Abstract
Background
Patients with myeloproliferative neoplasms (MPN) are reported to be at increased risk for thrombotic events, however, no population-based study has estimated the excess thrombotic risk compared to matched controls.
Objective
To assess the risk of arterial and venous thrombosis in MPN patients compared to matched controls.
Design
Matched cohort study.
Setting
Population-based setting in Sweden 1987–2009 with follow-up to 2010.
Patients
9,429 MPN patients and 35,820 matched population controls.
Measurements
Primary outcome was rate of arterial and venous thrombosis. Flexible parametric models were used to calculate hazard ratios (HRs) and cumulative incidence with 95% confidence intervals (CIs).
Results
The HRs of arterial thrombosis among MPN patients compared to controls at 3 months, 1- and 5 years were 3.0 (95% CI, 2.7 to 3.4), 2.0 (CI, 1.8 to 2.2), and 1.5 (CI, 1.4 to 1.6), respectively. The corresponding HRs for venous thrombosis were 9.7 (CI, 7.8 to 12.0), 4.7 (CI, 4.0 to 5.4), and 3.2 (CI, 2.9 to 3.6). The rate was significantly elevated across all age groups and was similar between MPN subtypes. The 5-year cumulative incidence of thrombosis in MPN patients showed an initial rapid increase followed by more leveled increases during follow up. The HR of venous thrombosis decreased during more recent calendar periods.
Limitations
No information on individual laboratory results or treatment.
Conclusion
Patients with MPN across all age groups are at a significantly increased rate of arterial and venous thrombosis compared to matched population controls, with rates highest at and shortly following diagnosis. Decreases in the rate of venous thrombosis over time most likely reflects advances in clinical management.
INTRODUCTION
Myeloproliferative neoplasms (MPNs) are bone marrow malignancies characterized by excess clonal hematopoiesis resulting in elevated peripheral blood counts. MPNs consist of the subtypes polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The acquired mutation JAK2V617F and mutations in CALR, MPL, and JAK2 exon 12, are found in the majority of patients with MPNs. (1–8) Even though most MPNs have an indolent disease course, the life expectancy is generally shorter compared to the general population and the diseases can be associated with various complications.(9–12)
Among physicians, there is a clinical impression that the thrombotic risk is elevated in MPN patients, however, no population-based study has estimated the excess thrombotic risk compared to matched population controls. Whereas there are numerous reports on the incidence of thrombosis and risk scores for predicting thrombosis in PV, ET, and PMF, the majority of the published studies are hampered by varying degrees of patient selection and lack of control population. (13–15) Thus, the magnitude of the risk of thrombosis in MPNs patients in relation to the general population remains largely unknown. Moreover, there is limited information on the patterns of thrombotic risk in relation to follow up time after MPN diagnosis. Therefore, we conducted a comprehensive population-based study to assess the relative risk of thrombosis in MPN patients compared to matched controls overall and in relation to clinical features and follow-up time.
METHODS
Registers and databases
In Sweden, the entire population of approximately 10 million people has access to universal health care. The Swedish Cancer Register was founded in 1958 and it is mandatory for every health care provider to report all new cancer cases diagnosed at clinical, morphological, other laboratory examinations to the register.(16) The Swedish National Inpatient Register, established in 1964 with complete coverage from 1987, holds information on all hospital discharge diagnoses.(17) Since 2001, all hospital outpatient visits are reported to the Outpatient Register.(17) All dates and causes of death are recorded in the Cause of Death Register.(18) In Sweden, the Polycythemia Vera Study Group (PVSG) criteria were used for diagnosing PV, ET, and PMF from the 1970s to the early 2000s.(12, 19, 20) From 2001, the PVSG criteria have gradually been replaced by the World Health Organization (WHO) criteria.(21–23) MPN unclassifiable (MPN-U) was introduced in the Swedish Cancer Register in 1993.(10)
Participants
All patients diagnosed with MPN and reported to the Swedish Cancer Register between January 1st, 1987 and December 31st, 2009 were identified and included in the study. Although the Swedish Cancer Register generally has a high level of coverage, there has been a certain degree of underreporting of indolent diseases such as MPNs. Therefore, we also retrieved information on all MPN patients from the Inpatient Register to capture additional patients not reported to the Cancer Register. For each MPN patient, four population controls matched by age, sex, and calendar period of diagnosis were randomly selected from the Total Population Register using stratified simple random sampling. All control subjects had to be alive and without preceding hematological malignancy at time of MPN diagnosis for the corresponding patient.
Outcome measures
Information on non-fatal and fatal events of arterial and venous thrombosis was obtained from the Inpatient and Outpatient Registers and the Cause of Death Register. Arterial thrombosis was defined as myocardial infarction, ischemic stroke, or peripheral arterial thromboembolism. Venous thrombosis was defined as pulmonary embolism, deep venous thrombosis, abdominal, i.e. liver or splanchnic venous thrombosis, cerebral venous sinus thrombosis, or other venous thromboembolism. Separate analyses were performed for all thrombosis, arterial thrombosis which was further categorized into myocardial infarction and ischemic stroke, and venous thrombosis overall and subdivided into pulmonary embolism, deep venous thrombosis, and abdominal thrombosis.
In this matched cohort study, patients and controls were followed until the first event within each category of thrombosis. Thus, recurrent events within the same category of thrombosis were not included in the analyses, and events of other categories of thrombosis were ignored in each analysis. Patients and controls were followed for 20 years of follow-up, or until end of follow-up (December 31st, 2010), or at the competing event of death.
Statistical analyses
To estimate the hazard ratios (HRs) with 95% confidence intervals (CIs) of thromboembolic events comparing MPN patients to controls, flexible parametric models were used, allowing for non-proportional hazards for the effect of MPN status (i.e. a time-varying effect).(24) Time since diagnosis (or matching time for controls) was used as the underlying time scale. To avoid detection bias at time of diagnosis, analyses of thromboembolic events during follow-up started 30 days after diagnosis. As the HR varied with follow-up time, the HRs at 3 months, 1 year, and 5 years are presented for each outcome. All analyses were adjusted for age (in groups 18–49, 50–59, 60–69, 70–79 and ≥80 years at MPN diagnosis), sex, and calendar period of MPN diagnosis (1987–1992, 1993–1998, 1999–2004 and 2005–2009). All covariates were time-fixed and proportional hazards were assumed for the adjusting factors. Analyses were performed for MPN patients overall and separately by subtypes. Additional analyses were also performed that included interactions between MPN status and age group, MPN status, and calendar period, respectively. Cumulative incidence of thrombosis for each calendar period, age group, and sex was calculated by transforming the hazard rates of thrombosis obtained from the flexible parametric model while also taking the competing risk of death into account. To do this, a flexible parametric model was again used, to model the rate of death, including the same covariates as the model for thrombosis.(24)
The odds ratios of all thrombosis, and separately for arterial and venous thrombosis, at +/−30 days of MPN diagnosis, were assessed using logistic regression. In a sensitivity analysis, the HR of thrombosis during follow-up was calculated in MPN patients excluding those who had had a thrombosis during the first +/− 30 days of MPN diagnosis. Furthermore, we also assessed the impact of traditional risk factors; age ≥60 years and history of thrombosis in analyses only including MPN patients.
To evaluate the degree of confounding necessary to explain away the difference in rate of thrombosis in MPN patients to controls, we performed a sensitivity analysis as described by VanderWeele and Ding.(25) Their method is used to estimate the value of association needed, the E-value, between a confounder and the exposure and the confounder and outcome, to totally explain the observed exposure-outcome association observed.
All analyses were carried out using Stata version 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.).
Ethics
The study was approved by the Regional Ethical Review Board in Stockholm. Informed consent was waived because we had no contact with study patients and the data used for analyses did not contain any personal identifiers.
Role of the funding source
This work was funded by the Cancer Research Foundations of Radiumhemmet, Blodcancerfonden, the Swedish Research Council, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Adolf H. Lundin Charitable Foundation, and the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748). The funding sources had no role in the design, data collection, conduct, or analysis of this study or the decision to submit the manuscript for publication.
RESULTS
In total, 9,429 MPN patients (PV=3,001, ET=3,462, PMF=1,488, and MPN-U=1,478) and 35,820 matched controls were identified and included in the study. Forty-six percent were men and median age at MPN diagnosis was 72 years. Eighty percent of MPN patients were identified through the Cancer Register and 20% were identified through the Inpatient Register. For ten percent of MPN patients, less than four controls were available (Table 1).
Table 1.
Characteristics of patients with myeloproliferative neoplasms and matched controls
| MPN patients N (%) | Controls N (%) | |
|---|---|---|
| Sex | ||
| Men | 4,297 (46) | 16,080 (45) |
| Women | 5,132 (54) | 19,740 (55) |
| Age at diagnosis | ||
| 19–49 | 920 (10) | 3,680 (10) |
| 50–59 | 1,193 (13) | 4,772 (13) |
| 60–69 | 1,965 (21) | 7,859 (22) |
| 70–79 | 3,100 (33) | 12,261 (34) |
| ≥80 | 2,251 (24) | 7,248 (20) |
| Median age | 72 | 71 |
| Calendar period of diagnosis | ||
| 1987–1992 | 1,655 (18) | 6,366 (18) |
| 1993–1998 | 2,206 (23) | 8,358 (23) |
| 1999–2004 | 2,845 (30) | 10,746 (30) |
| 2005–2009 | 2,723 (29) | 10,350 (29) |
| MPN subtype | ||
| PV | 3,001 (32) | - |
| ET | 3,462 (37) | - |
| PMF | 1,488 (16) | - |
| MPN-U | 1,478 (16) | - |
| Total | 9,429 (100) | 35,820 (100) |
MPN=myeloproliferative neoplasm, PV=polycythemia vera, ET=essential thrombocythemia, PMF=primary myelofibrosis, MPN-U=myeloproliferative neoplasm unclassifiable
The rate of thrombosis was significantly elevated in MPN patients compared to controls and the highest hazard ratio was observed shortly after the MPN diagnosis (Table 2, Figure 1A and 1B). In analyses excluding the first 30 days after MPN diagnosis, the HRs of any thrombosis at 3 months after diagnosis was 4.0 (95% CI 3.6 to 4.4, p<0.001), in MPN patients compared to corresponding controls (Table 2). The HRs of thrombosis at 1 and 5 years after MPN diagnosis were 2.4 (CI, 2.3 to 2.6) and 1.8 (CI, 1.6 to 1.9), respectively. The hazard ratio of thrombosis was similar for men and women in relation to their corresponding controls.
Table 2.
Thrombosis during follow-up in MPN patients
| Type of thrombosis | Time after MPN diagnosis | All MPN HR 95% CI |
PV HR 95% CI |
ET HR 95% CI |
PMF HR 95% CI |
|---|---|---|---|---|---|
| Overall | 3 months | 4.0 (3.6–4.4) | 4.2 (3.5–5.0) | 3.5 (2.9–4.2) | 4.7 (3.6–6.0) |
| 1 year | 2.4 (2.3–2.6) | 2.5 (2.2–2.8) | 2.2 (1.9–2.5) | 2.7 (2.2–3.2) | |
| 5 years | 1.8 (1.6–1.9) | 1.8 (1.6–2.0) | 1.7 (1.5–1.9) | 2.0 (1.7–2.4) | |
| All arterial thrombosis | 3 months | 3.0 (2.7–3.4) | 2.7 (2.2–3.3) | 2.7 (2.2–3.3) | 4.4 (3.4–5.9) |
| 1 year | 2.0 (1.8–2.2) | 1.9 (1.6–2.1) | 2.0 (1.7–2.3) | 2.3 (1.9–2.9) | |
| 5 years | 1.5 (1.4–1.6) | 1.5 (1.3–1.7) | 1.4 (1.3–1.6) | 1.6 (1.3–1.9) | |
| Myocardial infarction | 3 months | 2.5 (2.1–2.9) | 2.1 (1.5–2.9) | 1.8 (1.3–2.4) | 5.3 (3.5–7.8) |
| 1 year | 1.8 (1.6–2.0) | 1.5 (1.2–1.9) | 1.5 (1.2–1.8) | 2.6 (2.0–3.5) | |
| 5 years | 1.4 (1.2–1.5) | 1.2 (1.0–1.5) | 1.4 (1.1–1.6) | 1.6 (1.2–2.2) | |
| Ischemic stroke | 3 months | 3.7 (3.1–4.4) | 3.7 (2.8–5.0) | 3.8 (2.8–5.1) | 3.7 (2.4–5.7) |
| 1 year | 2.3 (2.0–2.6) | 2.3 (1.9–2.8) | 2.6 (2.1–3.2) | 1.8 (1.3–2.5) | |
| 5 years | 1.5 (1.3–1.6) | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) | 1.5 (1.1–2.0) | |
| All venous thrombosis | 3 months | 9.7 (7.8–12.0) | 13.1 (8.7–19.6) | 8.5 (5.6–12.8) | 6.8 (4.0–11.4) |
| 1 year | 4.7 (4.0–5.4) | 5.4 (4.1–7.0) | 3.5 (2.6–4.5) | 4.3 (3.0–6.2) | |
| 5 years | 3.2 (2.9–3.6) | 3.6 (3.0–4.4) | 2.9 (2.4–3.6) | 4.0 (2.9–5.5) | |
| Pulmonary embolism | 3 months | 9.0 (6.4–12.5) | 11.8 (5.7–24.3) | 7.4 (4.2–13.2) | 8.1 (3.7–17.9) |
| 1 year | 5.3 (4.2–6.6) | 5.8 (3.8–8.8) | 4.1 (2.8–6.1) | 5.5 (3.1–9.7) | |
| 5 years | 3.4 (2.8–4.1) | 3.5 (2.5–4.9) | 3.2 (2.4–4.4) | 4.9 (3.1–7.7) | |
| Deep venous thrombosis | 3 months | 7.3 (5.2–10.3) | 9.9 (5.5–17.8) | 7.4 (4.0–13.8) | 5.2 (2.4–11.1) |
| 1 year | 3.7 (3.0–4.6) | 4.6 (3.1–6.7) | 3.0 (2.0–4.4) | 3.8 (2.2–6.6) | |
| 5 years | 3.3 (2.8–3.9) | 4.5 (3.4–5.9) | 2.5 (1.9–3.3) | 3.6 (2.3–5.8) |
HR=hazard ratio, CI=confidence interval, MPN=myeloproliferative neoplasm, PV=polycythemia vera, ET=essential thrombocythemia, PMF=primary myelofibrosis
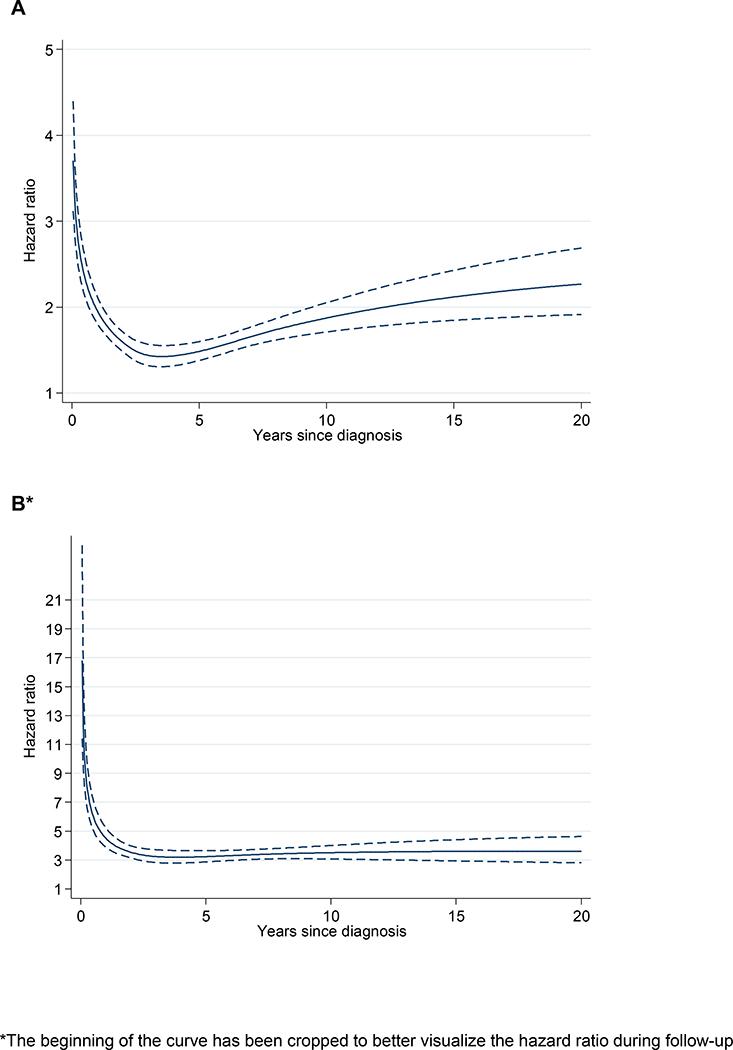
Figure 1.
Arterial (A) and venous (B) thrombosis in MPN patients vs matched controls during follow-up
In analysis of arterial thrombosis, overall and for myocardial infarction and ischemic stroke, there was a similar pattern with the highest HRs observed shortly after diagnosis which then decreased with follow-up time (Table 2). The HRs of arterial thrombosis at 3 months, 1 year, and 5 years were 3.0 (CI, 2.7 to 3.4), 2.0 (CI, 1.8 to 2.2), and 1.5 (CI, 1.4 to 1.6), respectively (Table 2 and Figure 1A). The rate ratio of arterial thrombosis was overall similar between the MPN subtypes apart from a trend towards higher HRs shortly after diagnosis in patients with PMF (Table 2). Moreover, the absolute number of first arterial events (n=2,078) was greater than the number of venous thrombotic events (n=1,106).
There was a near 10-fold elevation in the rate of venous thrombosis, HR 9.7 (CI, 7.8 to 12.0) at 3 months after MPN diagnosis. The HR of venous thrombosis decreased with follow-up time but was significantly elevated also at 1 and 5 years after MPN diagnosis (Table 2 and Figure 1B). The HRs for pulmonary embolism and deep venous thrombosis were significantly elevated and the HRs decreased in a similar pattern at 3 months, 1 year, and 5 years after MPN diagnosis (Table 2). In addition, the rate of abdominal thrombosis was substantially increased around the time of MPN diagnosis (HRs 81.1, CI, 22.0 to 300), 12.5 (CI, 6.4 to 24.5), and 4.3 (CI, 2.7 to 6.8) at 3 months, 1 year, and 5 years after MPN diagnosis, respectively, compared to controls. The hazard ratio of venous thrombosis was overall similar between the MPN subtypes (Table 2). After more than 5-years of follow-up, the HR for venous thrombosis remained stable up to 20 years after MPN diagnosis (Figure 1B).
The rate of both arterial and venous thrombosis was significantly elevated in MPN patients of all age groups (Table 3). The highest HRs of arterial and venous thrombosis were observed in younger patients. However, the absolute number of events was lower in these age groups which also was reflected in the lower cumulative incidence (Figure 3).
Table 3.
Thrombosis during follow-up according to age at MPN diagnosis
| Age at MPN diagnosis (years) | Time after MPN diagnosis | HR of arterial thrombosis with 95% CI | HR of venous thrombosis with 95% CI |
|---|---|---|---|
| 18–49 | 3 months | 15.2 (9.1–25.5) | 66.8 (42.5–105) |
| 1 year | 6.0 (3.9–9.2) | 14.6 (9.4–22.6) | |
| 5 years | 2.8 (1.9–4.1) | 6.0 (4.1–8.8) | |
| 50–59 | 3 months | 5.7 (3.8–8.6) | 20.5 (13.1–32.0) |
| 1 year | 3.0 2.3–4.0 | 9.0 (6.3–12.9) | |
| 5 years | 2.0 (1.5–2.5) | 4.9 (3.6–6.7) | |
| 60–69 | 3 months | 3.4 (2.6–4.4) | 9.1 (6.4–13.0) |
| 1 year | 2.0 (1.7–2.5) | 5.4 (4.2–7.0) | |
| 5 years | 1.5 (1.2–1.7) | 3.6 (2.9–4.5) | |
| 70–79 | 3 months | 2.4 (2.0–2.8) | 7.9 (6.0–10.5) |
| 1 year | 1.7 (1.5–1.9) | 4.3 (3.5–5.2) | |
| 5 years | 1.4 (1.2–1.5) | 3.0 (2.5–3.5) | |
| ≥80 | 3 months | 3.0 (2.5–3.5) | 6.2 (4.5–8.6) |
| 1 year | 2.1 (1.9–2.4) | 3.1 (2.5–3.9) | |
| 5 years | 1.5 (1.3–1.7) | 2.4 (1.9–3.2) |
MPN=myeloproliferative neoplasm, HR=hazard ratio, CI=confidence interval
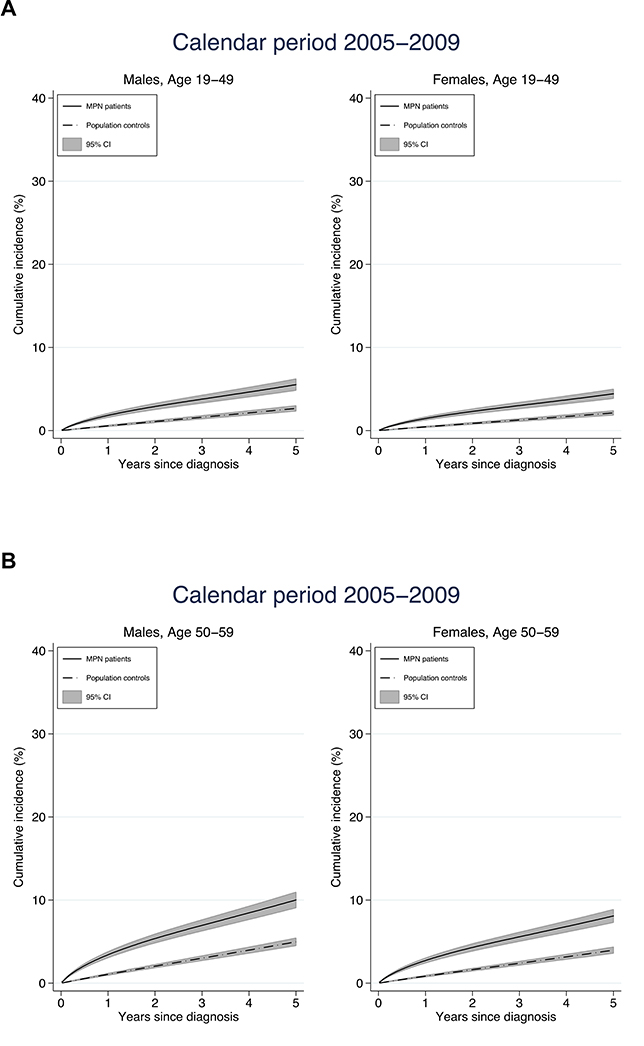
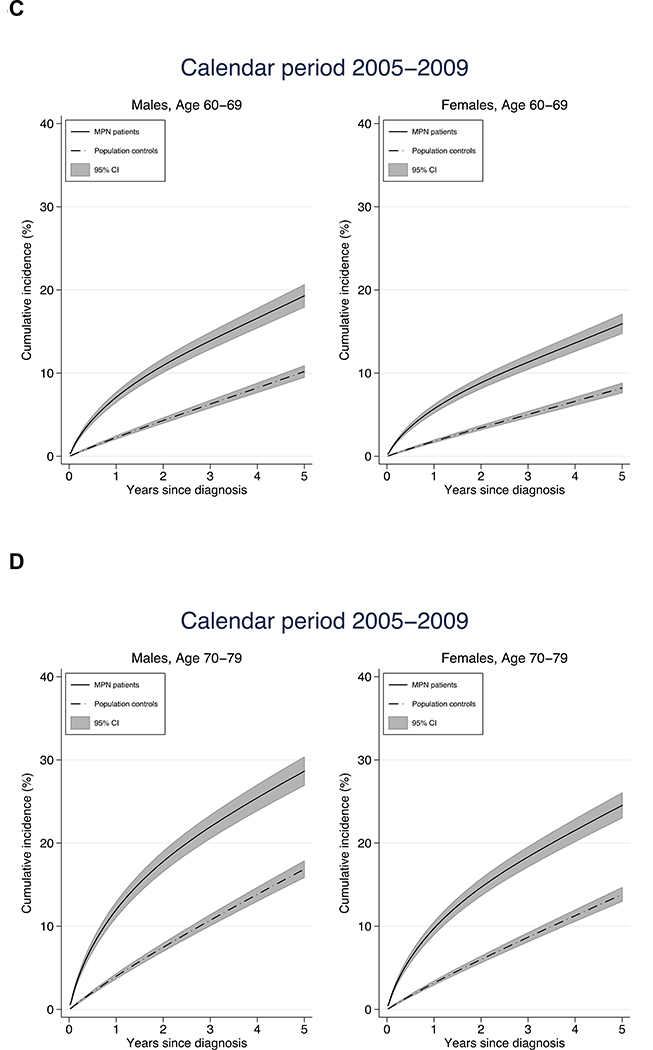
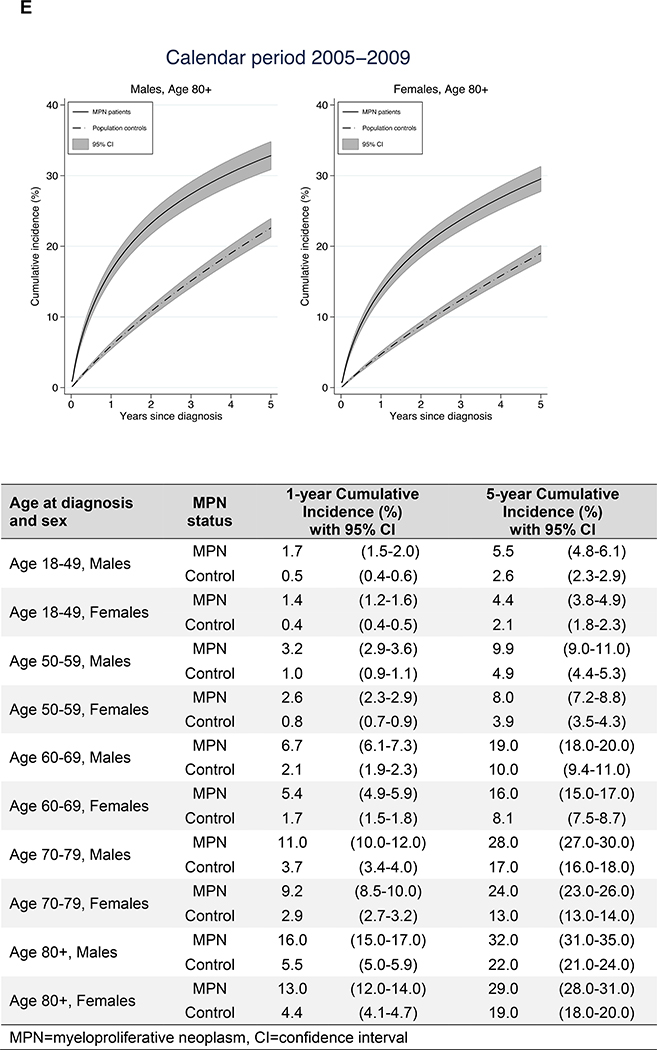
Figure 3.
Cumulative incidence of arterial and venous thrombosis in relation to age at diagnosis and sex
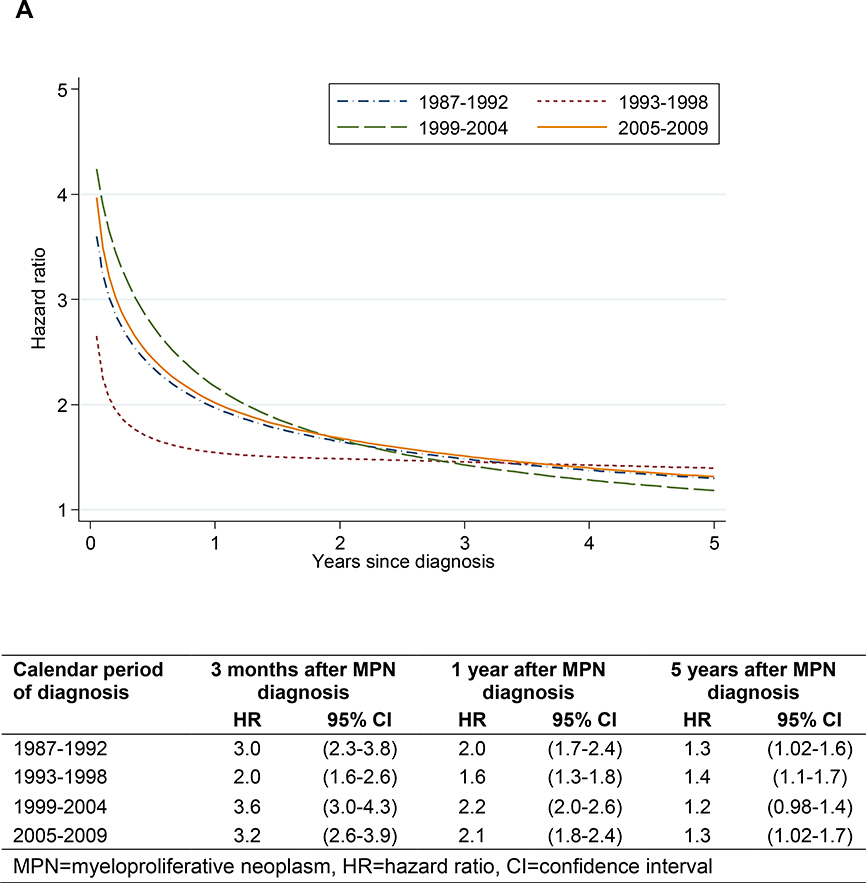
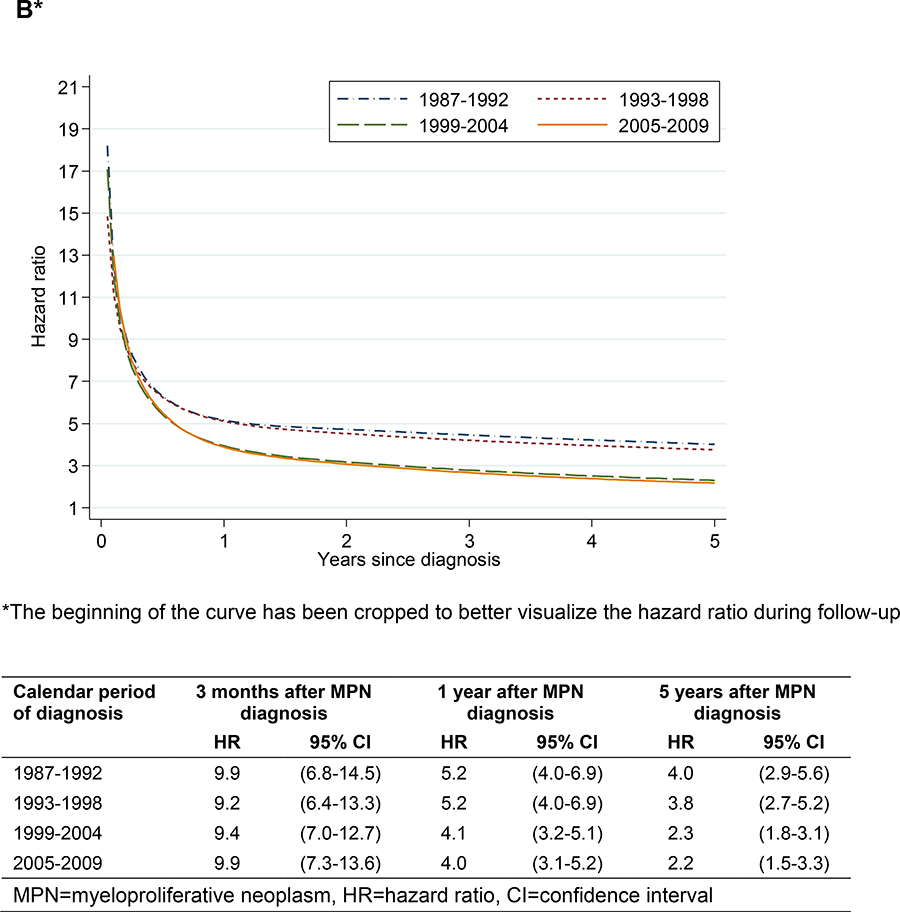
In analyses of thrombotic rate in relation to calendar period of MPN diagnosis, the HR was fairly stable over time for arterial thrombosis, whereas the HR for venous thrombosis was lower during the two latter calendar periods beyond the first 6 months after diagnosis (Figure 2).
Figure 2.
Arterial (A) and venous (B) thrombosis during follow-up in MPN patients vs matched controls in relation to calendar period of diagnosis
The cumulative incidence of thrombosis the first five years after diagnosis was higher in MPN patients compared to controls. The increase in cumulative incidence was more rapid in MPN patients, reflecting the higher rate of thrombosis during the initial follow up period. The 1-year cumulative incidence was an overall three times higher in MPN patients while the 5-year cumulative incidence was two times higher indicating that the rapid initial increase leveled off to a certain extent (Figure 3). As an example, the 1- and 5- year cumulative incidence for male MPN patients diagnosed between the ages of 60–69 years were 6.7% and 19.0% while the corresponding cumulative incidence for matched controls were 2.1 and 10.0%.
Nearly 10% of MPN patients had a thromboembolic event at the time of MPN diagnosis +/−30 days. The odds ratios for arterial and venous thrombosis at MPN diagnosis +/− 30 days were 15.6 (CI, 13.1 to 18.6) and 29.5 (21.3 to 40.7), respectively in MPN patients compared to controls (Supplementary Figure 1). In a sensitivity analysis, where all MPN patients who had a thrombosis during the initial period of +/− 30 days of diagnosis were excluded, there was only slight variations in the HRs of arterial and venous thrombosis during follow-up compared to the main analysis.
When addressing the traditional risk factors, age ≥60 years and history of thrombosis, the HR in MPN patients aged ≥60 years was 2.4 (CI, 2.1 to 2.6, p<0.001) compared to MPN patients <60 years. MPN patients with a history of thrombosis had a 2.7-fold increased rate of thrombosis (HR 2.7, CI 2.5 to 2.9, p<0.001) compared to MPN patients with no previous thrombosis. In patients that were ≥60 years and had a history of thrombosis, the HR of thrombosis was 7.0 (CI, 6.2 to 8.0).
A sensitivity analysis was performed in order to assess the degree of unmeasured confounding needed to fully explain away the difference in HR of any thrombosis at five years after diagnosis (HR 1.8; CI, 1.6 to 1.9). The reported HR could only be explained away by an unmeasured confounder that was associated with both MPN and thrombosis by a risk ratio of 2.37 each, but weaker confounding could not do so. The lower confidence limit of 1.6 has, similarly, an E-value of 2.11, which requires a confounder that is associated with both MPN and thrombosis by a risk ratio of 2.11 each for the confidence limit to get below 1. Most of the reported HRs (and their lower confidence limits) in this study are similar or higher than the HR for which we performed the sensitivity analyses, and would therefore have similar or higher E-value.
DISCUSSION
In this, largest to date, population-based study of risk of thrombosis in MPN patients, the rate of arterial and venous thrombosis was significantly increased in MPN patients compared to the general population. The highest rate ratio was observed at the time of MPN diagnosis and shortly thereafter, 3-fold and 10-fold elevated, respectively, for arterial and venous thrombosis. The HR thereafter decreased but remained significantly elevated throughout follow-up after MPN diagnosis. Moreover, the rate of thrombosis was significantly increased in MPN patients across all age groups. The long-term HR of venous thrombosis decreased in patients diagnosed during more recent calendar periods.
The overall high risk of thrombosis in MPN patients is well known but the pattern of thrombotic risk in relation to time after diagnosis in MPN patients has not been thoroughly assessed previously. We found a substantially elevated odds of thrombosis at the time of MPN diagnosis, the HR of thrombosis then decreased during the first year after diagnosis likely due effective thromboprophylactic and cytoreductive treatment of the MPN. Whilst the hazard ratio of venous events was greater, arterial events in MPN patients were twice as common compared to venous events, similar to earlier reports.(14, 15, 26, 27) Overall the HRs were similar across MPN subtypes which confirms previous findings of similar incidences of thrombosis in patients with ET and PMF and further emphasizes that vascular events are major contributors to excess morbidity and mortality in MPN patients.(13, 28–31) Using two different measures; hazard ratios over time and cumulative incidence, we conclude that the relative rate and risk of thrombosis in MPN patients is highest shortly after diagnosis and remains significantly elevated throughout follow-up time. This novel finding underlines the importance of initiating phlebotomies as well as thromboprophylactic and cytoreductive treatment, when indicated, as soon as the MPN has been diagnosed.
Traditional risk factors for thrombosis in MPN are age >60 years and prior thrombosis, both of which were confirmed in this study. The presence of both these risk factors was associated with a 7-fold increased risk of thrombosis. Furthermore, the risk of arterial and venous thrombosis was significantly elevated in MPN patients of all age groups and was in our study not restricted to those over the age of 60 years. Similar observations of elevated thrombotic risks in young MPN patients have been reported previously.(14, 15, 31, 32) However, due to limited number of events, further analysis of subgroups within the youngest age interval was not feasible and the results should be interpreted with some caution. Additional factors such as hematocrit ≥45% in PV patients, elevated leukocyte count, as well as concomitant cardiovascular risk factor have been associated with an increased risk of thrombosis.(13, 14, 26, 27, 33–35) Thrombocytosis has on the contrary not been correlated to an elevated thrombotic risk in MPN patients.(15, 27, 35, 36) There is emerging evidence that JAK2V617F positivity is associated with a higher risk while patients harboring CALR are at a lower risk of thrombosis compared to those who are negative for these mutations, respectively.(8, 37–41) Neither the Swedish Cancer Register nor the Patient Register includes individual clinical information on treatment, blood counts, or mutational status. Nevertheless, there are more complex mechanisms to take into consideration when assessing the thrombotic risk in MPN patients than age and prior thrombosis.
The excess rate of venous thrombosis decreased during more recent calendar periods implying a positive effect of improved treatment strategies. Additionally, there has been an overall decrease in the absolute risk of arterial thrombosis in both MPN patients and in the general population over the recent decades. (31, 33, 42–45) Advances in MPN management during the study period include increasing use of aspirin as primary prophylaxis, better management of cardiovascular risk factors, and more stringent adherence to recommendations for phlebotomies and cytoreductive treatment.(9, 33, 36, 43) In addition, JAK2-inhibitors, primarily ruxolitinib, and interferon may be effective in further reducing the risk of thrombosis.(46–48)
Strengths of this study are the population-based design, the high-quality Swedish Cancer Register, and the large number of patients over a study period of 23 years, all ensuring generalizability of our findings. The Swedish population consists mainly of Caucasian whites but up to 20% of individuals have a foreign background.(49, 50) Limitations to the study include possible underestimation of thrombotic events since only inpatient events were recorded before 2001. Another limitation concerns the possibility to adjust for co-morbidities and potential confounders. Since the Swedish Patient Registers does not include primary care visits, where the majority of population controls are seen, information on co-morbidities may not be captured equally for MPN patients and controls. We therefore refrained from including co-morbidities to avoid misclassification between patient and controls. Information on smoking is unfortunately not included in any of the registers. Although our results might be confounded by unmeasured factors, the estimated E-value suggests that such factor would have to have a large impact. Since there are no such strong risk factors for MPN, it is unlikely that there are any confounders with magnitude enough to explain away the mostly high HRs presented in this study.
This large population-based study is, to our knowledge, the first to quantify the excess rate of thrombosis in MPN patients compared to the general population, and the rate ratio changes over follow up time since diagnosis. We found that MPN patients of all age groups have an elevated rate of thrombosis especially shortly after the MPN diagnosis, thereafter the HR decreased but remained significantly elevated throughout follow-up time. Importantly, the hazard ratio of thrombotic events decreased with time after diagnosis likely due to effective treatment of the underlying MPN, and the hazard ratio of venous thrombosis was lower in patients diagnosed during more recent calendar periods. These are encouraging results and we believe that further refining the risk scoring systems, e.g. by including time after MPN diagnosis and biomarkers, as well as rethinking recommendations for young MPN patients, in combination with emerging more effective treatments will further improve the outcomes for patients with MPN.
Supplementary Material
Acknowledgments
Funding sources: The Cancer Research Foundations of Radiumhemmet, Blodcancerfonden, the Swedish Research Council, the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Adolf H. Lundin Charitable Foundation, and the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748).
Footnotes
Reproducible Research Statement
Study protocol: Available from Dr. Hultcrantz (malin.hultcrantz@ki.se). Statistical code: Available upon request from Drs. Hultcrantz or Andersson (malin.hultcrantz@ki.se, therese.m-l.andersson@ki.se). Data set: Not available but for specific questions, please contact Drs. Hultcrantz or Andersson.
References
- 1.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. [DOI] [PubMed] [Google Scholar]
- 3.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. [DOI] [PubMed] [Google Scholar]
- 5.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3(7):e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356(5):459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. [DOI] [PubMed] [Google Scholar]
- 9.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol. 2011;29(17):2410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorkholm M, Hultcrantz M, Derolf AR. Leukemic transformation in myeloproliferative neoplasms: therapy-related or unrelated? Best Pract Res Clin Haematol. 2014;27(2):141–53. [DOI] [PubMed] [Google Scholar]
- 12.Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(24):2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood. 2010;115(4):778–82. [DOI] [PubMed] [Google Scholar]
- 14.Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120(26):5128–33; quiz 252. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweden Statistics. Cancerincidens i Sverige 2013 : nya diagnosticerade cancerfall år 2013. Stockholm: Socialstyrelsen; 2014. [Google Scholar]
- 17.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swedish National Board of Health and Welfare (Socialstyrelsen). Dödsorsaksstatistik historik, produktionsmetoder och tillförlitlighet. In: Socialstyrelsen, ed; 2010. [Google Scholar]
- 19.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol 1975;12(4):339–51. [PubMed] [Google Scholar]
- 20.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol 1997;34(1):29–39. [PubMed] [Google Scholar]
- 21.Jaffe ESHN, Stein H, Vardiman JW, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2001. [Google Scholar]
- 22.Swerdlow SH, Jaffe ES, International Agency for Research on Cancer., World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 23.Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: Clinical and molecular advances. Blood Rev. 2016. [DOI] [PubMed] [Google Scholar]
- 24.Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol. 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 26.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353(1):33–45. [DOI] [PubMed] [Google Scholar]
- 27.Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, et al. Risk factors for arterial and venous thrombosis in WHO-defined essential thrombocythemia: an international study of 891 patients. Blood. 2011;117(22):5857–9. [DOI] [PubMed] [Google Scholar]
- 28.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29(23):3179–84. [DOI] [PubMed] [Google Scholar]
- 29.Cervantes F, Dupriez B, Pereira A, Passamonti F, Reilly JT, Morra E, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901. [DOI] [PubMed] [Google Scholar]
- 30.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23(10):2224–32. [DOI] [PubMed] [Google Scholar]
- 31.Hultcrantz M, Wilkes SR, Kristinsson SY, Andersson TM, Derolf AR, Eloranta S, et al. Risk and Cause of Death in Patients Diagnosed With Myeloproliferative Neoplasms in Sweden Between 1973 and 2005: A Population-Based Study. J Clin Oncol 2015;33(20):2288–95. [DOI] [PubMed] [Google Scholar]
- 32.Stein BL, Saraf S, Sobol U, Halpern A, Shammo J, Rondelli D, et al. Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymphoma. 2013;54(9):1989–95. [DOI] [PubMed] [Google Scholar]
- 33.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. [DOI] [PubMed] [Google Scholar]
- 34.Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109(6):2446–52. [DOI] [PubMed] [Google Scholar]
- 35.Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian JJ, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120(7):1409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176–84. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Larran A, Arellano-Rodrigo E, Reverter JC, Domingo A, Villamor N, Colomer D, et al. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann Hematol. 2008;87(4):269–76. [DOI] [PubMed] [Google Scholar]
- 38.Finazzi G, De Stefano V, Barbui T. Are MPNs vascular diseases? Curr Hematol Malig Rep. 2013;8(4):307–16. [DOI] [PubMed] [Google Scholar]
- 39.Robertson B, Urquhart C, Ford I, Townend J, Watson HG, Vickers MA, et al. Platelet and coagulation activation markers in myeloproliferative diseases: relationships with JAK2 V6I7 F status, clonality, and antiphospholipid antibodies. J Thromb Haemost. 2007;5(8):1679–85. [DOI] [PubMed] [Google Scholar]
- 40.Vannucchi AM, Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost. 2013;39(5):496–506. [DOI] [PubMed] [Google Scholar]
- 41.Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35(5):702–11. [DOI] [PubMed] [Google Scholar]
- 42.Barbui T, Carobbio A, Rumi E, Finazzi G, Gisslinger H, Rodeghiero F, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124(19):3021–3. [DOI] [PubMed] [Google Scholar]
- 43.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350(2):114–24. [DOI] [PubMed] [Google Scholar]
- 44.Barbui T, Vannucchi AM, Carobbio A, Thiele J, Rumi E, Gisslinger H, et al. Patterns of presentation and thrombosis outcome in patients with polycythemia vera strictly defined by WHO-criteria and stratified by calendar period of diagnosis. Am J Hematol. 2015;90(5):434–7. [DOI] [PubMed] [Google Scholar]
- 45.Statistics Sweden, Hjärtsjukvård : öppna jämförelser och utvärdering 2009. Stockholm: Socialstyrelsen; 2009. [Google Scholar]
- 46.Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–72. [DOI] [PubMed] [Google Scholar]
- 47.Samuelson BT, Vesely SK, Chai-Adisaksopha C, Scott BL, Crowther M, Garcia D. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis: a meta-analysis. Blood Coagul Fibrinolysis. 2016;27(6):648–52. [DOI] [PubMed] [Google Scholar]
- 48.Verstovsek S, Vannucchi AM, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101(7):821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Statistics Sweden, Befolkningsstatistik. 1993 Stockholm Örebro: SCB; Publikationstjänsten, SCB; 1994. [Google Scholar]
- 50.Statistics Sweden. Tabeller över Sveriges befolkning 2009 [Tables on the population in Sweden 2009]. Örebro: SCB-tryck; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.