Highlights
-
•
The prevalence of SARS-CoV-2 in blood from patients with COVID-19 was determined.
-
•
SARS-CoV-2 was found in 5.9 % of the tested patients.
-
•
Plasma should not be used as primary sample to identify SARS-CoV-2 infection.
Keywords: COVID, SARS-CoV-2, Viremia, Plasma, Blood
Abstract
Correct and reliable identification of SARS-CoV-2 in COVID-19 suspected patients is essential for diagnosis. Respiratory samples should always be tested with real-time PCR for SARS-CoV-2. In addition, blood samples have been tested, but without consistent results and therefore the added value of this sample type is unknown. The aim of this study was to determine the prevalence of SARS-CoV-2 by real-time PCR in blood samples obtained from PCR-proven COVID-19 patients and in addition to elaborate on the potential use of blood for diagnostics. In this single center study, blood samples drawn from patients at the emergency department with proven COVID-19 infection based on a positive SARS-CoV-2 PCR in respiratory samples were tested for the presence of SARS-CoV-2. Samples from 118 patients were selected, of which 102 could be included in the study (median age was 65 (IQR 10), 65.7 % men). In six (5.9 %) of the tested samples, SARS-CoV-2 was identified by real-time PCR.
In conclusion, SARS-CoV-2 can be detected by real-time PCR in plasma samples from patients with proven COVID-19, but only in a minority of the patients. Plasma should therefore not be used as primary sample in an acute phase setting to identify SARS-CoV-2 infection. These findings are important to complete the knowledge on possible sample types to test to diagnose COVID-19.
1. Introduction
Late December 2019, cases of viral pneumonia of unknown cause were identified in Wuhan, China, as reported by the WHO’s country office in China. Approximately 2 months later, the virus responsible for the disease was well characterized and named SARS-CoV-2, whereas the associated infection was named COVID-19 [1]. By mid-March 2020, the virus had spread worldwide, showing high severity, which led to the assessment by the WHO that COVID-19 could be characterized as a pandemic [2].
For diagnosis, primarily nasopharyngeal and oropharyngeal swabs are obtained and tested using real-time PCR. However, false negative results can be obtained when testing solely these samples, and diagnosis might only be due by testing a sample obtained from the lower respiratory tract [5]. In addition to respiratory samples, SARS-CoV-2 could be detected in both plasma and stool [6]. In all of these studies however, plasma samples were collected on an irregular basis with variable results. So far, the presence of SARS-CoV-2 in plasma from patients has not been studied consistently, whereas earlier studies showed a high prevalence of 2003 SARS-CoV in plasma samples [7,8]. In this study, we aimed to determine the prevalence of SARS-CoV-2 in plasma obtained from patients with proven COVID-19 and in addition to elaborate on the potential use of plasma as primary or additional clinical sample for diagnostics.
2. Material and methods
The study population was part of the MACARON study (MArkers in COVID-19 And Relations to Outcomes in the Netherlands). Blood samples were routinely collected over a one month period (March 23 – April 20, 2020) from all patients (age >18 years) presenting to the emergency department of our hospital, that were suspected of SARS-CoV-2 infection based on clinical, radiological and biochemical markers. To study the prevalence of SARS-CoV-2, remnant plasma was used from 118 different patients with proven COVID-19 based on PCR positivity in respiratory samples. Plasma of 20 COVID-19 negative patients obtained in the same period, which tested negative for SARS-CoV-2 in respiratory samples, were included as negative controls. All samples were stored at −70 °C before testing.
DNA was extracted from 200 μL plasma with the QIASymphony system (Qiagen, Venlo, the Netherlands) using the internally validated DSP DNA Mini Kit (Qiagen), yielding a 200 μL eluate. Correct extraction was controlled by the addition of a fixed amount of phocine distemper virus (PDV) as described earlier [9]. SARS-CoV-2 was tested using a real-time PCR assay targeting the E-gene [10]. In short, a PCR mix consisting of 6.25 μL 4x Fast Virus 1-step mastermix (Thermofisher, Waltham, Massachusetts, USA), 12.5 pmol of each of the primers, and 6.25 pmol of the probe was tested in a 25 μL reaction including 5 μL of the eluate. Real-time PCR was tested on the Quantstudio5 (Applied Biosystems, Foster City, USA) using a cycling protocol consisting of an initial step of 5 min 50 °C and 20 s 95 °C, followed by 45 cycli of 95 °C (3 s) and 60 °C (30 s). A test was defined as SARS-CoV-2 positive when a signal with cycle threshold (Ct-)value of <45 was found.
3. Results
In 116 out of 118 patients sufficient plasma for testing was available. Of 102 of these patients, a combined nasopharyngeal/oropharyngeal swab testing positive for SARS-CoV-2 was obtained the same day as the plasma sample or within 12 h. These patients were included in the study. The study population consisted of 67 men and 35 women (Table 1 ). Information on the duration of symptoms was available for 99 patients, ranging from 0 to 30 days (median 8 days, Table 1).
Table 1.
Patients included in this study.
| COVID-19 |
No COVID-19 |
||||||
|---|---|---|---|---|---|---|---|
| n | Agea | Duration of symptoms in days | SARS-CoV-2 positive | n | Agea | SARS-CoV-2 positive | |
| Men | 67 | 64 (IQR: 19) | 9 (IQR: 8) | 4 (6.0 %) | 9 | 66 (IQR: 15) | 0 |
| Women | 35 | 68 (IQR: 23) | 8 (IQR: 7) | 2 (5.7 %) | 11 | 64 (IQR: 24) | 0 |
| Total | 102 | 65 (IQR: 20) | 8 (IQR: 9) | 6 (5.9 %) | 20 | 65 (IQR: 20.8) | 0 |
Median age, inter quartile range (IQR) in brackets.
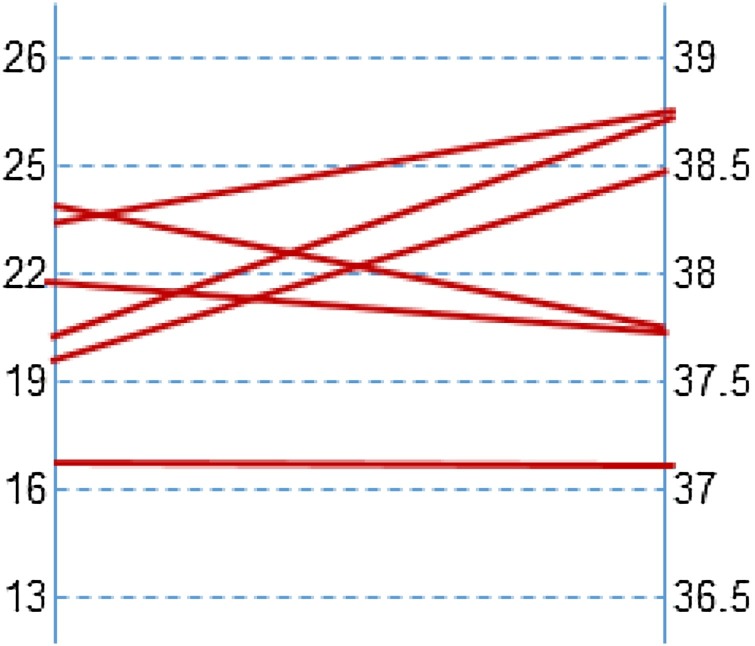
Six (5.9 %) of the plasma samples tested positive. Ct-values were high (37.1–38.8) indicating a low viral load. Mean Ct-value of the SARS-CoV-2 PCR as tested on the combined nasopharyngeal/oropharyngeal swabs was 21.0 for the plasma-positive patients, ranging from 16.5 – 24.1. No correlation between Ct-values found in nasopharynx/oropharyngeal swab and plasma was observed (Fig. 1 ). In contrast, the mean Ct-value of the SARS-CoV-2 PCR of the combined swabs from patients that tested negative in plasma was 28.9 (range 14.9–38.4). None of the plasma samples included as negative control tested positive for SARS-CoV-2. No inhibition was observed in all specimens.
Fig. 1.
Correlation of Ct-values as obtained in the nasopharyngeal/oropharyngeal swab (left) and plasma (right).
4. Discussion
As the SARS-CoV-2 pandemic unfolds, more information becomes available about the virus and its properties. Although clinical and radiological parameters can be highly predictive of COVID-19, detection of viral RNA using NAATs is essential to confirm infection with SARS-CoV-2 in the acute phase [11]. For primary diagnosis, mostly nasopharyngeal and/or oropharyngeal swabs are used. However, not all infections can be identified when testing only swabs from the upper respiratory tract [5]. Studies including multiple samples per patient show differences per tested sample type, both in positivity and in viral load [6,12]. Many studies have included plasma for detection of SARS-CoV-2 by NAAT, but with varying results [6,[13], [14], [15]]. However, plasma has not been tested consistently in early stages of disease and therefore the true added value of this type of sample in diagnosis is unclear. Our study aimed to address this knowledge gap, as we evaluated the prevalence of SARS-CoV-2 by real-time PCR in plasma from patients with proven COVID-19 at the time of their visit to the emergency department.
A total of 116 samples with sufficient plasma for testing were analyzed. Fourteen were excluded since these patients had tested positive for SARS-CoV-2 before visiting the emergency department and no information was available on the duration of symptoms. These plasma samples were nevertheless tested and SARS-CoV-2 was not identified in any of these (data not shown).
The remaining 102 plasma samples were included in this study, of which six (5.9 %) tested positive for SARS-CoV-2. This is low compared to the 41 % as reported in an earlier study that estimated the prevalence of SARS-CoV-2 in serum samples from patients after admission [16]. However, over 50 % of the specimens included in our study were obtained within the first week after onset of symptoms, whereas the referred study showed that the positive rate in serum samples gradually increased from the first week and decreased from the third week.
The 5.9 % of SARS-CoV-2 found in plasma in our study is significantly lower than found with the 2003 SARS-CoV in which studies showed a prevalence of 79 % in the first 3 days after onset of symptoms and 50 % within one week respectively [7,8]. Symptoms of the six patients that tested positive for SARS-CoV-2 in plasma started 4 days (n = 2), 7 days (n = 3) and 14 days (n = 1) before sample collection. Evidence has been found of 2003 SARS-CoV replicating in mononuclear cells, possibly explaining the higher prevalence [17].
With only a small proportion of plasma samples testing positive for SARS-CoV-2, that might also be dependent on the timing of sampling since onset of symptoms, it should be concluded that detection of SARS-CoV-2 in plasma is inadequate as primary method for identification. However, it might well be used as indicator for clinical severity. Earlier studies reported a correlation of serum viral RNA with the disease severity [13,16]. It was not the scope of this study to evaluate clinical outcome or evaluate underlying diseases, but all 6 patients with SARS-CoV-2 detected in plasma had a severe course of illness: one died 4 days after visiting the emergency department, 3 were ICU hospitalized, and 2 were hospitalized at non-ICU departments for 6 weeks and 1 week respectively.
In conclusion, SARS-CoV-2 can be detected in plasma samples from patients presenting at the emergency department suspected of COVID-19, but only in a minority of the patients. Plasma should therefore not be used as primary or even additional sample in an acute phase setting to identify SARS-CoV-2 infection, but might be of added value to determine or predict severity of disease.
CRediT authorship contribution statement
R.H.T. Nijhuis: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. A. Russcher: Conceptualization, Writing - original draft, Writing - review & editing. G.J. de Jong: Methodology, Investigation, Writing - review & editing. E. Jong: Methodology, Writing - review & editing. G.J.M. Herder: Methodology, Writing - review & editing. J.A. Remijn: Conceptualization, Methodology, Writing - review & editing. S.P. Verweij: Conceptualization, Methodology, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
None.
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . 2020. Timeline of WHO’s Response to COVID-19. [Google Scholar]
- 5.Hase R., Kurita T., Muranaka E. A case of imported COVID-19 diagnosed by PCR-positive lower respiratory specimen but with PCR-negative throat swabs. Infect. Dis. (Lond) 2020;52:423–426. doi: 10.1080/23744235.2020.1744711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant P.R., Garson J.A., Tedder R.S. Detection of SARS coronavirus in plasma by real-time RT-PCR. N. Engl. J. Med. 2003;349:2468–2469. doi: 10.1056/NEJM200312183492522. [DOI] [PubMed] [Google Scholar]
- 8.Ng E.K., Hui D.S., Chan K.C. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin. Chem. 2003;49:1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poelman R., Scholvinck E.H., Borger R. The emergence of enterovirus D68 in a dutch university medical center and the necessity for routinely screening for respiratory viruses. J. Clin. Virol. 2015;62:1–5. doi: 10.1016/j.jcv.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in china: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu F., Yan L., Wang N. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W., Lan Y., Yuan X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes Infect. 2020;9:469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie C., Jiang L., Huang G. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Du RH Li B. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9:386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng S., Fan J., Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, china, January-March 2020: retrospective cohort study. Bmj. 2020;369 doi: 10.1136/bmj.m1443. m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L., Wo J., Shao J. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J. Clin. Virol. 2003;28:239–244. doi: 10.1016/S1386-6532(03)00195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]