Abstract
Purpose
The aim of this study was to evaluate 1-year quantitative changes in specific inflammatory parameters on optical coherence tomography (OCT) / optical coherence tomography angiography (OCTA) in diabetic macular edema (DME) treated with subthreshold micropulse laser (SMPL).
Methods
Thirty-seven patients / eyes with previously treatment-naïve DME treated with SMPL were prospectively evaluated at 3, 6, and 12 months. Fifteen fellow eyes with only microaneurysms (MAS) not eligible for treatment were controls. Evaluated OCT / OCTA parameters included: central macular thickness (CMT); hyper-reflective retinal spots (HRS); disorganization of inner retinal layers (DRILs); MA in the superficial / deep capillary plexuses (SCP/DCP); cysts in the area at the SCP / DCP; and macular perfusion parameters (MATLAB, version 2017b).
Results
In the treated group, mean best corrected visual acuity (BCVA) progressively increased from 69.4 ± 12.0 to 76.0 ± 9.1 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (P < 0.001) at 12 months; HRS decreased from baseline (80.75 ± 20.41) at 3 (73.81 ± 17.1, P = 0.002), 6 (69.16 ± 16.48, P < 0.0001), and 12 months (66.29 ± 18.53, P < 0.0001). MA decreased at 3 months in the DCP (P = 0.015), at 6 and 12 months in both plexuses (P ≤ 0.0007). BCVA, HRS, and MA remained stable in the controls during all follow-ups. DRIL was present in 18 of 37 patients at baseline and progressively decreased from 557.0 ± 238.7 to 387.1 ± 282.1 μm (P = 0.01). The area of cyst decreased both in the SCP (P = 0.03) and the DCP (P = 0.02). CMT and perfusion parameters did not change.
Conclusions
SMPL reduced the number of HRS (sign of activated microglia cells in the retina), MA, DRIL extension, and the area of cysts. Further studies are needed to confirm these preliminary data on the anti-inflammatory effect of SMPL, and to explore the mechanism of action.
Translational Relevance
The follow-up of OCT/OCTA noninvasive biomarkers offers a unique insight in the mechanism of laser action, suggesting an anti-inflammatory effect of SMPL.
Keywords: diabetic macular edema, inflammation, optical coherence tomography, optical coherence tomography angiography, subthreshold micropulse laser
Introduction
The subthreshold micropulse laser (SMPL) is a relatively novel retinal laser technique with documented higher safety for retinal tissue compared to the conventional continuous wavelength laser.1–6 SMPL does not induce protein coagulation, and, therefore, prevents formation of retinal scars and tissue damage.1,7 Even if SMPL has proven to be an effective treatment for diabetic macular edema (DME), in terms of visual function improvement/stabilization and macular thickness decrease,3,4,8–15 the exact mechanism of action is still under investigation. Retinal pigment epithelium (RPE) has been considered the main target with the consequent release of “heat shock proteins” (Hsps), in particular Hsp 70.16,17 This stress-induced response results in the immunomodulation of retinal cells metabolism and function, activation of repair processes, and decrease in the production of inflammatory cytokines, vascular endothelium growth factor (VEGF), and matrix metalloproteinases.1,2,17,18 Recently, it has been reported that SMPL reduces the aqueous humour (AH) concentration of inflammatory cytokines secreted by retinal glial cells (GLCs), both Müller cells (MCs) and microglial cells (MGCs) in eyes with DME.19,20 A decrease in the inner nuclear layer thickness (INL), where the bodies of MCs are located, was reported after SMPL.21 Whether this effect on GLCs is direct or mediated by the RPE has still to be evaluated and confirmed; however, these findings suggest that SMPL might be able to downregulate the inflammatory retinal processes activated by hyperglycemia in diabetes mellitus (DM).19,20
The clinical effects of SMPL on the retina in eyes with DME have been evaluated using standard imaging techniques, such as color fundus photography, optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein angiography (FFA), and microperimetry.3,4,9–11,15 However, only one study reported on the use of OCT angiography (OCTA) in evaluating microvascular changes occurring after SMPL treatment in DME.18 In that study, our group documented early significant changes in some microvascular parameters (decrease in the area of FAZ, number of microaneurysms (MAs), and intraretinal area of cysts), in particular in the deep capillary plexus (DCP), during 6 months of follow-up after SMPL.18 No data are available on the long-term effect of SMPL on the retina using OCTA, by quantitative evaluation of specific perfusion parameters using different software programs.
The aim of the present new study was to evaluate, during a period of 12 months, and in a different cohort of patients, specific quantitative changes in inflammatory and microvascular macular parameters in patients with DME treated with SMPL, by swept source (SS)-OCT and OCTA. Moreover, novel quantitative methods of OCTA image analyses were adopted for evaluation of perfusion parameters, using both MATLAB and ImageJ software programs.
Methods
Population and Study Design
This study is a prospective, 12-month, longitudinal and consecutive case evaluation of 37 eyes (37 patients) with previously treatment-naïve DME treated with SMPL. All enrolled patients were evaluated at the Medical Retina Service, University Hospital Maggiore della Carità, Novara, Italy, between February 2017 and March 2019. Fifteen fellow eyes of the same patients with MAs in the central 3 mm of the macula (not requiring any treatment for the entire duration of the study) were recruited as the control group.
Inclusion criteria for the study were: men or women with age ≥ 18 years; no previous treatment for DME; central macular thickness (CMT) ≤ 400 μm; patients with best corrected visual acuity (BCVA) ≥ 78 letters Early Treatment Diabetic Retinopathy Study (ETDRS) score, or patients with BCVA < 78 letters ETDRS score who refused treatment with anti-VEGF intravitreal injections or could not have anti-VEGF treatment due to contraindications; and patients who accepted to participate in the study. Exclusion criteria were any other retinal condition different from diabetic retinopathy (DR); proliferative DR; any previous retinal surgery/laser treatment; cataract surgery in the previous 6 months and presence of any degree of cataract other than initial opacity of the lens (not significantly affecting visual acuity); glaucoma or history of ocular hypertension (intraocular pressure > 21 mm Hg); any systemic neurodegenerative disease (e.g. multiple sclerosis, Alzheimer's disease, and Parkinson's disease); uncontrolled systemic blood pressure (values ≥ 120/80); and significant media opacity precluding good quality fundus imaging.
All patients underwent a complete eye examination, including BCVA assessed at 4 meters using standard ETDRS protocol, dilated slit-lamp fundus examination with 90D lens, color fundus photography, FAF, swept source OCT/OCTA, and FFA. All examinations, except FFA, were performed at baseline, 3, 6, and 12 months after SMPL. FFA was performed only at baseline.
The standard treatment parameters and re-treatment criteria had already been described in detail (100 μm spot size, 5% duty cycle, and 250-mW power, confluent spots, using a subthreshold 577-nm yellow light micropulse laser, Iridex IQ 577; Laser System Iridex Corp, Mountain View, CA).18 This “high-density” treatment was used to cover the area of increased macular thickness. Criteria for retreatment were CMT ≥ 300 μm; decrease in retinal thickness in the treated quadrant <20% of the baseline value; and BCVA decrease ≥ 5 ETDRS letters.
The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional ethics committee (CE 123-2017); signed informed consent was obtained from all patients.
Imaging
Color fundus photography, FAF, SS-OCT, and SS-OCTA were performed using DRI OCT/OCT-A Triton plus (Topcon Medical Systems Europe, Milano, Italy). The characteristics of this device have been previously described in detail.18
The following scans were acquired: single 6-mm high-definition B-scan at 0 degrees and 90 degrees, 6-mm radial OCT scan (consisting of 12 scans 15 degrees apart) centered on the fovea, and 3-dimensional 3 × 3 mm OCTA map of the macula.
FFA at baseline was performed with Spectralis HRA-OCT (Heidelberg Engineering, Heidelberg, Germany).
OCT Parameters
Following pre-treatment and post-treatment OCT quantitative parameters were evaluated: CMT measured within 1 central mm using the instrument automatic segmentation (software version 10.07.003.03); total number of hyper-reflective retinal spots (HRS), determined within 3 central millimeters on the horizontal scan using the multipoint tool on ImageJ software (version 1.51, http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD), after adjusting image brightness and contrast to enhance HRS visibility; presence and extension of disorganization of retinal inner layers (DRILs) within 1 central millimiter.22
OCTA Parameters
The following quantitative parameters were evaluated on 3 × 3 mm automatic OCTA slabs before and after treatment: number of MAs in the superficial capillary plexus (SCP) and in the DCP; area of cysts in both the SCP and DCP; perfusion density (PD) in both the SCP and DCP; flow voids in the choriocapillaris (CC FVs); and perfused capillary density (PCD) in the full macula slab. All OCTA images were carefully reviewed to check automatic segmentation of different slabs, with particular attention to DCP and CC segmentation, as previously reported.18
MAs were manually counted on OCTA slabs using the reverse mode (black vessels on white background) allowing a better visualization.11 The area of the cysts was measured after manually delimiting the contours of single retinal cysts in the SCP and DCP using the caliper tool implemented in the machine software IMAGENET 6 (version 1.17.9720). All manual evaluations were made in a masked fashion to the clinical data of eyes by two retina specialists. In case of disagreement, the final adjudication was given by S.V.
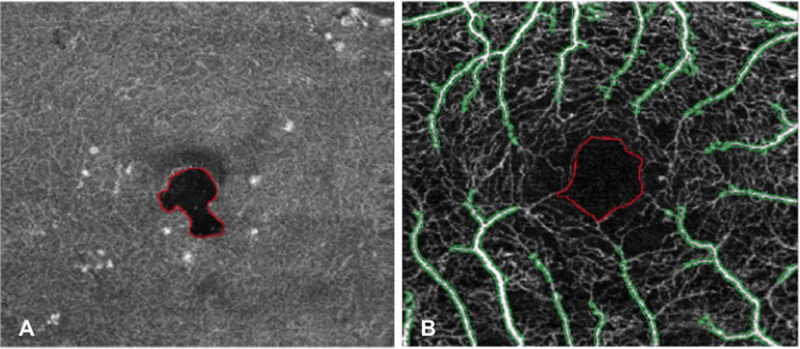
PDs at the SCP and DCP were computed after excluding the regions occupied by cysts in the corresponding retinal layer. CC FVs were evaluated after removing projection artifacts caused by the presence of cysts in the overlying retinal layers (in particular at the DCP level). These analyses were performed using MATLAB (version 2017b; MathWorks, Natick, MA) adopting a previously published method (Fig. 1A).23
Figure 1.
MATLAB (version 2017b, Mathworks, Natick, MA) analyses performed on OCTA images of the same patient (left eye) to evaluate macular perfusion parameters. (A) First method of analysis: automatic detection and demarcation of cyst area in the en-face DCP image; the area of the cyst was then excluded from final computation of perfusion parameters. (B) Second method of analysis: OCTA image of the full macula slab in which the area corresponding to FAZ (identified by red boundaries) and noncapillary blood vessels (identified by green boundaries) was excluded from final computation of PCD. OCTA, optical coherence tomography angiography; DCP, deep capillary plexus; FAZ, foveal avascular zone; PCD, perfused capillary density.
An additional analysis of PCD was performed applying a recently published method using the full macula slab.24 This further analysis aimed at strengthening data on retinal perfusion as less influenced by the presence of artifacts. First, each OCTA image full macula slab was opened in ImageJ analysis software and the FAZ profile was manually outlined using the free-hand selection tool in order to create an FAZ mask. Then, global thresholding was applied using MATLAB to obtain a binary image aimed at isolating noncapillary blood vessels (that had to be excluded from the final computation of PCD). After the removal of the area corresponding to the FAZ and noncapillary blood vessels, local thresholding was applied for the automatic segmentation of macular perfused capillaries and PCD (%) was quantified as the ratio between perfused capillary area and total remaining area × 100% (Fig. 1B).
Statistical Analyses
The study parameters have been summarized as mean and SD. Changes over time of quantitative variables were evaluated by means of analysis of variance for repeated measures (ANOVA). Patient group (treated and controls) and time elapsed from the first visit were assumed as independent factors. The interaction between patient's grouping and time was assessed to establish the presence of difference in temporal trends of the variable under consideration between treated and control patients.
Differences in the average baseline values between treated patients and controls were assessed by two-tailed unpaired t-test.
Differences in treated patients between average baseline values and values at 3, 6, and 12 months of follow-ups were assessed with two-tailed t-test for dependent variable with Bonferroni's correction for multiple comparisons.
All statistical analyses have been made using Statistica version 6.0 (StatSoft Inc., Tulsa, OK) using a two-sided type I error rate of P = 0.05.
Results
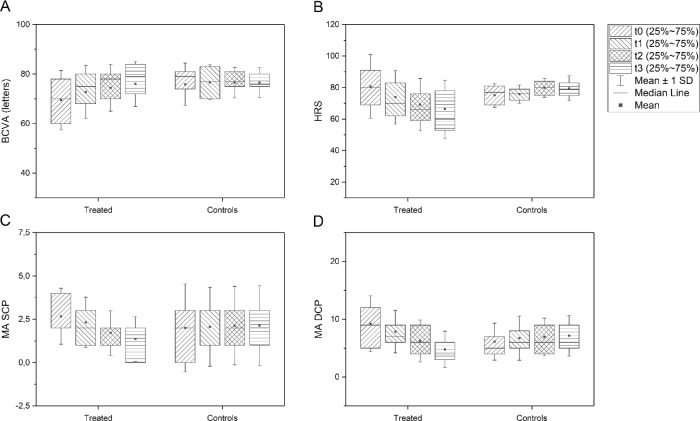
All 37 eyes treated with SMPL and 15 control eyes (with DM type 2) were included in the final analysis. All patients were of Caucasian ethnicity and had type 2 DM and moderate nonproliferative DR according to the International Clinical Diabetic Retinopathy Disease Severity Scale.25 All the patients were evaluated by an experienced retina specialist (S.V.). FFA performed at baseline excluded the presence of significant ischemic retinal areas in the posterior pole and periphery. In the treated group, 18 patients were men and 19 were women; in the control group, 12 were men and 3 were women. Mean age of patients was 69.1 ± 11.0 years, mean duration of DM was 15.9 ± 9.4 years, and HbA1c was 7.5 ± 1.18%. Mean BCVA in the treated group at baseline was 69.4 ± 12.0 ETDRS letters score with significant increase over time (72.8 ± 10.8 at 3 months, mean gain +3.4 ± 7.3 ETDRS letters, P = 0.009; 74.4 ± 9.4 at 6 months, mean gain +5.0 ± 8.3 ETDRS letters, P = 0.0007; and 76.0 ± 9.1 at 12 months, mean gain +6.6 ± 8.4 ETDRS letters, P < 0.0001). Mean BCVA in the control group remained stable over time (75.9 ± 8.6 ETDRS letters score at baseline, 76.7 ± 7.1 at 3 months, 76.5 ± 6.1 at 6 months, and 76.6 ± 6.0 at 12 months; P > 0.05). Mean BCVA at baseline did not significantly differ between the two groups (P = 0.06). BCVA change over time was significantly different between the two groups (P = 0.01; Fig. 2A).
Figure 2.
Box-plots showing BCVA (A), number of HRS (B), MA in the SCP (C), and MA in the DCP (D) at baseline, 3, 6, and 12 months after SMPL treatment and in the control group. BCVA, best corrected visual acuity; HRS, hyper-reflective retinal spots; MA, microaneurysm; SCP/DCP, superficial/deep capillary plexus; SMPL, subthreshold micropulse laser.
Tables 1 and 2 show mean values of all quantitative parameters evaluated on OCT and OCTA in the treated group during the period of 12 months. The number of HRS significantly decreased during the follow-up in the treated group (P = 0.002 at 3 months, and P < 0.0001 at 6 and 12 months), whereas it remained stable in the control group. HRS number change over time was significantly different between the two groups (P < 0.001; Fig. 2B). The number of MAs decreased significantly both in the SCP and in the DCP in the treated group. This decrease was detected at 6 months (P = 0.0007) and at 12 months (P < 0.0001) in the SCP; in the DCP, the significant decrease was detected as early as 3 months after treatment (P = 0.015) and was maintained during all follow-up visits (P < 0.0001 at 6 and 12 months). In the control group, MAs remained stable in the SCP (2.0 ± 2.5 at baseline, 2.1 ± 2.3 at 3 months, 2.1 ± 2.3 at 6 months, and 2.1 ± 2.3 at 12 months) and DCP (6.1 ± 3.2 at baseline, 6.7 ± 3.8 at 3 months, 6.9 ± 3.3 at 6 months, and 7.1 ± 3.5 at 12 months). The MA number change over time was significantly different between the two groups (P < 0.001; Figs. 2C, 2D). The inter-grader agreement was almost perfect (k > 0.9) for all manual evaluations.
Table 1.
OCT Parameters Before and After Subthreshold Micropulse Laser
| Parameter | Baseline | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| CMT | 304.95 ± 50.69 | 302.29 ± 44.53 | 304.13 ± 51.1 | 294.49 ± 39.8 |
| HRS | 80.75 ± 20.41 | 73.81 ± 17.1a | 69.16 ± 16.48b | 66.29 ± 18.53c |
| DRIL | 557.0 ± 238.7 | 465.5 ± 268.2 | 462.1 ± 312.1 | 387.1 ± 282.1c |
P = 0.002.
P < 0.0001.
P = 0.01.
P values are referred to baseline.
Values are represented in mean ± standard deviation. CMT and DRIL are expressed in micrometers.
OCT, optical coherence tomography; CMT, central macular thickness; SCP/DCP, superficial/deep capillary plexus; DRIL, disorganization of retinal inner layers.
Table 2.
OCT-Angiography Parameters Before and After Subthreshold Micropulse Laser
| Parameter | Baseline | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| MA SCP | 2.7 ± 1.6 | 2.3 ± 1.5 | 1.7 ± 1.3a | 1.3 ± 1.3b |
| MA DCP | 9.2 ± 4.9 | 7.9 ± 3.7c | 6.2 ± 3.7b | 4.8 ± 3.2b |
| Cysts area SCP | 96.65 ± 110.18 | 76.09 ± 109.49 | 62.96 ± 84.74d | 62.20 ± 100.77e |
| Cysts area DCP | 433.09 ± 397.10 | 377.27 ± 371.85 | 316.83 ± 272.92f | 308.98 ± 290.6g |
| PD SCP | 25.57 ± 3.07 | 25.47 ± 3.15 | 25.29 ± 2.8 | 25.88 ± 2.78 |
| PD DCP | 31.92 ± 8.65 | 29.94 ± 4.97 | 30.05 ± 4.67 | 31.97 ± 7.14 |
| PCD | 44.41 ± 4.81 | 43.81 ± 6.0 | 44.89 ± 4.21 | 44.2 ± 4.75 |
| FV CC | 34.9 ± 4.05 | 34.93 ± 4.83 | 35.37 ± 3.94 | 33.48 ± 5.87 |
P = 0.0007.
P < 0.0001.
P = 0.015.
P = 0.004.
P = 0.03.
P = 0.003.
P = 0.02. P values are referred to baseline.
Values are represented in mean ± standard deviation. Cysts area is expressed in square micrometers, PD, PCD, and FV in percentage.
OCT, optical coherence tomography; SCP/DCP, superficial/deep capillary plexus; MA, microaneurysm; PD, perfusion density; PCD, perfused capillary density; FV, flow void; CC, choriocapillaris.
At baseline, DRIL was present in 18 patients (48.6%) treated with SMPL. Mean DRIL extension at baseline was 557.0 ± 238.7 μm; it progressively decreased during the follow-up reaching statistical significance at 12 months (P = 0.01; Fig. 3). A decrease in DRIL extension was found in 15 of 18 patients (83.3%) and a complete resolution in 3 of 18 patients (16.6%).
Figure 3.
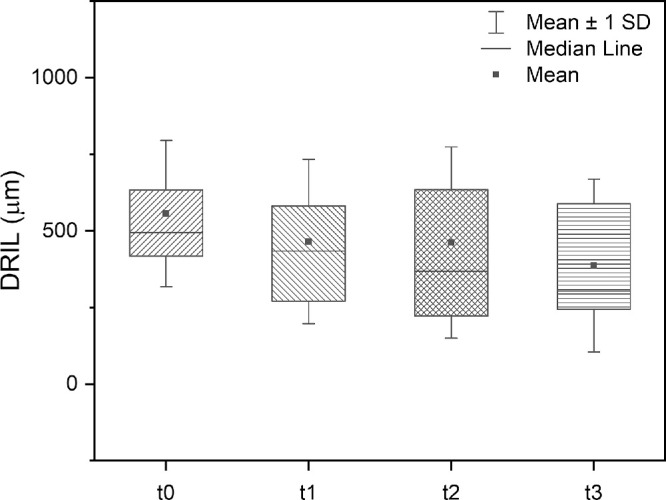
Box-plots showing DRIL extension at baseline, 3, 6, and 12 months after SMPL treatment in 18 patients with presence of DRIL in the one central mm at baseline. DRIL, disorganization of inner retinal layers; SMPL, subthreshold micropulse laser.
The area of the cysts significantly decreased starting from month 6 both in the SCP (P = 0.004 at 6 months and P = 0.03 at 12 months) and in the DCP (P = 0.003 at 6 months and P = 0.02 at 12 months).
No significant changes were found as for the CMT, PD in SCP and DCP, PCD, and CC FV.
Thirty-one patients (83.8%) needed re-treatment. Mean number of SMPL treatments was 2.19 ± 0.7 during 12 months. No FAF signs of treatment were detected at any examination.
No eye, neither in the treated group nor in the control group, needed any additional treatment (i.e. anti-VEGF, steroids, and/or conventional laser) for DME and/or DR during the entire duration of the study.
Discussion
In the present study, we report on changes in macular inflammatory and microvascular parameters in eyes with DME, up to 1 year after SMPL treatment (Fig. 4 and Fig. 5). In particular, a significant decrease in the number of HRS, extension of DRIL, number of MAs (especially in the DCP), and the area of the cysts, was documented. To the best of our knowledge, only one previous study (from our group), using a different cohort of patients with DME and with shorter follow-up, evaluated the use of OCTA to investigate the clinical effects of SMPL, documenting earlier and more pronounced changes in some macular parameters (FAZ area, number of MAs, and area of the cysts) in the DCP than in the SCP up to 6 months after treatment.18
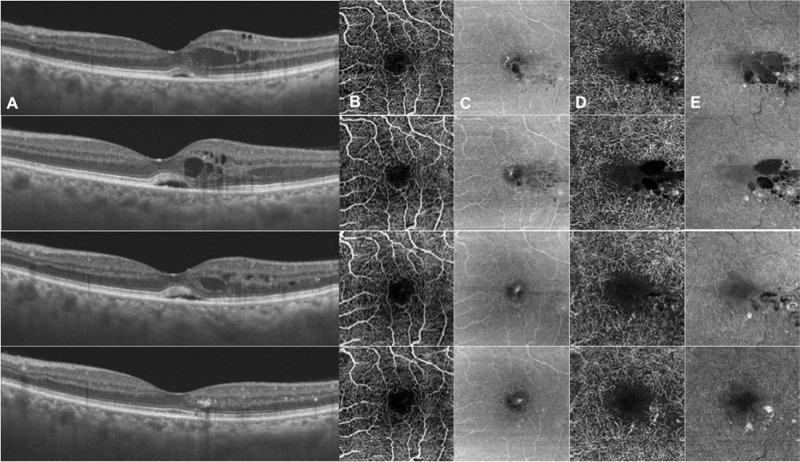
Figure 4.
Images of the left eye of a patient with DME at baseline (top row) and 3 (second row), 6 (third row), and 12 months (bottom row) after SMPL treatment showing a progressive reduction of intraretinal cysts and MAs in the DCP and resolution of serous neuroretinal detachment. In this patient, DRIL was not present at baseline. (A) Horizontal structural OCT scan; (B) OCTA SCP slab; (C) en face SCP slab; (D) OCTA DCP slab; and (E) en face DCP slab. DME, diabetic macular edema; SMPL, subthreshold micropulse laser; MAs, microaneurysms; DCP, deep capillary plexus; HRS, hyper-reflective retinal spots; DRIL, disorganization of inner retinal layers; OCTA, optical coherence tomography angiography; SCP, superficial capillary plexus.
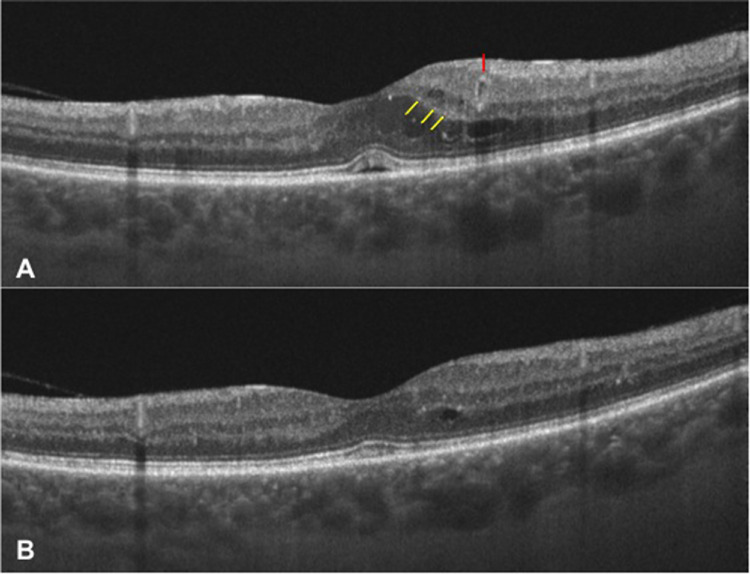
Figure 5.
Images of the left eye of a patient with DME at baseline (A) and 12 months after SMPL treatment (B) showing the reduction of HRS (just a few of the HRS highlighted by yellow arrows at baseline), complete resolution of serous retinal detachment and disappearance of a perifoveal microaneurysm (highlighted by a red arrow at baseline). At the 12-month visit, all retinal layers are clearly discernible and no DRIL is present. DME, diabetic macular edema; SMPL, subthreshold micropulse laser; HRS, hyper-reflective retinal spots; DRIL, disorganization of inner retinal layers.
DR is a complex multifactorial disease, in which inflammation plays an important role.26,27 Several noninvasive imaging biomarkers of inflammation in the retina have been studied in patients with DR and DME.28 These include HRS, visible on OCT, and considered the signs of activated microglial cells in the retina.29 These HRS have peculiar features, such as dimension <3 0 μm, reflectivity similar to that of the nerve fiber layer, absence of back-shadowing, and location in both the inner and outer retina.29 Previous studies have demonstrated a decrease in the number of HRS after treatment with both intravitreal dexamethasone and anti-VEGF drugs23,30,31; however, no evidence has been reported so far regarding the effect of SMPL on HRS during long-term follow-up. In this study, a progressive decrease in the number of HRS was documented up to 1 year after treatment with SMPL. This may support the hypothesis of a possible anti-inflammatory effect of SMPL on the retina, as postulated in recent studies reporting a reduction in pro-inflammatory molecules released by GLC in the AH of eyes with DME (glial fibrillary acidic protein, Kir 4.1, regulated and normal T cell expressed and secreted, macrophage inflammatory protein-1α, Fas ligand, and VEGF), suggesting that the metabolic activity of both the macroglia and the microglia may be improved by SMPL.19,20 However, the RPE has been considered the main target of SMPL with consequent modifications of retinal cells’ metabolic activity and changes of gene expression and protein secretion.1,2,17,18 As the present study was not designed to evaluate the anatomic target of SMPL, we could only speculate on a possible effect on GLC through reduction of HRS and MA in the DCP.18 Further studies are needed to confirm this hypothesis and determine whether this action is direct on GLC or indirect through the stimulation of RPE.
The number of MAs decreased significantly both in the SCP and DCP, despite not being directly treated by SMPL, as previously reported.18 MA decrease was more precocious in the DCP (starting from the third month after treatment) and continued during the entire follow-up (12 months). Recent studies by Parravano et al. demonstrated that MAs visible on OCTA in the DCP may correspond to hyper-reflective MAs on structural OCT and were interpreted as leaking MAs with a high blood flow rate.32 It has been speculated that hyper-reflective MAs could have a higher inflammatory component resulting in early and acute blood-retinal barrier impairment.33 MCs have a key role in the regulation of retinal homeostasis and their activation in response to neuroinflammatory local changes can influence the blood-retinal barrier function at the level of intermediate capillary plexus and DCP (all located and interconnected with the bodies of MCs at the level of the INL).34 Thus, improvement of MC function can have a beneficial effect also at the DCP level,26,35 with consequent reduction in the number of MAs.18 Moreover, a previous study evaluating single retinal layer changes after SMPL, had documented the greatest reduction in thickness at the level of the INL at 12 months.21 In the present study, no significant reduction in CMT was documented after treatment. However, the thickness of single retinal layers has not been evaluated due to the intrinsic limit of the used OCT instrument. Even if no reduction in CMT (evaluated only in the onecentral millimeter of the macula) was found, the area of the cysts (evaluated in the entire 3 × 3 mm en face image) significantly decreased both in the SCP and in the DCP starting from month 6 and with greater decrease in the DCP.
The re-treatment was needed in 83.8% of patients during the 12 months of follow-up, according to the re-treatment criteria described in the Methods section. However, this fact does not increase the risk of retinal damage when standard laser settings are used (as in the present study).11
Almost half of the patients (48.6%) had DRIL in the 1 central millimeter at baseline. DRIL isent considered to be a sign of neurodegeneration in DR and it was proposed to present disorganization/destruction of cells located within inner retinal layers, leading to dysfunction of visual pathways between photoreceptors and ganglion cells.22,36 A decrease in DRIL extension is considered an important prognostic and predictive factor of visual acuity recovery after treatment in DME.22,36 In this study, the extension of DRIL progressively decreased after treatment, reaching statistical significance at 12 months. In particular, a decrease in DRIL extension was found in 15 of 18 patients (83.3%) and a complete resolution in 3 of 18 patients (16.6%). In recent studies, a positive effect on DRIL extension was documented after intravitreal Dexamethasone implant, which was probably due to the reduction in retinal inflammation.23,37 This anti-inflammatory effect was hypothesized through both suppression of microglial reactivity, with a consequent protective effect on retinal neurons, and improvement in Müller cells’ architecture.23,37
In the present study, no changes were detected in retinal and choroidal perfusion parameters after compensating for possible artifacts and using different methods of analysis. This may further strengthen the hypothesis of a predominant action of SMPL on the inflammatory component of DME. However, data on macular perfusion obtained through the analyses of OCTA images should be interpreted with caution, as a lack of finding significant changes after treatment could be due to insufficient sensitivity of the instrument itself and not to the effective lack of modification of a specific parameter.38 There are limited data in the literature on the use of OCTA in the evaluation of DME treatment.23,39 Lee et al. were the first to evaluate separately the SCP and DCP in DME after treatment with anti-VEGF reporting a greater extent of microvascular damage in the DCP (reduced perfusion and a higher number of MAs) in eyes with poor response to treatment.40 In addition, later studies confirmed the usefulness of OCTA in the assessment of DME, despite the risk of misinterpretation of data due to artifacts determined by the presence of intraretinal fluid.41 However, to the best of our knowledge, this is the first study to apply different quantitative and mainly automatic methods of analyses on different retinal layers and choroid to evaluate treatment response to SMPL.
Major limits of the present study include the relatively low number of examined eyes and the presence of the control group for BCVA, HRS, and MAs (the fellow eyes with MA in the macula not needing any treatment during the study). We would like to acknowledge that this study did not aim at evaluating the efficacy of SMPL treatment, thus it was not designed as a randomized controlled trial. However, the use of a prospective longitudinal design, methods aimed at compensating for possible artifacts, and different methods of analysis in the evaluation of OCTA images strengthen the obtained results.
In conclusion, the present study aimed to evaluate long-term quantitative changes in OCT and OCTA parameters in DME treated with SMPL. A significant decrease in the number of HRS and MAs, in particular in the DCP, the area of the cysts, and extension of DRIL was found. These findings may be due to a possible anti-inflammatory effect of SMPL through the improvement of GLC metabolism/activity. Thus, this study documented the presence of quantifiable retinal biomarkers of response to SMPL treatment. Further studies are needed to confirm these preliminary data.
Acknowledgments
Disclosure: S. Vujosevic, None; C. Toma, None; E. Villani, None; M. Brambilla, None; E. Torti, None; F. Leporati, None; A. Muraca, None; P. Nucci, None; S. De Cilla, None
References
- 1. Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev. 2012; 8: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vujosevic S, Martini F, Convento E, et al.. Subthreshold laser therapy for diabetic macular edema: metabolic and safety issues. Curr Med Chem. 2013; 20: 3267–3271. [DOI] [PubMed] [Google Scholar]
- 3. Figueira J, Khan J, Nunes S, et al.. Prospective randomized controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009; 93: 1341–1344. [DOI] [PubMed] [Google Scholar]
- 4. Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010; 30: 908–916. [DOI] [PubMed] [Google Scholar]
- 5. Sivaprasad S, Sandhu R, Tandon A, Sayed-Ahmed K, McHugh DA. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Experiment Ophthalmol. 2007; 35: 640–644. [DOI] [PubMed] [Google Scholar]
- 6. Luttrull JK, Sinclair S.. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014; 34: 2010–2020. [DOI] [PubMed] [Google Scholar]
- 7. Su D and Hubschman JP. A review of subthreshold micropulse laser and recent advances in retinal laser technology. Ophthalmol Ther. 2017; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005; 89: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004; 88: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavinsky D, Cardillo JA, Melo LA Jr, Dare A, Farah ME, Belfort R Jr. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011; 52: 4314–4323. [DOI] [PubMed] [Google Scholar]
- 11. Vujosevic S, Martini F, Longhin E, Convento E, Cavarzeran F, Midena E. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema. Retina. 2015; 35: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 12. Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Subthreshold micropulse diode laser versus conventional laser photocoagulation for diabetic macular edema: a meta-analysis of randomized controlled trials. Retina. 2016; 36: 2059–2065. [DOI] [PubMed] [Google Scholar]
- 13. Jorge EC, Jorge EN, Botelho M, et al.. Monotherapy laser photocoagulation for diabetic macular oedema. Cochrane Database Syst Rev. 2018; 10: CD010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Y, Ai P, Ai Z, et al.. Subthreshold diode micropulse laser versus conventional laser photocoagulation monotherapy or combined with anti-VEGF therapy for diabetic macular edema: a Bayesian network meta-analysis. Biomed Pharmacother. 2018; 97: 293–299. [DOI] [PubMed] [Google Scholar]
- 15. Donati MC, Murro V, Mucciolo DP, et al.. Subthreshold yellow micropulse laser for treatment of diabetic macular edema: comparison between fixed and variable treatment regimen. Eur J Ophthalmol. 10.1177/1120672120915169. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Li Z, Song Y, Chen X, et al.. Biological modulation of mouse RPE cells in response to subthreshold diode micropulse laser treatment. Cell Biochem Biophys. 2015; 73: 545–552. [DOI] [PubMed] [Google Scholar]
- 17. Inagaki K, Shuo T, Katakura K, Ebihara N, Murakami A, Ohkoshi K. Stimulation with a micropulse laser induces heat shock protein expression in ARPE-19 cells. J Ophthalmol. 2015; 2015: 729792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vujosevic S, Gatti V, Muraca A, et al.. Optical coherence tomography angiography changes after subthreshold micropulse yellow laser in diabetic macular edema. Retina. 2020; 40: 312–321. [DOI] [PubMed] [Google Scholar]
- 19. Midena E, Bini S, Martini F, et al.. Changes of aqueous humour Müller cells’ biomarkers in human patients affected by diabetic macular edema after sub-threshold micropulse laser treatment. Retina. 2020; 40: 126–134. [DOI] [PubMed] [Google Scholar]
- 20. Midena E, Micera A, Frizziero L, Pilotto E, Esposito G, Bini S. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci Rep. 2019; 9: 10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vujosevic S, Frizziero L, Martini F, et al.. Single retinal layer changes after subthreshold micropulse yellow laser in diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2018; 49: e218–e225. [DOI] [PubMed] [Google Scholar]
- 22. Sun JK, Radwan SH, Soliman AZ, et al.. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015; 64: 2560–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vujosevic S, Toma C, Villani E, et al.. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020; 57: 287–296. [DOI] [PubMed] [Google Scholar]
- 24. Rosen RB, Andrade Romo JS, Krawitz BD, et al.. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019; 203: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkinson CP, Ferris FL, Klein RE, et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110: 1677–1682. [DOI] [PubMed] [Google Scholar]
- 26. Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. [DOI] [PubMed] [Google Scholar]
- 27. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015; 122: 1375–1394. [DOI] [PubMed] [Google Scholar]
- 28. Vujosevic S, Simó R.. Local and systemic inflammatory biomarkers of diabetic retinopathy: an integrative approach. Invest Ophthalmol Vis Sci. 2017; 58: BIO68–BIO75. [DOI] [PubMed] [Google Scholar]
- 29. Vujosevic S, Bini S, Torresin T, et al.. Hyperreflective retinal spots in normal and diabetic eyes: b-scan and en face spectral domain optical coherence tomography evaluation Retina. 2017; 37: 1092–1103. [DOI] [PubMed] [Google Scholar]
- 30. Vujosevic S, Torresin T, Bini S, et al.. Imaging retinal inflammatory biomarkers after intravitreal steroid and anti-VEGF treatment in diabetic macular oedema. Acta Ophthalmol. 2017; 95: 464–471. [DOI] [PubMed] [Google Scholar]
- 31. Bonfiglio V, Reibaldi M, Pizzo A, et al.. Dexamethasone for unresponsive diabetic macular edema: optical coherence tomography biomarkers. Acta Ophthalmol. 2019; 97: 540–544. [DOI] [PubMed] [Google Scholar]
- 32. Parravano M, De Geronimo D, Scarinci F, et al.. Diabetic microaneurysms internal reflectivity on spectral-domain optical coherence tomography and optical coherence tomography angiography detection. Am J Ophthalmol 2017; 179: 90–96. [DOI] [PubMed] [Google Scholar]
- 33. Parravano M, De Geronimo D, Scarinci F, et al.. Progression of diabetic microaneurysms according to the internal reflectivity on structural optical coherence tomography and visibility on optical coherence tomography angiography. Am J Ophthalmol. 2019; 198: 8–16. [DOI] [PubMed] [Google Scholar]
- 34. Newman E, Reichenbach A.. The Müller cell: a functional element of the retina. Trends Neurosci. 1996; 19: 307–312. [DOI] [PubMed] [Google Scholar]
- 35. Bandello F, Tejerina AN, Vujosevic S, et al.. Retinal layer location of increased retinal thickness in eyes with subclinical and clinical macular edema in diabetes type 2. Ophthalmic Res. 2015; 54: 112–117. [DOI] [PubMed] [Google Scholar]
- 36. Sun JK, Lin MM, Lammer J, et al.. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014; 132: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 37. Zur D, Iglicki M, Sala-Puigdollers A, et al.. Disorganization of retinal inner layers as a biomarker in patients with diabetic macular oedema treated with dexamethasone implant. Acta Ophthalmol. 2020; 98: e217–e223. [DOI] [PubMed] [Google Scholar]
- 38. Vujosevic S, Toma C, Villani E, et al.. Early detection of microvascular changes in patients with diabetes mellitus without and with diabetic retinopathy: comparison between different swept-source OCT-A instruments. J Diabetes Res. 2019; 2019: 2547216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toto L, D'Aloisio R, Di Nicola M, et al.. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int J Mol Sci. 2017; 18: E1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016; 123: 2368–2375. [DOI] [PubMed] [Google Scholar]
- 41. AttaAllah HR, Mohamed AAM, Ali MA. Macular vessel density in diabetic retinopathy: quantitative assessment using optical coherence tomography angiography. Int Ophthalmol. 2019; 39: 1845–1859. [DOI] [PubMed] [Google Scholar]