA3.1-SNHG12 + si-COL11A1. *P<0.05. SNHG12, small nucleolar RNA host gene 12; COL11A1, collagen type XI α1 chain; miR, microRNA; NC, negative control; si, small interfering RNA; RT-qPCR, reverse transcription-quantitative PCR; 7-AAD, 7-Aminoactinomycin D.
To confirm whether SNHG12 exerted its role through COL11A1, A498 and 786O cell lines were transfected with the pcDNA3.1-SNHG12 overexpression vector or si-COL11A1. The transfection efficiency was proven to be successful (Fig. 6B and C), and MTT and apoptotic assays were subsequently performed. The cell viability was significantly decreased and the apoptotic rate was significantly increased in cells co-transfected with both pcDNA3.1-SNHG12 and si-COL11A1 compared with the cells transfected with pcDNA3.1-SNHG12 alone (Fig. 6D and E). These findings indicated that the effect of SNHG12 on the growth of RCC cell lines may be mediated, at least in part, by COL11A1.
Discussion
Previous studies have indicated that lncRNAs were vital to physiological and pathological processes (28–30). In fact, several lncRNAs have been associated with RCC; for example, in one study, the lncRNA metastasis associated lung adenocarcinoma transcript 1 interacted with miR-205 to promote aggressive RCC (31), whereas in another study, the lncRNA HOTAIR regulated HIF-α/AXL signaling by inhibiting miR-217 (9). Nevertheless, to the best of our knowledge, the role of SNHG12 in RCC remains unclear.
SNHG12 has been extensively investigated in several types of cancer, including papillary thyroid carcinoma, cervical cancer, lung cancer, osteosarcoma and hepatocellular carcinoma (15,16,18,32,33). A previous study has reported that SNHG12 promoted tumor progression in non-small cell lung carcinoma by binding to miR-218 and miR-181a, which regulated Slug/zinc finger E-box binding homeobox (ZEB)2 and mitogen activated protein kinase/Slug signaling (34).
The present study demonstrated that SNHG12 expression levels were upregulated in the tissues from patients with RCC compared with the normal kidney tissues. This finding was further validated in the A498 and 786O RCC cell lines and patient samples. In addition, it was observed that SNHG12 knockdown significantly suppressed cell viability and invasion and promoted apoptosis in vitro. Moreover, analysis of TCGA data indicated that high SNHG12 expression levels were associated with poor clinical outcomes, a lower histological differentiation, advanced clinical stages and metastasis. Overall, these results suggested that SNHG12 may serve as an oncogene in RCC.
The ceRNA theory is widely accepted in the study of lncRNAs. According to the ceRNA theory, lncRNAs regulate gene expression by functioning as miRNA sponges, thereby interfering in the binding between miRNAs and target genes (35). Several studies have suggested that miR-200c-5p may function as a tumor suppressor gene in RCC (36–40). Indeed, miR-200c-5p inhibited RCC cell migration and invasion and participated in demethylation drug-induced decreased cell migration and invasion (41). Moreover, miR-200c-5p was reported to serve as a sponge for the lncRNA HOTAIR in the ceRNA network, whereby it regulated RCC progression through several oncogenes, including VEGFA, ZEB1 and ZEB2 (40). In the present study, miR-200c-5p was also discovered to interact with SNHG12, as validated by luciferase reporter assay, thus the findings suggested that SNHG12 may function as an oncogene in RCC through regulating COL11A1 expression levels by sponging miR-200c-5p. This was validated using a luciferase reporter assay, which demonstrated that the 3′-UTR of both SNH12 and COL11A1 were strongly bound to miR-200c-5p.
COL11A1, a primary interstitial extracellular matrix constituent, is one of the most frequently upregulated genes identified in chemoresistant tumors relative to normal tissues (42). Notably, COL11A1 was previously discovered to be associated with poor survival in patients with RCC, owing to its high expression levels in the tumor tissues compared with the normal kidney tissue (27). COL11A1 also promoted tumor progression in ovarian cancer (43) and it was associated with poor survival in multiple other types of cancer (e.g. pancreatic and colon cancer) (44–46). In addition, a previous study suggested that COL11A1 may be a highly specific biomarker and molecular signature of cancer-associated fibroblasts, as it was detected in several epithelial cancers, including lung cancer, ovarian cancer, head and neck cancer, brain cancer, glioma, colon cancer, liver cancer, bladder cancer, adrenocortical cancer, kidney cancer and mesothelioma, according to data from TCGA database (44). Thus, targeting COL11A1 or COL11A1-associated genes may serve as an attractive therapeutic target for RCC.
The function of miR-200c-5p and COL11A1 was further analyzed in RCC cells using rescue assays, which indicated that miR-200c-5p upregulation increased the rate of cell apoptosis and decreased cell viability. COL11A1 knockdown also led to the same results, which was consistent with previous findings (27).
In conclusion, the findings of the present study suggested that SNHG12 may contribute to RCC progression by serving as an oncogene, sponging miR-200c-5p and thereby upregulating the expression levels of COL11A1. Thus, the present study provided further insight into the role of SNHG12 in RCC development.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are available in the TCGA database (portal.gdc.cancer.gov/).
Authors' contributions
CX, YW, SL and XW conceived and designed the study, performed experiments, and drafted and revised the manuscript. HL and LS performed experiments and collected data. JZ performed experiments. XK funded the study and interpreted the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of Hainan General Hospital (Haikou, China). Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin. 2017;67:507–524. doi: 10.3322/caac.21411. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 3.Garcia JA, Rini BI. Recent progress in the management of advanced renal cell carcinoma. CA Cancer J Clin. 2007;57:112–125. doi: 10.3322/canjclin.57.2.112. [DOI] [PubMed] [Google Scholar]
- 4.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chi Y, Wang D, Wang J, Yu W, Yang J. Long non-coding RNA in the pathogenesis of cancers. Cells. 2019;8:1015. doi: 10.3390/cells8091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan Y, Chen Z, Li Y, He A, He S, Gong Y, Li X, Zhou L. Long non-coding RNA DANCR promotes malignant phenotypes of bladder cancer cells by modulating the miR-149/MSI2 axis as a ceRNA. J Exp Clin Cancer Res. 2018;37:273. doi: 10.1186/s13046-018-0921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DL, Lu YX, Zhang JX, Wei XL, Wang F, Zeng ZL, Pan ZZ, Yuan YF, Wang FH, Pelicano H, et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–4849. doi: 10.7150/thno.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Q, Li O, Zheng W, Xiao WZ, Zhang L, Wu D, Cai GY, He JC, Chen XM. LncRNA HOTAIR regulates HIF-1α/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis. 2017;8:e2772. doi: 10.1038/cddis.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zhou W, Ye XL, Xu J, Cao MG, Fang ZY, Li LY, Guan GH, Liu Q, Qian YH, Xie D. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci Signal. 2017;10:eaak9557. doi: 10.1126/scisignal.aak9557. [DOI] [PubMed] [Google Scholar]
- 11.Trimarchi T, Bilal E, Ntziachristos P, Fabbri G, Dalla-Favera R, Tsirigos A, Aifantis I. Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell. 2014;158:593–606. doi: 10.1016/j.cell.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atianand MK, Hu W, Satpathy AT, Shen Y, Ricci EP, Alvarez-Dominguez JR, Bhatta A, Schattgen SA, McGowan JD, Blin J, et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165:1672–1685. doi: 10.1016/j.cell.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamang S, Acharya V, Roy D, Sharma R, Aryaa A, Sharma U, Khandelwal A, Prakash H, Vasquez KM, Jain A. SNHG12: An LncRNA as a potential therapeutic target and biomarker for human cancer. Front Oncol. 2019;9:901. doi: 10.3389/fonc.2019.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin XJ, Chen XJ, Zhang ZF, Hu WS, Ou RY, Li S, Xue JS, Chen LL, Hu Y, Zhu H. Long noncoding RNA SNHG12 promotes the progression of cervical cancer via modulating miR-125b/STAT3 axis. J Cell Physiol. 2019;234:6624–6632. doi: 10.1002/jcp.27403. [DOI] [PubMed] [Google Scholar]
- 16.Dong J, Wang Q, Li L, Xiao-Jin Z. Upregulation of long non-coding RNA small nucleolar RNA host gene 12 contributes to cell growth and invasion in cervical cancer by acting as a sponge for MiR-424-5p. Cell Physiol Biochem. 2018;45:2086–2094. doi: 10.1159/000488045. [DOI] [PubMed] [Google Scholar]
- 17.Jiang B, Hailong S, Yuan J, Zhao H, Xia W, Zha Z, Bin W, Liu Z. Identification of oncogenic long noncoding RNA SNHG12 and DUXAP8 in human bladder cancer through a comprehensive profiling analysis. Biomed Pharmacother. 2018;108:500–507. doi: 10.1016/j.biopha.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, Li L, Li Y, Sun H, Zeng C. Long noncoding RNA SNHG12 mediates doxorubicin resistance of osteosarcoma via miR-320a/MCL1 axis. Biomed Pharmacother. 2018;106:850–857. doi: 10.1016/j.biopha.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Liu J, Chu L, Yang W, Liu H, Li C, Yang J. Long noncoding RNA SNHG12 facilitates the tumorigenesis of glioma through miR-101-3p/FOXP1 axis. Gene. 2018;676:315–321. doi: 10.1016/j.gene.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Chen Q, Zhou W, Du SQ, Gong DX, Li J, Bi JB, Li ZH, Zhang Z, Li ZL, Liu XK, Kong CZ. Overexpression of SNHG12 regulates the viability and invasion of renal cell carcinoma cells through modulation of HIF1α. Cancer Cell Int. 2019;19:128. doi: 10.1186/s12935-019-0782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang O, Yang F, Liu Y, Lv L, Ma R, Chen C, Wang J, Tan Q, Cheng Y, Xia E, et al. C-MYC-induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple-negative breast cancer. Am J Transl Res. 2017;9:533–545. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZB, Tang C, Jin X, Liu SH, Pi W. Increased expression of lncRNA SNHG12 predicts a poor prognosis of nasopharyngeal carcinoma and regulates cell proliferation and metastasis by modulating Notch signal pathway. Cancer Biomark. 2018;23:603–613. doi: 10.3233/CBM-181873. [DOI] [PubMed] [Google Scholar]
- 23.Xian HP, Zhuo ZL, Sun YJ, Liang B, Zhao XT. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol Lett. 2018;16:4689–4698. doi: 10.3892/ol.2018.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: Renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, Bowlby R, Gibb EA, Akbani R, Beroukhim R, et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 2018;23:313–326.e315. doi: 10.1016/j.celrep.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Boguslawska J, Kedzierska H, Poplawski P, Rybicka B, Tanski Z, Piekielko-Witkowska A. Expression of genes involved in cellular adhesion and extracellular matrix remodeling correlates with poor survival of patients with renal cancer. J Urol. 2016;195:1892–1902. doi: 10.1016/j.juro.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14:42–54. doi: 10.1016/j.gpb.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarani R, Mirza AH, Kaur S, Pociot F. The emerging role of lncRNAs in inflammatory bowel disease. Exp Mol Med. 2018;50:1–14. doi: 10.1038/s12276-018-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 2015;75:1322–1331. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan T, Ma W, Hong Z, Wu L, Chen X, Yuan Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36:11. doi: 10.1186/s13046-016-0486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding S, Qu W, Jiao Y, Zhang J, Zhang C, Dang S. LncRNA SNHG12 promotes the proliferation and metastasis of papillary thyroid carcinoma cells through regulating wnt/β-catenin signaling pathway. Cancer Biomark. 2018;22:217–226. doi: 10.3233/CBM-170777. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Chen D, Ma H, Li Y. LncRNA SNHG12 contributes to multidrug resistance through activating the MAPK/Slug pathway by sponging miR-181a in non-small cell lung cancer. Oncotarget. 2017;8:84086–84101. doi: 10.18632/oncotarget.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: Possible functions and clinical implications. J Med Genet. 2015;52:710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 36.Maolakuerban N, Azhati B, Tusong H, Abula A, Yasheng A, Xireyazidan A. MiR-200c-3p inhibits cell migration and invasion of clear cell renal cell carcinoma via regulating SLC6A1. Cancer Biol Ther. 2018;19:282–291. doi: 10.1080/15384047.2017.1394551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang J, Yi BO, Ding S, Sun J, Cao W, Liu M. Demethylation drug 5-Aza-2′-deoxycytidine-induced upregulation of miR-200c inhibits the migration, invasion and epithelial-mesenchymal transition of clear cell renal cell carcinoma in vitro. Oncol Lett. 2016;11:3167–3172. doi: 10.3892/ol.2016.4364. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Wang X, Chen X, Han W, Ruan A, Chen L, Wang R, Xu Z, Xiao P, Lu X, Zhao Y, et al. miR-200c targets CDK2 and suppresses tumorigenesis in renal cell carcinoma. Mol Cancer Res. 2015;13:1567–1577. doi: 10.1158/1541-7786.MCR-15-0128. [DOI] [PubMed] [Google Scholar]
- 39.Gao C, Peng FH, Peng LK. MiR-200c sensitizes clear-cell renal cell carcinoma cells to sorafenib and imatinib by targeting heme oxygenase-1. Neoplasma. 2014;61:680–689. doi: 10.4149/neo_2014_083. [DOI] [PubMed] [Google Scholar]
- 40.Ding J, Yeh CR, Sun Y, Lin C, Chou J, Ou Z, Chang C, Qi J, Yeh S. Estrogen receptor β promotes renal cell carcinoma progression via regulating LncRNA HOTAIR-miR-138/200c/204/217 associated CeRNA network. Oncogene. 2018;37:5037–5053. doi: 10.1038/s41388-018-0175-6. [DOI] [PubMed] [Google Scholar]
- 41.Ying G, Wu R, Xia M, Fei X, He QE, Zha C, Wu F. Identification of eight key miRNAs associated with renal cell carcinoma: A meta-analysis. Oncol Lett. 2018;16:5847–5855. doi: 10.3892/ol.2018.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu YH, Chang TH, Huang YF, Chen CC, Chou CY. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPβ pathway and PDK1 stabilization. Oncotarget. 2015;6:23748–23763. doi: 10.18632/oncotarget.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33:3432–3440. doi: 10.1038/onc.2013.307. [DOI] [PubMed] [Google Scholar]
- 44.Jia D, Liu Z, Deng N, Tan TZ, Huang RY, Taylor-Harding B, Cheon DJ, Lawrenson K, Wiedemeyer WR, Walts AE, et al. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016;382:203–214. doi: 10.1016/j.canlet.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Wu J, Zhang J, Yang Z, Jin W, Li Y, Jin L, Yin L, Liu H, Wang Z. Integrated bioinformatics analysis of key genes involved in progress of colon cancer. Mol Genet Genomic Med. 2019;7:e00588. doi: 10.1002/mgg3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, Fendrich V, Beaty R, Mullendore M, Karikari C, Bardeesy N, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut. 2008;57:1420–1430. doi: 10.1136/gut.2007.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available in the TCGA database (portal.gdc.cancer.gov/).