The advances of immunology in the last decades have been spectacular, both at the basic and the translational level. This progress has led to the discovery of many immunotherapies for autoimmune diseases and cancer. Besides, whole-genome sequencing studies have confirmed that the genetic susceptibility for autoimmune diseases such as multiple sclerosis (MS) is mainly driven by the HLA and other immune genes regulating the adaptive and innate immune response (1). However, the same level of advances has not materialized for understanding how autoimmune responses are generated and damage the central nervous system (CNS) in patients with MS (2). Decades of efforts searching for the antigen targeted in autoimmune CNS diseases have provided some successes, such as the identification of aquaporin-4 in the case of neuromyelitis optica (3) or myelin oligodendrocyte glycoprotein (MOG) in MOG antibody-associated encephalomyelitis (4). However, antigens responsible for the autoimmune response in MS have remained elusive (5, 6), even though new candidate antigens have been recently discovered (7, 8) which may involve cross-reactivity or epitope spreading processes (9). Identification of target antigens is critical for the development of antigen-specific tolerization as a path for restoring the homeostasis of the immune system and preventing tissue damage (10). Similarly, the search for the cells and molecules responsible for CNS damage in MS has been intensive. Still, it has failed to provide definite evidence for the involvement of one or several encephalitogenic immune cells that drives the autoimmune response against the CNS or orchestrates the chronic compartmentalized inflammation inside the CNS (11). However, the high efficacy of anti-CD20 therapies in MS has placed B cells in the spotlight (12).
There are many reasons behind the lack of success for identifying such autoimmune signature in MS, including 1) the heterogeneity of MS patients in terms of genetic, infection-related, lifestyle-related, and environmental factors (13); 2) the prodromal phase of the disease that delays the diagnosis and therefore the opportunity for identifying the first and triggering events (14); 3) the more frequent analysis of blood compared with cerebrospinal fluid, lymph nodes, or CNS tissue where the immune response is organized due to accessibility to the sample; or 4) the fact that the immune system is a complex network of cells, molecules, and pathways that self-organize to define the response, making it a very robust system but also more difficult to identify specific culprits (15).
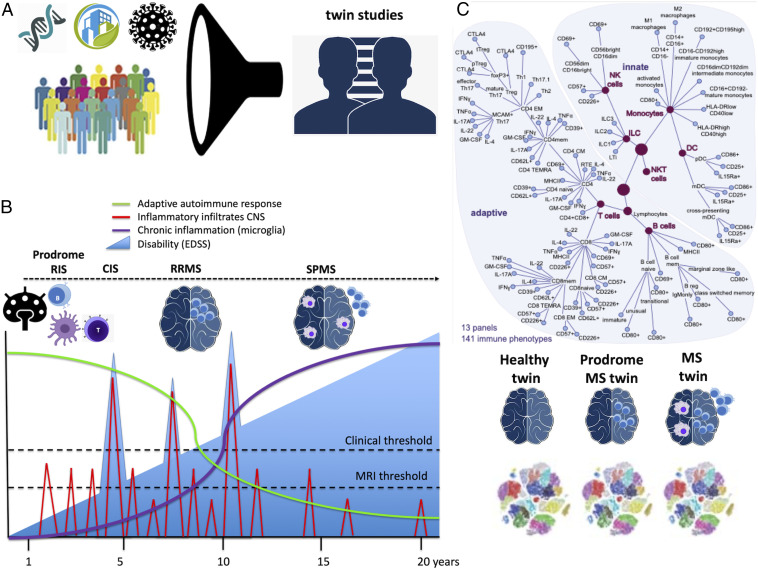
In PNAS, Gerdes et al. (16) address the search for MS immune signatures by controlling several variables and overcoming previous limitations. They reduced genetic and environmental heterogeneity by making use of homozygous twins discordant for MS and phenotyping blood cell subpopulations using an extended cytometry profiling (Fig. 1A). The 43 twin pairs participating in this study shared, in addition to the genetic background, the environment and lifestyle because all grew up in the same household. They found that twinship was the most important factor, explaining 56% (11 to 99%) of immune subpopulations variability. In contrast, all other factors explained a minor fraction of the variability, namely, 2% for sex, 4% for age, 3% for cytomegalovirus status, and only 1% for the MS diagnosis. Therefore, this study supports the previous evidence that immune responses have a significant heritability (17).
Fig. 1.
The MS immune signature in discordant twins. (A) MS patient heterogeneity is due to genetics, infections, lifestyle, and other environmental factors. Using homozygous twins discordant for MS who shared the genetic background, the environment, and lifestyle because all grew up in the same household, patient heterogeneity and noise can be significantly reduced. (B) Prodromal MS allows studying the early events of the disease: The autoimmune process starts early in the disease with the generation of encephalitogenic antigen-specific T and B cells in the lymph nodes that can migrate to the brain and produce acute inflammatory infiltrates, being observed as contrast-enhancing lesions or T2 lesions on the MRI or as clinical relapses depending on the size, location, and severity of the damage (MRI or clinical thresholds). Therefore, the early events of MS may only be observed in the disease's prodromal stage, such as in radiologically isolated syndromes (RISs) or in the twins in this study with either oligoclonal bands on the CSF or presence of MS lesions on MRI. After the onset of the disease, either clinically isolated syndrome (CIS) or relapsing-remitting MS (RRMS) it is very likely that the autoimmune process has already evolved including new antigen-specific T and B cells, being responsible for new autoimmune attacks (relapses) as well as the onset of chronic compartmentalized inflammation. With the evolution of the disease, the autoimmune attack evolves from acute inflammatory infiltrates to chronic inflammation inside the brain and meninges, corresponding with the development of secondary progressive MS (SPMS). Adapted from ref. 25, which is licensed under CC BY 4.0. (C) Immune cell subpopulations were assessed by cytometry. Surprisingly, the main differences in immune cell frequencies were attributable to twinship, whereas the disease only explained a minority of the differences. However, MS twins shared the immune signature with the prodromal twins and differed from the healthy twins. Adapted from ref. 16, which is licensed under CC BY-NC-ND 4.0.
Surprisingly, the twins with MS did not show a substantial difference in the immune subpopulation frequencies than the healthy twins. To address another source of confusion, namely, the prodromal period of MS (Fig. 1B), they analyzed the healthy twins and identified a subgroup (10 out 40 unaffected twins) with subclinical–prodromal MS based on the presence of lesions on brain MRI or oligoclonal bands in the cerebrospinal fluid (CSF). They found that MS prodromal twins have a closer immune signature to MS twins than to healthy twins for CD4+ and CD8+ T cell subsets and especially for Th17 and Th1, but neither for B cells nor the major innate cell populations (Fig. 1C). Finally, they validated the subclinical MS signature in early MS cases (55 clinically isolated syndrome and 60 early untreated relapsing-remitting MS), finding significant MS-related changes in some of the previously identified immune traits from the twin approach, such as effector Th17, migratory Th17 (MCAM+), and Th17.1 cells, as wells as migratory Th1 (CD195+) cells.
In contrast, in previous work studying a subset of this MS twin cohort, the team examined CSF cells by single-cell RNA sequencing (RNA-seq), and identified clonally expanded CD8+ T cells, plasmablasts, and, to a lesser extent, CD4+ T cells with characteristics of activated tissue-resident memory T cells in the prodromal phase and in MS (18). These results highlight the importance of examining both peripheral blood and CSF, a compartment that may more closely reflect immune cells within MS brain. Overall, the results from the twin cohort are in agreement with recent studies using well-standardized and comprehensive cytometry panels that identified blood immune signatures, also involving Th17 cells but also B-memory/B-regulatory cells (19)
However, the main questions remain—which are the immune cells responsible for the onset of the autoimmune attack in MS, and which are driving the transition from relapsing autoimmune attacks to chronic compartmentalized inflammation within the CNS? Probably the answer will lie in some lessons biology uses to teach us, namely that biological processes are very specific, and at the same time, the immune system contains many redundancies and checkpoints to be efficient. The first lesson suggests that unless we identify the antigen-specific cell triggering autoimmunity in the tissue (either lymph node or CNS tissue) at the onset of the disease, it is very likely we will miss such a signal within the noise of the overall immune response. Precisely, the function of the adaptive immune system is to identify specific chemical patterns (antigens), and the innate immune system provides the tissue context (danger or healthy) in a dose-dependent probabilistic process (15). For this reason, it is critical to identify such antigen-specific cells triggering MS in a given patient. The complexity of the immune system organization allows it to be very robust for defining the immune response. Still, autoimmunity may appear as a slow process that deviates from homeostasis, making it very difficult to identify such changes (20). Fortunately, technological advances are coming to the help of researchers and patients. For example, RNA-seq, T cell receptor/B cell receptor sequencing, high-resolution cytometry, or mass cytometry are now powerful tools allowing to interrogate thousands of single cells even from small samples like the CSF (21, 22). Myeloid-specific exosomes (extracellular vesicles) in the CSF enable us to collect molecular information from previously hidden immune cells (23). Finally, clinical MS researchers are now paying significant attention to the early stages of MS, including the prodromal phase, such as the radiologically isolated syndromes (24), which may permit capturing cases at the very first onset of the autoimmune responses. Therefore, powerful single-cell technologies to study well-controlled populations like monozygotic twins at early stages of the disease should pay dividends in the near future, elucidating the mechanisms that generate the autoimmune response in patients with MS and other autoimmune diseases.
Footnotes
Competing interest statement: P.V. holds stock and has received consultancy payments from Accure Therapeutics SL, Spiral Therapeutics Inc., Health Engineering SL, Attune Neurosciences Inc., QMenta Inc., C Light Inc., and NeuroPrex Inc. S.S.Z. served on data safety monitoring boards of Opexa, BioMS, Teva, and Eli Lilly; is a member of the clinical advisory board of the Myelin Repair Foundation; received speaker honoraria from Biogen and Teva; is a deputy editor of Neurology: Neuroimmunology and Neuroinflammation; consulted for Biogen, Teva, EMD Serono, Genzyme, Novartis, and Roche; serves on the speaker's bureau of Advanced Health Media and Biogen; and received research support from Biogen, Teva, the NIH, the National Multiple Sclerosis Society, the Weill Institute, and Alexander M. and June L. Maisin Foundation.
See companion article, “Immune signatures of prodromal multiple sclerosis in monozygotic twins,” 10.1073/pnas.2003339117.
References
- 1.Canto E., Oksenberg J. R., Multiple sclerosis genetics. Mult. Scler. 24, 75–79 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Dendrou C. A., Fugger L., Friese M. A., Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salama S., Khan M., Pardo S., Izbudak I., Levy M., MOG antibody-associated encephalomyelitis/encephalitis. Mult. Scler. 25, 1427–1433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohlfeld R., Dornmair K., Meinl E., Wekerle H., The search for the target antigens of multiple sclerosis, Part 1: Autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 15, 198–209 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Hohlfeld R., Dornmair K., Meinl E., Wekerle H., The search for the target antigens of multiple sclerosis, Part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 15, 317–331 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Planas R., et al. , GDP-l-fucose synthase is a CD4+ T cell-specific autoantigen in DRB3*02:02 patients with multiple sclerosis. Sci. Transl. Med. 10, eaat4301 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Jelcic I., et al. , Memory B cells activate brain-homing, autoreactive CD4+ T cells in multiple sclerosis. Cell 175, 85–100.e23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatino J. J. Jr, Zamvil S. S., T cells take aim at a ubiquitous autoantigen in multiple sclerosis. Sci. Transl. Med. 10, eaau8826 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Steinman L., Ho P. P., Robinson W. H., Utz P. J., Villoslada P., Antigen-specific tolerance to self-antigens in protein replacement therapy, gene therapy and autoimmunity. Curr. Opin. Immunol. 61, 46–53 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff R. M., Hafler D. A., Lucchinetti C. F., Multiple sclerosis-a quiet revolution. Nat. Rev. Neurol. 11, 134–142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenfield A. L., Hauser S. L., B-cell therapy for multiple sclerosis: Entering an era. Ann. Neurol. 83, 13–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson T., Barcellos L. F., Alfredsson L., Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 13, 25–36 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Tremlett H., Marrie R. A., The multiple sclerosis prodrome: Emerging evidence, challenges, and opportunities. Mult. Scler., 10.1177/1352458520914844 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Iranzo J., Villoslada P., Autoimmunity and tumor immunology: Two facets of a probabilistic immune system. BMC Syst. Biol. 8, 120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerdes L. A., et al. , Immune signatures of prodromal multiple sclerosis in monozygotic twins. Proc. Natl. Acad. Sci. U.S.A. 117, 21546–21556 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patin E. et al.; Milieu Intérieur Consortium , Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat. Immunol. 19, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Beltrán E., et al. , Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J. Clin. Invest. 129, 4758–4768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cellerino M., et al. , Impact of treatment on cellular immunophenotype in MS: A cross-sectional study. Neurol. Neuroimmunol. Neuroinflamm. 7, e693 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Germain R. N., The art of the probable: System control in the adaptive immune system. Science 293, 240–245 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Papalexi E., Satija R., Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 18, 35–45 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Newell E. W., Davis M. M., Beyond model antigens: High-dimensional methods for the analysis of antigen-specific T cells. Nat. Biotechnol. 32, 149–157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verderio C., et al. , Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72, 610–624 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Hosseiny M., Newsome S. D., Yousem D. M., Radiologically isolated syndrome: A review for neuroradiologists. AJNR Am. J. Neuroradiol., 10.3174/ajnr.A6649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotelnikova E., et al. , Dynamics and heterogeneity of brain damage in multiple sclerosis. PLOS Comput. Biol. 13, e1005757 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]