Abstract
Infection with the severe acute respiratory syndrome coronavirus 2 causes severe acute lung injury in approximately 5% of infected adults, but few reports have been made of severe pediatric disease. We present an adolescent patient who contracted severe acute respiratory syndrome coronavirus 2 one week after a paternal haplo-identical hematopoietic stem cell transplant, with development of severe hyperferritinemic acute lung injury and macrophage activation-like syndrome. We present her case and a comparison of her laboratory data with those of a cohort of pediatric patients with coronavirus disease 2019 without severe disease.
Key Words: bone marrow transplant, COVID-19, macrophage activation-like syndrome, pediatric acute respiratory distress syndrome, pediatrics
Abbreviations: COVID-19, coronavirus disease 2019; HSCT, hematopoietic stem cell transplant; MALS, Macrophage activation-like syndrome
The coronavirus disease 2019 (COVID-19) has infected 3 million people worldwide, with varying disease phenotype.1 Data from China suggest that critical cases occur in 0.6% of infected children, and they have reported only one pediatric death.2 This contrasts with adult data describing critical illness in 5% of patients and mortality rates of 2.3% to 7%.3, 4, 5 Patients who have hematopoietic stem cell transplantation (HSCT) are at high risk for severe infectious complications.
Case Report
A 15-year-old Hispanic girl in New York City developed pneumonitis 8 days after a paternal-donor haplo-identical HSCT for myelodysplastic syndrome. She had no other medical history before transplantation. Her conditioning regimen consisted of fludarabine 25 mg/m2 IV daily days -14 to -11, busulfan 0.8 mg/mg IV Q6 × 16 days -10 to -7, donor T cells on day -6, and cyclophosphamide 60 mg/kg IV daily days -3 and -2. She received CD34-selected peripheral blood stem cells. Graft-vs-host disease prophylaxis consisted of tacrolimus and mycophenolate mofetil.
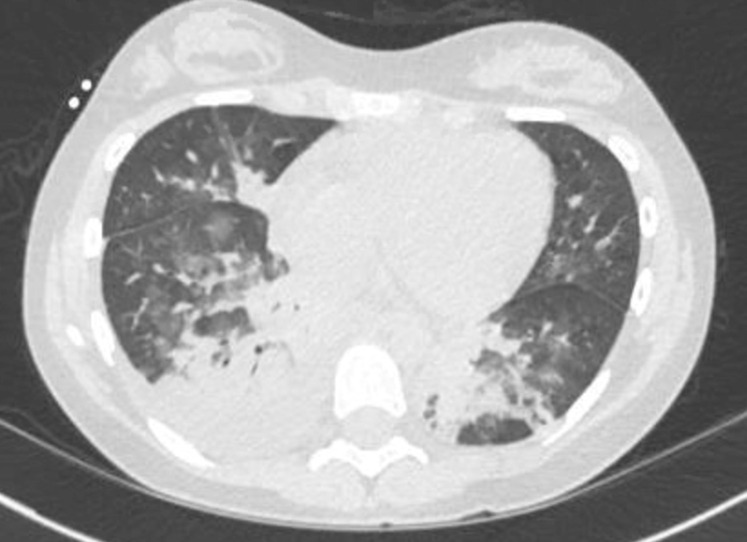
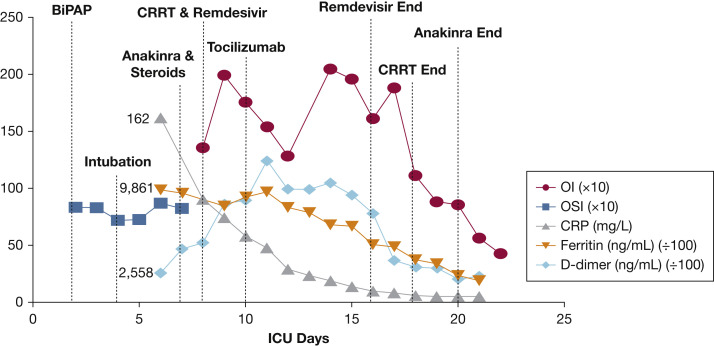
She became febrile 8 days posttransplantation. She had no other complications from the pretransplantation or immediate posttransplantation period. Chest CT findings were consistent with those of COVID-19 (Fig 1 ).6 She was on biphasic positive airway pressure for 4 days, when escalating oxygen requirements necessitated intubation. She developed severe pediatric ARDS and presumed septic shock. A sputum sample tested positive for COVID-19 by polymerase chain reaction. All other infectious studies were negative. Adult medication dosing was used because she weighed 59 kg. Treatment began with hydroxychloroquine 400 mg twice daily and azithromycin 250 mg twice daily, as was common practice at that time. On intubation, she had features suggestive of macrophage activation-like syndrome (MALS) or secondary hemophagocytic lymphohistiocytosis with fever, splenomegaly, high ferritin, and elevated soluble IL-2 receptor. Anakinra 100 mg q6h and dexamethasone 10 mg/m2 daily were started per secondary hemophagocytic lymphohistiocytosis institutional standards. Her C-reactive protein and ferritin decreased significantly (Fig 2 ). A 7-day course of remdesivir, an experimental antiviral, was given through compassionate use protocol. There was an interruption in remdesivir because of a continuous renal replacement therapy requirement on ICU days 8 through 18 for oliguric renal failure. In the setting of severe PARDS and shock, there was concern for anakinra treatment failure, and tocilizumab 480 mg was administered (PICU day 10). Given her elevated D-dimer and reports of microthrombi in COVID-19, she was anticoagulated with therapeutic enoxaparin.7 Her renal function normalized. She underwent tracheostomy placement after 26 days of intubation and one failed extubation attempt. She awaits rehabilitation transfer with the goal of eventual decannulation.
Figure 1.
CT of the chest showing bibasilar patchy ground glass opacities, a right lower lobe consolidation, and a right pleural effusion. The patchy lower-lobe ground glass appearance is typical of coronavirus disease 2019 seen in adults.
Figure 2.
Trend of inflammatory markers and oxygenation index over time. OI and OSI are powered to 101. CRP = C-reactive protein; CRRT = continuous renal replacement therapy; OI = oxygenation index; OSI = oxygen saturation index.
Discussion
We present a teenage girl with immunosuppression due to HSCT who developed hyperferritinemic acute lung injury, multisystem organ dysfunction, and MALS secondary to COVID-19. She was treated with anakinra and dexamethasone, with some improvement in inflammatory markers.8 Because her clinical condition remained critical, tocilizumab was administered, using treatment protocols for cytokine release syndrome.9 Recent data suggest that early IL-6 blockade impairs host ability to suppress viral replication.10 Indications for and appropriate timing of IL-1 and IL-6 blockade in COVID-19-associated cytokine release syndrome remain to be established. Therapeutic enoxaparin was administered given reports of thrombi in COVID-19.7
With Northwell Health institutional review board approval (20-0318-CCMC), we compared the patient’s laboratory values with those of five randomly selected PICU patients with COVID-19 who did not require mechanical ventilation and were discharged without complication. Her inflammatory markers were pointedly higher than those of the healthier cohort (Table 1 ).
Table 1.
Laboratory Values With Comparison With a Randomly Selected PICU Cohort With Mild Coronavirus Disease 2019
| Laboratory Value | Peak Value (PICU Day) | Posttreatment (PICU Day 18) | Median Mild Disease Severity ICU Cohort (Range) (n = 5) | Laboratory Reference Value |
|---|---|---|---|---|
| CRP, mg/L | 162 (6) | <5.0 | 14 (7.7-36) | <5.0 |
| Ferritin, ng/mL | 9,861 (6) | 1,513 | 302 (127-579) (n = 3) | 15-150 |
| D dimer, ng/mL | 12,396 (11) | 1,021 | 300 (207-438) (n = 3) | <230 |
| Fibrinogen, mg/dL | 666 (7) | 175 | 550 (500-601) (n = 2) | 15-150 |
| Triglycerides, mg/dL | 526 (2) | 103 | 119 (66-172) (n = 4) | 10-149 |
| LDH, U/L | 795 (11) | 689 | 361 (178-593) | 135-225 |
| Soluble IL-2 receptor, U/mL | 1,096 (8) | 137-838 |
CRP = C-reactive protein; LDH = lactate dehydrogenase.
Reports from China concluded that severe disease in children is rare.11 Parri et al12 described characteristics of 100 admitted Italian children; the median age was 3.3 years, and only one patient presented with hypoxia.12 Our case contrasts with reports of pediatric disease in China and Italy, given the patient's age and severe disease. The United States has one of the highest uses of HSCT worldwide, with a rate six times that of China.13 The higher rates of comorbidities in American children may explain the seemingly higher rate of severe illness in the United States.14 The COVID-19 Global Rheumatology Alliance reported a mortality rate similar to that reported in general population studies in patients with autoimmune disorders, including those on immunosuppressive medications. Whether immunosuppression is associated with a more severe phenotype of COVID-19 remains unknown; some have postulated the cytokine storm of COVID-19 may be dampened in these patients.15 , 16
COVID-19 has been reported in HSCT adult patients in Europe. Challenges exist in differentiating COVID-19 from other opportunistic infections in HSCT patients.17 There has been discussion of delaying or modifying chemotherapy in pediatric cancer patients during the pandemic.18 Given that the severity of disease herein was likely modified by the immune dysregulation associated with HSCT, delaying HSCT in nonemergent cases should be considered until the severe acute respiratory syndrome coronavirus 2 pandemic is better controlled.
Conclusion
We present a teenager post-HSCT with COVID-19-induced hyperferritinemic acute lung injury, multisystem organ dysfunction, and MALS. Further studies are needed to elucidate optimal therapy for these patients, because the patient received multiple targeted medications. This case suggests that infection in the immune-dysregulated adolescent can cause severe illness.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. F. L. has stock in Pfizer. M. D. T. receives NIGMS grant funding [K08GM132794]. None declared (G. F., A. H., J. S., C. A. C., J. D. F., J. A. B.).
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met. We thank Dr Indira Sahdev for providing her insight into management decisions.
References
- 1.Dong E., Du H., Garnder L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Novel coronavirus pneumonia emergency response epidemiology T. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 6.Liu M., Song Z., Xiao K. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020;44(3):311–313. doi: 10.1097/RCT.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang T., Sun L.X., Feng R.E. [Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E040. doi: 10.3760/cma.j.cn112147-20200311-00312. [DOI] [PubMed] [Google Scholar]
- 8.Kyriazopoulou E., Leventogiannis K., Norrby-Teglund A., et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15(1):172. doi: 10.1186/s12916-017-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng F., Liao C., Fan Q.H., et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parri N., Lenge M., Buonsenso D. Children with COVID-19 in pediatric emergency departments in Italy. New Engl J Med. 2020;383(2):187–190. doi: 10.1056/NEJMc2007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratwohl A., Baldomero H., Aljurf M., et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 Data: North American pediatric ICUs virtual pediatric systems: Children’s Hospital Association. https://covid19.myvps.org/ Accessed July 8, 2020.
- 15.Gianfrancesco M.A., Hyrich K.L., Gossec L., et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020;19(5):102523. doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahu K.K., Jindal V., Siddiqui A.D., Cerny J. Facing COVID-19 in the hematopoietic cell transplant setting: a new challenge for transplantation physicians. Blood Cells Mol Dis. 2020;83:102439. doi: 10.1016/j.bcmd.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotecha R.S. Challenges posed by COVID-19 to children with cancer. Lancet Oncol. 2020;21(5):e235. doi: 10.1016/S1470-2045(20)30205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]