Abstract
Cell therapy, along with intensive rehabilitation has been shown to significantly improve outcomes in amyotrophic lateral sclerosis (ALS), in addition to standard therapy. We present a 40-years-old male ALS patient, suffering for the past four years, who underwent multiple doses of cell therapy at our institution. Along with riluzole treatment and lithium co-administration, his treatment involved multiple intrathecal transplants of autologous bone marrow-derived mononuclear cells, followed by multidisciplinary neurorehabilitation. The outcome measures of ALSFunctional Rating Scale Revised score remained stable, and importantly, Six Minute Walk Test distance improved from 475.2 m to 580.8 m, over a span of 16 months. Improved outcomes are indicative of slowing down of disease progression. Multiple doses of intrathecal autologous cell therapy along with rehabilitation and lithium, in addition to standard riluzole treatment is a novel approach for decelerating disease progression and qualitatively improving living conditions for ALS patients and their caregivers.
Key words: Amyotrophic lateral sclerosis, bone marrow cells, cell therapy
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that selectively targets upper and lower motor neurons, and is clinically characterized by wasting, fasciculating musculature that is eventually fatal. Prognosis depends upon numerous factors, such as type of ALS, gender, and age of symptom onset.1 Thorough neurological and electrophysiological investigations are required to confirm the diagnosis of ALS on the basis of the revised EL Escorial Criteria for ALS diagnosis.2 Worldwide, the annual incidence of ALS is approximately 1-2.6 cases per 100,000.3
ALS treatment demands a comprehensive approach for slowing down disease progression and improving quality of life due to the multifarious pathophysiology of ALS. Studies implicate a variety of genetic mutations, misfolded protein aggregates, excessive free radicals, and excitotoxic agents in the pathology of ALS.1 Current pharmacological treatment strategies only provide symptomatic relief: riluzole curbs glutamate excitotoxicity; dextromethorphan/ quinidine treats pseudobulbar affect; and edaravone scavenges free radicals, but only in the early disease stages.4 Multidisciplinary rehabilitation, respiratory management, assistive devices, adaptive equipment and an experienced rehabilitation team at each disease stage are currently the standard of ALS care.5 There is an urgent need worldwide to formulate safe, effective, and holistic treatment options for patients living with ALS that can significantly slow down or reverse the disease pathology.
Autologous bone marrow-derived mononuclear cells (BMMNCs) have proven to be a safe and effective therapeutic intervention for treating neurological disorders including ALS.6,7 Transplanted cells tend to integrate better into target tissue when a rehabilitative regimen follows the transplant. 8-10 We present a case of 40-year-old male with Definite ALS, who underwent multiple intrathecal autologous BMMNC transplants with lithium and standard treatment at our institution. In this unique case, we are evaluating 6 Minute Walk Test (6MWT) scores along with ALS-Functional Rating Scale Revised (ALSFRSr). To our knowledge, there are no previous studies that show therapeutic effects of this therapy on the 6MWT in ALS.
Case Report
A 40-year-old male, with no family history of ALS or psychological disorders, noticed weakness in the right wrist in April 2014. Fasciculations started by June 2014, bilaterally in upper and lower limbs. Muscle cramps were noted in neck and legs. By September 2014, forearm muscle weakness ensued, along with left wrist weakness. At this point in time, he underwent an Electromyography (EMG) study, which showed diffuse denervation and reinnervation of multiple upper limb muscles with no conduction block.
A repeat EMG and Nerve Conduction Study (NCS) in December 2014 detected lower limb fasciculations. By January 2015, he started showing proximal muscle weakness in bilateral upper limbs. There were no facial or tongue fasciculations, dysphagia, dysarthria, sialorrhea, or dyspnea. Lower limb muscle strength was intact. Despite being treated with riluzole, supplements, rehabilitation, and undergoing hyperbaric oxygen therapy, his condition deteriorated.
Magnetic resonance imaging (MRI) using diffusion tensor imaging of the brain was normal. MRI cervical spine revealed mild/moderate spinal canal stenosis at C5-6. EMG and NCS showed evidence of a diffuse progressive disorder of motor neurons or their axons, and this was most severely affecting the cervical segments.
On assessment in November 2016, ALSFRSr score was 40/48; Functional Independence Measure (FIM) score was 109/126; and, 6MWT distance was 475.2 m. Upper motor neuron involvement manifested as hyperreflexia in knee jerk reflexes bilaterally in lower limbs; lower motor neuron signs were evident with hypotonia of the upper limbs, subluxation of shoulders, muscle atrophy and depressed deep tendon (biceps, triceps, and supinator jerk) reflexes; and bilateral wasting of pectoral, scapular, forearm, thenar and hypothenar muscles. Muscle weakness was also observed in the neck. Additionally, he noticed cramping in both upper and lower limbs. Bilateral gastrocnemius-soleus and hamstring tightness, and weakness in upper extremities was noted. He also underwent psychological evaluation and had normal cognitive function. There was no family history and no genetic tests were done.
Another motor neuron disease, progressive muscular atrophy (PMA), was considered in the differential diagnosis; but he was eventually diagnosed as Definite ALS due to upper (hyperreflexia) and lower (hypotonia) motor neuron signs in multiple segments of the body according to the revised El Escorial criteria.2 His chief complaints at assessment included difficulty in performing instrumental activities of daily living (ADLs), like writing, typing, driving, cutting food, gripping and lifting objects; fine motor ADL movements like buttoning shirts, tying shoelaces; overhead movements such as those involved in bathing, transition activities such as getting up from the bed, and fatigue.
The patient reported minimal neck pain, but no pain radiating from the neck into the upper limbs. He also observed transient muscular chest pain.
Neuroregenerative therapy
Pre-intervention
The Institutional Ethics Committee provided the ethical approval for this treatment; it was in concordance with the World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.11 A written informed consent was obtained from the patient after explaining the procedure in detail. An experienced team of doctors and therapists thoroughly examined the patient. Anesthetic and surgical fitness wasconfirmed via pre-surgical routine blood tests, urinalysis, and chest X-ray.
Bone marrow aspiration
Forty-eight hours and 24 hours prior to autologous BMMNCs transplant, 300 μg of Granulocyte-Colony Stimulating Factor (GCSF) injections were administrated subcutaneously, as G-CSF mobilizes BMMNCs from their niche, stimulates CD34+ cells and increases their survival and multiplication rate.12 Local anesthesia was administered in the region of anterior superior iliac spine; 110 mL of bone marrow was aseptically aspirated, collected in heparinized tubes, and transferred to regenerative medicine laboratory.
Separation of autologous bone marrowderived mononuclear cells
BMMNCs were separated from the aspirated bone marrow by aseptic differential centrifugation. The BMMNCs, obtained as a separate layer, were washed, resuspended in normal saline, and counted. Viability estimate of the isolated BMMNCs was obtained using Trypan blue vital dye on a hemocytometer for total and viable count. 1.2.108 BMMNCs were obtained at a viability rate of 98%. This was further confirmed by TALI cell counter. CD34+ analysis was done using Fluorescence Activated Cell Sorting using CD34 PE antibody, and the CD34+ fraction was found to be 5.38%.
Administration of autologous bone marrow- derived mononuclear cells
Immediately post separation, isolated BMMNCs were administered intrathecallyat L4-L5 level; simultaneously, 1 gm methylprednisolone in 500 mL Ringer’s Lactate solution was injected intravenously to reduce local inflammation. The patient was closely monitored for any immediate adverse events during his stay at the hospital.
Neurorehabilitation therapy
In physiotherapy, the patient was administered strength training exercises for upper limbs, lower limbs and trunk; sitting and standing balance training exercises, gait training, and respiratory exercises to improve lung capacity and stamina; bed mobility exercises; and energy conservation techniques. Exercises to improve fine and gross motor skills of upper limbs, and ADL retraining were done as part of occupational therapy.
Exercises to improve fine and gross motor skills of upper limbs, and ADL retraining were done as part of occupational therapy. Additionally, psychological and family counseling was performed to help the patient and his family to cope with the difficult diagnosis of ALS. Post discharge, he was put on a home exercise program.
Table 1.
Symptomatic analysis of disease progression depicts a stable condition.
| Measurements or symptoms | At assessment | At 4 months after 1st transplant (just before 2nd transplant) | At 10 months after 1st transplant (just before 3rd transplant) | At 16 moths after 1st transplant |
|---|---|---|---|---|
| Ambulation | Ambulatory with some difficulties | Maintained | Maintained | Maintained. Stamina has improved. He can perform 25 mins of brisk walking on the treadmill at inclination level 3, at 5.5 km/h |
| Leg movements | Difficulty during ambulation | Maintained | Maintained | Lower limbs have become stronger |
| Maximum inspiratory | 2500 | 2500 | 2500 | 2500 |
| volume (mL) | ||||
| Peak expiratory flow | 540 | 500 | 500 | 680 |
| rate (L/min) | ||||
| Chest expansion (in cm) | Maintained: | Maintained: | Maintained: | |
| Upper | 6 | 6 | 6 | 6 |
| Middle | 5 | 5 | 5 | 5 |
| Lower | 6 | 5 | 5 | 5 |
| Stamina/Level of fatigue | Fatiguability present | Same as before | Same as before | Improved walking stamina and exercising ability |
| Reach test (in inches) | Maintained: | Maintained: | Improved: | |
| Forward | 6.5 | 6.5 | 6.5 | 12 |
| Backward | 6.5 | 6.5 | 6.5 | 10 |
| Left | 7 | 7 | 7 | 11 |
| Right | 7 | 7 | 7 | 11 |
Medical management
He was prescribed to continue 50 mg riluzole twice a day. In addition, 300 mg lithium was prescribed once a day, and serum lithium levels were maintained at 0.5 to 0.8 mmol/L for 6 weeks.
Based on improvements seen after the first transplant (Tables 1 and 2; Figures 1 and 2), the patient underwent a second transplant four months later (1.6.108 total autologous intrathecal BMMNCs; cell viability- 98%; CD34+ fraction-2.06%.), and a third transplant 10 months after the first transplant (3.5.108 total autologous intrathecal BMMNCs; cell viability-98%; CD34+ fraction-3.02%). Each transplant was followed by a comprehensive neurorehabilitative routine as described above. The serial scores on different outcome measures were evaluated and compared to natural disease progression to understand the effect of the treatment.
Post-transplant, his gross motor skills improved. At four months (second transplant) and 10 months (third transplant) since the first transplant, his ambulation, stamina and pulmonary function remained stable. At 16 months since the first transplant, his ambulation improved, owing to increased lower limb strength. His pulmonary function improved, as did his stamina and reach. His condition was otherwise maintained. Functionally, he still requires some assistance in his activities of daily living. However, his fine motor skills and overhead activity showed some deterioration.
Symptomatic analysis of the patient over the course of 16 months was performed (Table 1). Improvements in the patient’s outcome measures were charted, depicting his stability over 16 months (Table 2; Figures 1 and 2).
The FIM scores of the patient dropped from 109 to 99 over 16 months. This change was observed primarily in the motor section of the FIM scale. This patient reports to have some deterioration only in his distal upper limbs, possibly due to lack of upper limb exercises, and his disease had not progressed significantly. FIM is limited only to functional independence and is not a reliable measure of disease progression in this case.
Discussion and Conclusions
Numerous clinical publications advocate the safety and efficacy of adult autologous BMMNCs as a therapy for neurological disorders, including ALS.6,13 No irreversible adverse events have been reported post transplant. These cells are a safe treatment modality because they are autologous adult cells, do not elicit an immune response,14 and have no tumorigenic properties or ethical issues. The intrathecal delivery of cells diluted in autologous cerebrospinal fluid is particularly attractive, because it is a minimally invasive procedure that facilitates efficient homing of BMMNCs across the blood-brain barrier in a relatively immune-privileged environment. 15
Lithium improves the survival and potency of BMMNCs, as well as their integration into target tissue,16 while being well tolerated by ALS patients.17 We have also published a study that showed that as compared to controls there was higher survival (by 30.38 months) of ALS patients who underwent intrathecal autologous BMMNC transplant followed by six weeks of lithium.7 Subgroup analysis showed that the patients who received lithium showed a clinically significant higher mean survival of 106.73 (±15.69) months as compared to the ones that did not receive lithium, 66.83 (±7.52) months (P=0.121). However, 6MWT was not used in this study.
Table 2.
Changes in outcome measures over 16 months.
| Outcome measures | At assessment before 1st transplant | At 4 months after 1st transplant (just before 2nd transplant) | At 16 months after 1st transplant (6 months after 3rd transplant) |
|---|---|---|---|
| 6MWT (in m) | 475.2 | 514.8 | 580.8 |
| FIM (out of 126) | 109 | 104 | 99 |
| ALSFRSr (out of 48) | 40 | 40 | 38 |
Figure 1.
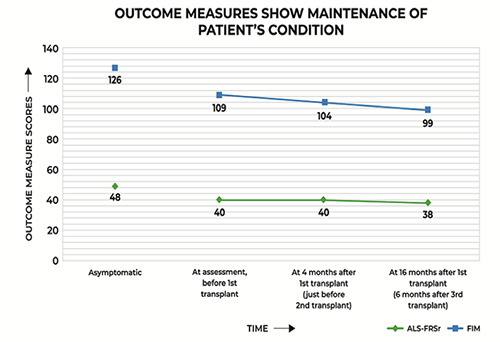
The patient’s 6 Minute Walk Test (6MWT) distance improved as time progressed post intervention. 6MWT distance in the asymptomatic state is the median distance obtained from values measured by Sanjak et al.23 in healthy subjects aged 50 to 85 years, measured in meters.
Figure 2.
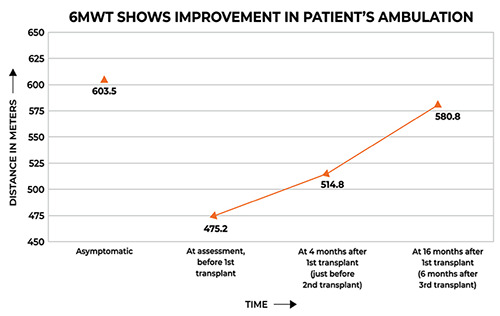
Data points show score values measured at different intervals. Initial points indicate asymptomatic scores for each outcome, i.e. complete functionality. ALS-Functional Rating Scale Revised is scored out of a total of 48 points, and FIM is scored out of 126 points.
BMMNCs revitalize the degenerating nervous system by migrating and differentiating into different cell types in vivo.18 These cells also regulate cellular microenvironments by the modulation of cytokines like vascular endothelial growth factor (VEGF), ciliary neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF),19 platelet-derived growth factor-BB (PDGF-BB), granulocytemacrophage colony-stimulating factor (GM-CSF),20 tumor necrosis factor (TNF)-α and interleukins (IL-1α, IL-β, IL-6, IL- 10).21
Rehabilitation and exercise post transplant have several neurological merits. Exercise at moderate intensity increases synaptogenesis and dendritic branching inmultiple brain regions, in addition to BDNF, IGF-I, and NGF secretion.8 Conversely, lack of exercise impedes endogenous neurogenesis and downregulates neurotrophic factor production.9 Further, several studies attest to the beneficial effects of moderate exercise in ALS patients.10
Prabhakar et al. monitored the disease progression for a period of 12 months using ALSFRSr of 10 ALS patients treated with autologous BMMNCs intrathecally.22 They show that a four-point deterioration on the ALSFRSr scale took a median of 16.7 months. Here, the ALSFRSr score decreased by a mere two points in a span of 16 months post intervention.
6MWT is a pertinent monitor of ALSrelated decline. Sanjak et al. demonstrate that the mean walking distance ranges from 576 m to 631 m during 6MWT (median distance= 603.5 m; see Asymptomatic 6MWT data point in Figure 1), in healthy subjects aged 50 to 85 years.23 They also report that as the disease progressed, the distance covered by ALS patients reduced significantly. Here, the patient’s 6MWT distance increased from 475.2 m at pre-assessment to 580.8 m at 16 months. The 6MWT provides an additional, robust measure of cardiopulmonary function, and concurs with the inference derived from the ALSFRSr scores.
This case report demonstrates the stabilization of a male Definite ALS patient via multiple doses of cell therapy and lithium co-administration, along with standard riluzole treatment and neurorehabilitation. Autologous BMMNCs along with lithium and standard treatment may have mitigated disease progression, and improved the condition of this patient. Randomized controlled clinical trials with scientific rigor and a large sample size are required for deriving conclusive evidence and standardizing this therapeutic regimen as treatment for this devastating disorder.
References
- 1.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 2013;14:248-64. [DOI] [PubMed] [Google Scholar]
- 2.World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293-9. [DOI] [PubMed] [Google Scholar]
- 3.Talbott E, Malek A, Lacomis D. The epidemiology of amyotrophic lateral sclerosis. Handbook Clin Neurol 2016;138:225-38. [DOI] [PubMed] [Google Scholar]
- 4.Scott A. Drug therapy: On the treatment trail for ALS. Nature 2017;550:S120-1. [DOI] [PubMed] [Google Scholar]
- 5.Simon NG, Huynh W, Vucic S, et al. Motor neuron disease: current management and future prospects. Intern Med J 2015;45:1005-13. [DOI] [PubMed] [Google Scholar]
- 6.Gokulchandran N, Sharma A, Sane H, et al. Stem cell therapy as a treatment modality for neurotrauma. Indian J Stem Cell Therapy 2015;1:21-6. [Google Scholar]
- 7.Sharma A, Sane H, Paranjape A, et al. The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis - a retrospective controlled study. Am J Stem Cells 2015;4:50-65. [PMC free article] [PubMed] [Google Scholar]
- 8.Ploughman M, Austin MW, et al. The effects of poststroke aerobic exercise on neuroplasticity: a systematic review of animal and clinical studies. Transl Stroke Res 2015;6:13-28. [DOI] [PubMed] [Google Scholar]
- 9.Yasuhara T, Hara K, Maki M, et al. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience 2007;149:182-91. [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Montes J, Mitsumoto H. The role of exercise in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am 2008;19:545-57. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [DOI] [PubMed] [Google Scholar]
- 12.Yoon SH, Shim YS, Park YH, et al. Complete Spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophagecolony stimulating factor: Phase I/II clinical trial. Stem Cells 2007;25:2066-73. [DOI] [PubMed] [Google Scholar]
- 13.Moura MC, Novaes MRCG, Zago YSSP, et al. Efficacy of stem cell therapy in amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Clin Med Res 2016;8:317-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem Cells Transl Med 2012;1:403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakshi A, Hunter C, Swanger S, et al. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine 2004;1:330-7. [DOI] [PubMed] [Google Scholar]
- 16.Richman CM, Kinnealey A, Hoffman PC. Granulopoietic effects of lithium on human bone marrow in vitro. Exp Hematol 1981;9:449-55. [PubMed] [Google Scholar]
- 17.UKMND-LiCALS Study Group. Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2013;12:339-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002;416:542-5. [DOI] [PubMed] [Google Scholar]
- 19.Bhasin A, Srivastava MVP, Mohanty S, et al. Paracrine mechanisms of intravenous bone marrow-derived mononuclear stem cells in chronic ischemic stroke. Cerebrovasc Dis Extra 2016;6:107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moniche F, Montaner J, Gonzalez-Marcos J-R, et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGFBB, and MMP-2 serum levels in stroke patients: results from a clinical trial. Cell Transplant 2014;23:S57-64. [DOI] [PubMed] [Google Scholar]
- 21.Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middleaged rats. J Cereb Blood Flow Metab 2010;30:140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhakar S, Marwaha N, Lal V, et al. Autologous bone marrow-derived stem cells in amyotrophic lateral sclerosis: a pilot study. Neurol India 2012;60:465-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanjak M, Langford V, Holsten S, et al. Six-Minute Walk Test as a measure of walking capacity in ambulatory individuals with amyotrophic lateral sclerosis. Arch Phys Med Rehabil 2017;98:2301-7. [DOI] [PubMed] [Google Scholar]


