Abstract
Emerging evidence has identified the association between gut microbiota and various diseases, including cardiovascular diseases (CVDs). Altered intestinal flora composition has been described in detail in CVDs, such as hypertension, atherosclerosis, myocardial infarction, heart failure, and arrhythmia. In contrast, the importance of fermentation metabolites, such as trimethylamine N-oxide (TMAO), short-chain fatty acids (SCFAs), and secondary bile acid (BA), has also been implicated in CVD development, prevention, treatment, and prognosis. The potential mechanisms are conventionally thought to involve immune regulation, host energy metabolism, and oxidative stress. However, numerous types of programmed cell death, including apoptosis, autophagy, pyroptosis, ferroptosis, and clockophagy, also serve as a key link in microbiome-host cross talk. In this review, we introduced and summarized the results from recent studies dealing with the relationship between gut microbiota and cardiac disorders, highlighting the role of programmed cell death. We hope to shed light on microbiota-targeted therapeutic strategies in CVD management.
1. Introduction
Cardiovascular disease (CVD), with its rising prevalence rate and mortality, entails both health threats and economic burdens to our society. As a chronic progressive condition, the development of CVDs often begins with risk factors like obesity, type 2 diabetes, and hypertension, most of which would irreversibly damage vascular structure and eventually lead to detrimental clinical outcomes like arterial thrombosis and ischemic stroke. While heredity can only be blamed for less than 20% occurrence of CVDs, dietary and nutritional statuses are two stimuli with more profound and lasting impacts [1]. Therefore, increasing evidence has suggested a close relationship between gut microbiota and CVD development [2].
The gut microbiota refers to trillions of commensal microorganisms located in the intestine in a certain proportion, whose balance is easily disturbed by food intake, lifestyle, and environment [3]. Considered a complex organ, the microbial community is required in the committed step through which food would be converted into small compounds and metabolites, thus modulating intestine structure, gut barrier integrity, inflammatory status, and host metabolism both directly and indirectly [4]. Since Hippocrates claimed that “all diseases begin in the gut” centuries ago, a great body of research has demonstrated the interplay between intestinal microbiota and diseases, including colorectal cancer [5], cerebral ischemia-reperfusion injury [6], liver fibrosis [7], and CVDs [8]. The gut microbiota accounts for 0.2–2.0 kg of the weight of an adult and approximately 50% of the dry weight of adult feces. The enormous genome of microbial genes and their functions are described as the microbiome, which outnumbers the human genome tremendously [3, 9]. Although the characteristics of the gut community may be inherited in early life, the composition could also be altered by external conditions [10, 11]. Appropriate gut microbiota structure and metabolite functions are essential in homeostasis maintenance, whereas gut dysbiosis contributes to atherosclerosis, hypertension, heart failure, arrhythmia, cardiac tumours, and others [12]. However, its underlying mechanisms are multifactorial and yet to be determined.
In this report, we introduce the role of gut microbiota in CVDs and summarize possible mechanisms, which may provide a theoretical basis and shed light on novel therapeutic strategies in the prevention and treatment of CVDs.
2. Mechanisms Underlying the Interaction between Gut Microbiota and the Host
The community of gut microbiota consists mostly of bacteria, fungi, and viruses in which the primary component is bacteria. There are 5 major families in the intestinal flora: Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia [13]. Although the variety of species is abundant, the architecture of gut microbiota is comparatively fixed in different sites. However, the differences in gut microorganism quantities between locations are significant, with the ascending colon containing the largest number [13]. Under physiological conditions, more than 90% of the bacteria comprise Bacteroidetes and Firmicutes, while an elevated Firmicutes/Bacteroidetes (F/B) proportion is associated with CVDs [14]. Koliada et al. found that with the body mass index (BMI) in Ukraine adult population increasing, their F/B ratio raised likewise after removing other confounders such as age or smoking [15]. Subsequently, evaluation of children's gut microbiota composition and BMI had confirmed F/B ratio as a key risk indicator for childhood obesity [16]. Additionally, the F/B ratio is related to low-grade inflammation leading diabetes mellitus [17]. These diseases serve as both risk factors and stimulatives for CVDs. In addition to intestinal integrity maintenance, gut metabolites serve as essential messengers in the communication between gut microbiota and the host. Here, we review the mechanisms underlying the interaction between gut microbiota and the host, especially in CVDs.
2.1. Immunoregulation
Generated by fiber fermentation in the colon, short-chain fatty acids (SCFAs) include three major products, namely, acetate, propionate, and butyrate, all of which contain less than six carbons [18]. Apart from being nutrients and energy sources for intestinal epithelial cells, these small-molecule metabolites could enter the blood circulation, participate in immune regulation and inflammation modulation either by binding to G protein-coupled receptors (GPCRs) or by inhibiting histone deacetylases (HDACs) [18], and thereby influence gut homeostasis and host diseases. Laurence et al. found that SCFAs induce NLRP3 inflammasome activation and subsequent abundant IL-18 secretion in a GPR43- and GPR109A-dependent manner, thus eliciting favourable effects on intestinal integrity maintenance [19]. Of note, GPR43 and GPR109A are two receptors that are expressed on intestinal epithelial cells and some immune cells, where GPR43 mainly binds to acetate and propionate, while GPR109A is specifically activated by butyrate [20]. Studies have demonstrated that SCFAs beneficially upregulate not only the proliferation and differentiation of regulatory T cells (Tregs) but also the anti-inflammatory IL-10 secreted from Foxp3+ Tregs, which are mediated through GPR43 (also known as Ffar2) activation and HDAC inhibition [21]. Additionally, butyrate was shown to suppress proinflammatory factors, including IL-6, IL-12, and NO, from intestinal macrophages by HDAC inhibition [18]. Likewise, Bartolomaeus et al. recently proved that the anti-inflammatory role of SCFAs such as propionate significantly reduced the number of effector memory T cells and T helper 17 cells, thus mitigating cardiovascular damage [22]. However, the proinflammatory functions mediated by GPR41 (also known as Ffar3) and GPR43 were reported elsewhere [23], indicating that SCFA-induced immunoregulatory effects are dependent on the distinct cell types.
Additionally, trimethylamine N-oxide (TMAO) is generally investigated as a risk indicator for cardiovascular diseases, diabetes mellitus, nonalcoholic fatty liver disease, and other metabolic events [24–26]. As the end-product of dietary choline and L-carnitine, TMAO is converted from trimethylamine (TMA) in the liver by flavin-containing monooxygenases (FMOs), especially FMO3 [24]. However, how exactly TMAO functions to regulate homeostasis is seldom discussed. According to Sun et al., TMAO induces inflammation by activating the ROS-TXNIP-NLRP3 inflammasome, thereby contributing to endothelial dysfunction in human umbilical vein endothelial cells [27]. Similarly, Yue et al. showed that TMAO promotes the release of the inflammatory cytokines IL-1β and IL-18 via activation of the NLRP3 inflammasome from foetal human colon cells in a time- and dose-dependent manner [28]. Moreover, injection of TMAO was shown to significantly increase inflammatory markers, including cyclooxygenase 2, IL-6, E-selectin, and ICAM1, through the MAPK and NF-κB signalling pathways, which then recruit leukocytes and induce vascular inflammation [29]. In these fine experiments in which treatments against TMAO were adopted, inflammatory damage was prevented. Taken together, the proinflammatory role of TMAO is established.
Plasma cholesterol, the key cellular membranes constituent and precursor of steroid hormones, vitamin D, and bile acids, is positively correlative with cardiovascular diseases. There are two main sources of cholesterol, with one-third being exogenous from daily dietary and the other two-third synthesized inside the body [30]. Confirmed with various models, microbial regulation is believed to be critically involved in cholesterol balance modulation [31]. To begin with, gut microbiome is reported to convert cholesterol into poorly absorbed coprostanol, reducing the risk of cardiovascular diseases [30, 32]. Further elucidation reveals that the presence of intestinal sterol metabolism A genes is responsible for such metabolism mediation [32]. Another key aspect the gut microbiota enrolled is bile acids metabolism. Bile acids deconjugation yields free bile acids as well as free glycine or taurine residues, which requires the participation of bile salt hydrolase enzymes (BSHs) [30]. The presence of BSHs was found within Clostridium, Bifidobacterium, Lactobacillus, and others. With higher degree of bile salts deconjugation, more free BAs were excreted into feces [30]. Primary bile acids refer to steroid molecules that result from the decomposition of cholesterol in the liver. Most of them are recycled back to the liver, while the rest enter the intestine, where they are converted into secondary bile acids by gut microbiota [33]. The most well-studied secondary bile acids are deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA), which often function through their receptors, including G protein-coupled BA receptor 1 (TGR5), farnesoid X receptor (FXR), and vitamin D receptor (VDR) [33]. When bound to the TGR5 receptor, secondary bile acids cause the activation of macrophages and then the production of inflammatory cytokines [34]. Interestingly, researchers found that low concentrations of secondary bile acids bring anti-inflammatory effects, while high concentrations would instead cause damage. For example, Wang et al. demonstrated that low-dose DCA mitigates the inflammatory response in birds [35].
Additionally, these products from commensal microbiota would trigger innate immune signalling, thereby communicating with the host. Microbial-associated molecular patterns (MAMPs) including LPS or peptidoglycan are recognized by receptors like Toll-like receptors (TLRs), NOD-like receptors (NLRs), and others [4]. The strong connection between TLRs and atherosclerosis was confirmed in genetic mice researches. In the TLR4-/- apoE-/- mice model fed with cholesterol-rich diet, the size of aortic plaque was significantly reduced [36]. Interestingly, deficiency of TLR2 in myeloid cells had no influence in the development of atherosclerosis, suggesting the role of endothelial TLR2 in atherogenesis [37]. Furthermore, the development of arterial thrombosis was relative to NOD2, TLR2, and TLR9 signalling in platelets as well as TLR2 and TLR4 pathways in endothelial cells [4].
2.2. Energy Metabolism and Homeostasis
Among the numerous risk factors contributing to CVD, abnormal immune regulation and metabolic disorders represent two major elements. Metabolic syndromes such as obesity, dyslipidosis, hyperglycaemia, and insulin resistance are closely related to the occurrence and development of CVD. In recent years, the link between gut microbiota, metabolism, and CVD has gained much attention. For instance, Den and his coworkers considered SCFAs to carry metabolic benefits for those with a high-fat diet through inhibition of peroxisome proliferator-activated receptor gamma (PPARγ), converting lipid synthesis to lipid oxidation [38]. Moreover, a fiber-rich diet upregulates the levels of SCFAs in the gut, which then promotes intestinal gluconeogenesis [39]. SCFAs accelerate the production of GLP-1 by binding to GPR41 and GPR43, therefore facilitating insulin secretion [39]. In contrast, TMAO aggravates triglyceride accumulation and lipogenesis in the livers of high-fat diet-fed mice [40]. Propionate was found to induce glycogenolysis and hyperglycaemia via the upregulation of glucagon and fatty acid-binding protein 4 (FABP4), thereby hindering the effects of insulin [41]. In mice with obesity, bile acid promotes GLP-1 secretion via the TGR5 pathway, thereby modulating blood sugar [42]. Notably, there is multiplicity in the associations between gut microbiota and their microbiome. For instance, TMAO could alter the bile acid profile and metabolism, thus contributing to liver steatosis and atherosclerosis [40, 43], whereas bile acid stimulates FMO3 expression via FXR, eventually resulting in TMAO production (Bennett et al., 2013). Moreover, butyrate was found to restore bile acid dysregulation and counteract hepatic inflammation [44].
To sum up, the gut microbiota communicates with the host through diverse manners. To begin with, SCFAs and secondary bile acids are two of the main products by gut microbiota. They play their immune-regulatory role either by directly affecting the proliferation of immune cells or by stimulating the production of cytokines. Moreover, SCFAs are involved in both lipid and sugar metabolism. Second, TMAO that primarily comes from L-carnitine and choline consumption participates in inflammatory modulation by promoting IL-18 and IL-1β release or activating MAPK/NF-κB signalling pathway, thus upregulating the levels of COX2, IL-6, and ICAM1. Moreover, MAMPs including LPS and peptidoglycan serve as another vital contributor in the development of atherosclerosis and arterial thrombosis, mainly through TLRs and NLRs (Figure 1).
Figure 1.
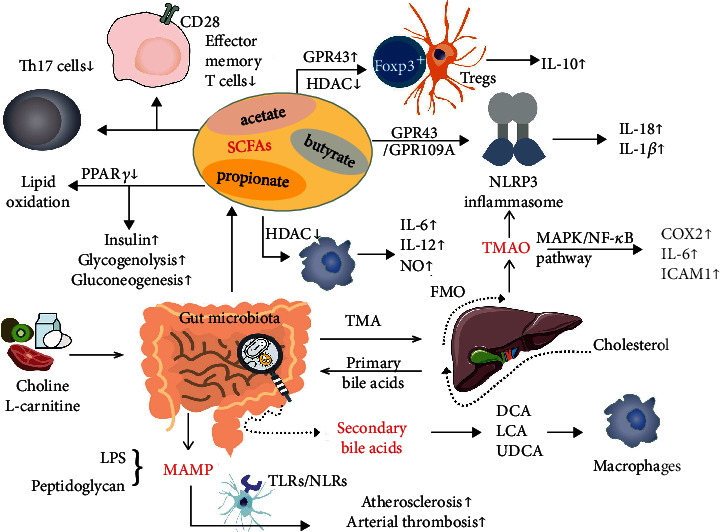
Mechanisms involved in gut microbiota-host communication. Short-chain fatty acids (SCFAs), mainly propionate, acetate, and butyrate, stimulate Fox3+ Tregs and macrophages via GPR43 activation and HDAC inhibition. Fox3+ Tregs subsequently produce the anti-inflammatory cytokine IL-10, while proinflammatory cytokines such as IL-6 and IL-12 are secreted by macrophages. Moreover, Th17 cells and effector memory T cells were downregulated by SCFAs. By suppressing PPARγ, SCFAs promote lipid oxidation. Although insulin production was enhanced by SCFAs, glycogenolysis and gluconeogenesis were both observed to occur even with SCFA treatment. L-carnitine and choline consumption contribute to the release of trimethylamine (TMA), which is then converted by FMO into trimethylamine N-oxide (TMAO). Both SCFAs and TMAO activate the NLRP3 inflammasome, leading to IL-18 and IL-1β release. Through the MAPK/NF-κB signalling pathway, TMAO increases the levels of COX2, IL-6, and ICAM1. Secondary bile acids such as deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) are produced in the intestine by gut microbiota and then participate in inflammatory modulation and blood sugar regulation.
2.3. Programmed Cell Death
Apart from the well-known immune and inflammation modulation properties of gut microbiota, accumulating evidence has revealed its potential in the determination of diverse manners of cell death (Figure 2).
Figure 2.
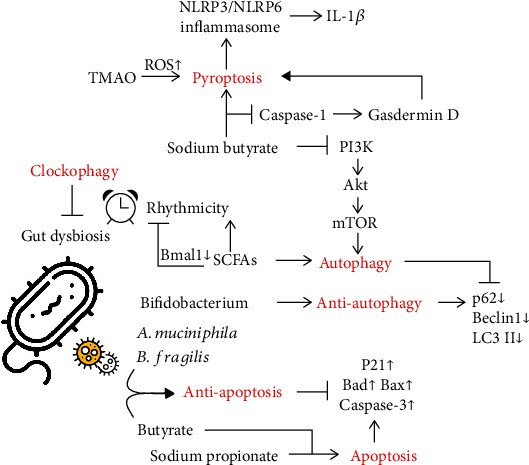
Manners of cell death induced by gut microbiota. A variety of gut flora have been demonstrated to be effective in regulating cell death. (a) Muciniphila and (b) fragilis were shown to counteract apoptosis. In contrast, sodium propionate has the ability to induce apoptosis. Interestingly, the effects of butyrate on apoptosis are controversial, manifesting elevated biomarkers such as P21, Bad, Bax, and caspase-3. In addition, SCFAs stimulate autophagy, while Bifidobacterium is autophagy-protective, with decreased expression of P62, Beclin1, and LC3II. Sodium butyrate promotes autophagy by inhibiting the PI3K/Akt/mTOR pathway. Additionally, it is involved in pyroptosis via regulation of the caspase-1/gasdermin D pathway. In addition, TMAO stimulates ROS activation and thus induces pyroptosis. Along with pyroptosis, the NLRP3/NLRP6 inflammasome and IL-1β are produced. Moreover, clockophagy can reverse gut dysbiosis. For instance, SCFAs are capable of controlling rhythmicity via clock genes such as Bmal1.
2.3.1. Apoptosis
Characterized by the formation of a distinctive apoptotic body, apoptosis is one of the most widely investigated programmed cell deaths. It is often observed in myocardial infarction, heart failure, and other vascular damage. Saito et al. found that Bacteroides fragilis (B. fragilis) is able to protect HT29 cells from apoptosis resulting from Shiga toxin [45]. Butyrate promotes vascular smooth muscle cell growth via proliferation arrest as well as apoptosis inhibition [46]. Notably, there are proapoptotic effects as well. Sodium propionate was reported to induce apoptosis in H1299 and H1703 lung cancer cells, as evidenced by increased protein expression of p21, Bad, and Bax as well as apoptosis markers, including cleaved PARP and cleaved caspase 3 [47]. According to Nie et al., Bifidobacterium (BIF) ameliorates TNF-α-induced cell apoptosis in Caco-2 cells [48]. Likewise, butyrate causes apoptosis and cell cycle arrest in kidney epithelial cells [49].
2.3.2. Autophagy
Nie et al. discovered that BIF ameliorates TNF-α-induced autophagy in Caco-2 cells by suppressing the level of p62 and inhibiting the expression of autophagy-related markers such as Beclin1 and LC3II [48]. According to their research, BIF may provide a therapeutic target aimed at the Kawasaki disease, which is highly related to acquired heart disease in children. Lannucci and his coworkers proved that SCFAs induce autophagy in hepatic cells by uncoupling protein 2 (UCP2) [50]. Accordingly, Qiao et al. demonstrated that sodium butyrate contributes to the reduction in α-synuclein both via the inhibition of the PI3K/Akt/mTOR autophagy pathway and enhancement of Atg5-mediated autophagy, manifested as elevated LC3II and reduced p62 expression [51].
2.3.3. Pyroptosis
As a type of proinflammatory cell death, pyroptosis is characterized by swollen cells, subcellular organelle damage, and the release of cytokines, including the NLRP3 inflammasome, NLRP6, an apoptosis-associated speck-like protein containing CARD (ASC), cysteinyl-aspartate-specific proteinase 1 (caspase-1), and gasdermin D. Data have shown that sodium butyrate is capable of breaking down the gingival epithelial barrier by inducing pyroptosis [52]. Similarly, TMAO promotes vascular endothelial cell pyroptosis via ROS production, thus resulting in the development of atherosclerosis [53]. However, Gu et al. proved the antipyroptosis effects of sodium butyrate on renal glomerular endothelial cells, protecting them from damage caused by high glucose [54]. From the perspective of the mechanism, the classic caspase-1-gasdermin D pathway and NF-κB/IκB-α signalling may both be involved [54]. Moreover, Cohen et al. confirmed that Vibrio proteolyticus (VPRH), a Gram-negative bacterium from the gut of a wood borer, induces pyroptosis by activating the NLRP3 inflammasome and caspase-1, thereby resulting in IL-1β secretion, suggesting that the NLRP3 inflammasome pyroptotic pathway can benefit the host during infection [55].
2.3.4. Ferroptosis
Induced by lipid reactive oxygen species accumulation, ferroptosis refers to another distinct kind of cell death mediated by mitochondria. Studies concerning whether gut microbiota are implicated in ferroptosis are rather rare. Until recently, Robert et al. proposed that supplementation of omega-3 polyunsaturated fatty acids (n-3 PUFAs) and butyrate may both facilitate mitochondrial Ca2+- and Gpx4-dependent ferroptosis [56]. Hopefully, this hypothesis may shed light on the link between gut microbiota and ferroptosis as well as accelerate related research.
2.3.5. Clockophagy
The circadian rhythm, namely, clockophagy, is controlled by a complex circadian clock gene network including the ARNTL, CLOCK, CRY2, and PER2 genes [57]. The interaction between circadian rhythms and diverse gut microbiota has been well studied, where the acute sleep-wake cycle shift alters the functional profiles of gut microbes. Together, the clock-microbial communities affect host homeostasis [58]. The circadian rhythm of SCFA production was observed by Segers et al. to cause rhythmicity in intestinal movement [59]. However, such effects were abolished by the deletion of Bmal1 [59]. Besides, Marques et al. found that in hypertensive mice, a high-fiber diet changes the composition of the gut microbiota and restores gut dysbiosis, which may be partially due to increased levels of clock genes in the heart and kidney [60]. Additionally, a negative correlation between the phylum Firmicutes and Bmal1 as well as a positive correlation between Bacteroidetes and Bmal1 was observed in mice [61].
3. Implications of Gut Microbiota in CVDs
To concisely describe the role of gut microbiota in cardiovascular disease, the positive or negative effects of gut microbiota on CVDs are listed in Table 1.
Table 1.
The exact role of different gut microbiota in CVDs.
| CVDs | Atherosclerosis | Myocardial infarction | Heart failure | Arrhythmia |
|---|---|---|---|---|
| Species | ||||
| Enterobacteriaceae | Negative | |||
| Ruminococcus gnavus | Negative | |||
| Eggerthella lenta | Negative | |||
| Roseburia intestinalis | Positive | |||
| Faecalibacterium cf. prausnitzii | Positive | |||
| Synergistetes phylum | Negative | |||
| Lachnospiraceae family | Negative | |||
| Spirochaetes phylum | Negative | |||
| Syntrophomonadaceae family | Negative | |||
| Tissierella and Soehngenia genera | Negative | |||
| Lactobacillus plantarum 299v | Positive | |||
| Faecalibacterium prausnitzii | Positive | |||
| Bacteroides fragilis | Positive | |||
| Ruminococcus | Negative | |||
| Streptococcus | Negative | |||
| Enterococcus | Negative | |||
| Faecalibacterium | Positive | |||
| Alistipes | Positive | |||
| Oscillibacter | Positive | |||
| Bilophila | Positive |
3.1. Hypertension
Hypertension (HTN) has been a key link in the occurrence and development of cardiovascular diseases. Although HTN is currently beyond cure, it is preventable and controllable. According to the mosaic theory advanced by Irvin Page, HTN is induced by multiple factors, including inheritance, diet, and environment [62]. HTN also has extensive impacts on various tissues and organs, such as endothelial cells, the kidneys, and brain. Moreover, in recent years, the value of gut microbiota in HTN has been widely investigated.
In the work conducted by Li et al., fecal transplantation was performed from hypertensive individuals to germ-free mice. Along with microbiota shift, blood pressure was also elevated in those mice, indicating the contributing role of gut microbiota in hypertension [63]. It has been demonstrated that butyrate-producing bacteria and butyrate levels are relatively low in patients with HTN, indicating that imbalanced host-microbiome cross talk is relevant to systolic blood pressure [64]. Accordingly, in mice pretreated with angiotensin II, supplementation with butyrate effectively lowered blood pressure [65]. Interestingly, the same team found that gut barrier dysfunction is another contributor to HTN, as evidenced by elevated levels of zonulin, a gut epithelial tight junction protein regulator [65]. However, the same metabolite may yield contradictory biological effects through different receptors. For instance, Jennifer et al. found that propionate may upregulate blood pressure via olfactory receptor 78 (Olfr78) while exerting hypotensive effects through activation of Gpr41 [66]. In-depth knowledge reveals that vascular inflammation and endothelial dysfunction are two key processes in the development of hypertension [67]. In mice fed with Western diet, endothelial dysfunction was associated with decreased proportion of Bifidobacterium spp., whereas antibiotic administration helped mitigate such vascular damage [68]. As compared with germ-free mice, the conventionally raised mice pretreated with Ang II presented with a higher level of IL-4 and IL-10, indicating a vascular inflammation-prone role of enteric flora [68]. In a meta-analysis of 8 studies, a higher circulating TMAO level was positively associated with hypertension risk, which was dose-dependent [69]. Liu and coworkers identified that administration of the Lactobacillus rhamnosus GG strain is an effective approach to prevent exacerbation of HTN, which is in part mediated by reducing TMAO levels [70]. However, it is worth noting that the application of TMAO alone would not alter blood pressure in normotensive rats but prolonged the hypertensive-prone effects of angiotensin II [71]. More recently, a novel mechanism different from inflammation or immunity regulation has been presented. In high salt-induced hypertensive mice, elevated blood pressure is closely related to increased levels of intestinal-derived corticosterone [72].
Taken together, these results established that the gut microbiota is involved in blood pressure regulation. However, the underlying mechanisms still await further validation.
3.2. Atherosclerosis and Arterial Thrombosis
Initially related to dyslipidaemia, abnormal accumulation of macrophages, and massive production of inflammatory cytokines, atherosclerosis is considered a chronic inflammatory disease that underlies end-stage CVDs such as myocardial infarction or heart failure. In recent years, people have started to consider gut microbiota potent regulators during the development of atherosclerotic lesions. Koren et al. first identified bacterial DNA in atherosclerotic plaques, and the amount of DNA was associated with the infiltration of leukocytes in the plaques [73]. Moreover, the altered composition of the gut microbiome was confirmed in a metagenome-wide association study encompassing 218 individuals with atherosclerosis and 187 healthy controls. Specifically, the abundances of Enterobacteriaceae, Ruminococcus gnavus, and Eggerthella lenta were significantly increased in those with atherosclerosis, whereas Roseburia intestinalis and Faecalibacterium cf. prausnitzii, both butyrate-yielding bacteria, were reduced [74]. The above findings strongly suggest correlations between gut microbiota and atherosclerosis.
With the use of atherosclerosis-prone germ-free mice and antibiotic treatments, the role of gut microbiota in atherosclerosis development was further elucidated (Table 2). First people suggested that bacterial or viral infection is necessary for the initiation of atherosclerosis. However, such hypothesis was overturned by Samuel and his colleagues' work [75]. Apolipoprotein (apo) E-/- murine model was often adopted for atherosclerosis research given the self-driven ability of atherosclerotic plaque formation. Samuel et al. compared the atherosclerosis lesion in germ-free apoE-/- animals with those raised in conventional environment, and they found no evident difference [75]. Alternatively, with the help of antibiotics to suppress gut microflora, choline-enhanced atherosclerosis in aorta was off-set along with reduced macrophage and scavenger receptor CD36 [76]. However, given the complexity of enteric flora, the pro- or antiatherosclerosis role of gut microbiota depends. Kasahara and his colleagues demonstrated that Roseburia intestinalis is capable of ameliorating atherosclerosis by shaping gene expression, enhancing fatty acid metabolism, and reducing the inflammatory response [77]. However, treatment with butyrate markedly mitigates the formation of atherosclerotic plaques via the upregulation of ABCA1 and subsequent cholesterol efflux [78]. In contrast, the production of TMAO by gut microbiota yields negative effects on atherosclerosis [79].
Table 2.
Researches of gut microbiota in CVDs.
| Diseases | Sample | Observations | Mechanism | Ref. |
|---|---|---|---|---|
| Hypertension | HTN patients | Decreased butyrate-producing bacteria and butyrate level | SCFA-dependent | [62] |
| Ang-II pretreated mice | Reduced BP after butyrate administration; increased zonulin level | SCFA-dependent; gut barrier dysfunction | [65] | |
| Mice | Increased BP after propionate treatment | Olfr78-dependent | [66] | |
| Mice | Decreased BP after propionate treatment | Gpr41-dependent | [66] | |
| Lactobacillus rhamnosus GG prevents HTN development | Reduced TMAO levels | [70] | ||
| Mice | High salt-induced HTN | Increased intestinal-derived corticosterone | [72] | |
| Atherosclerosis | Patients | Bacterial DNA observed in atherosclerotic plagues | / | [73] |
| Roseburia intestinalis ameliorates atherosclerosis | Alter gene expression, induce fatty acid metabolism, and reduce inflammation response | [77] | ||
| apoE-/- mice | Comparable atherosclerosis lesion in germ-free apoE-/- animals and their conventionally raised counterparts | / | [75] | |
| Choline-enhanced atherosclerosis in aorta was off-set by antibiotics | Reduced macrophage and scavenger receptor CD36 | [76] | ||
| apoE-/- mice with HFD | Butyrate mitigates atherosclerotic plaque formation | Upregulation of ABCA1 and subsequent cholesterol efflux | [78] | |
| Myocardial infarction | AMI rat model | Increased Synergistetes phylum, Lachnospiraceae family, Spirochaetes phylum, Syntrophomonadaceae family, and Tissierella and Soehngenia genera | In parallel with gut barrier impairment | [83] |
| STEMI patients | Over 12% plasma bacteria originated from the gut | Partially associated with an inflammatory response | [84] | |
| Patients presenting with chest pain | Predictive value of plasma TMAO levels for incident cardiovascular events | TMAO-related proinflammatory monocytes augment | [85] | |
| Mice | Improve cardiac repair and post-MI outcome though modulation of immune composition | Gut microbiota-derived SCFAs modulate immune composition | [86] | |
| Lactobacillus plantarum 299v improved ischemia tolerance and acute cardiac injury after MI | Reduce leptin level | [87] | ||
| Heart failure | Mice | Bacteroides fragilis reduces ventricular remodelling | Increased Foxp3+ Treg cells and anti-inflammatory cytokine | [92] |
| Depletion of SCFAs finally leads to HF | Intestinal barrier destruction, with endotoxin translocation | [93, 94] | ||
| Mice | TMAO alters cardiac muscle cells contractility | Promotion of calcium ions release | [95, 96] | |
| TMAO confers detrimental effects on adult cardiomyocytes | T-tubule network damage; Ca handling dysfunction | [97] | ||
| Mice | Pulmonary edema, cardiac enlargement, and decreased ejection fraction | TMAO-dependent | [98] | |
| Patients | TMAO increases susceptibility to HF | Induction of myocardial fibrosis | [99] | |
| Overload-induced HF mice | DMB ameliorates adverse cardiac structural remodelling | Downregulating TMAO levels | [100] | |
| Arrhythmia | Patients | Shared common features of gut microbiota dysbiosis | Alike ratio of Firmicutes and Bacteroidetes | [104, 105] |
| Patients | Thrombus formation; platelet hyperreactivity | Elevated TMAO level | [107] | |
| TMAO stimulates ischemia-induced VA | Release of proinflammatory markers such as IL-1β and TNF-α | [109] | ||
| Canine AF model | Gut microbes counteracts AF progression | TMAO production and CANS activation | [110] | |
| Mice | Reduced susceptibility to cardiac ventricular arrhythmias | SCFA-dependent | [22] |
Rupture of the atherosclerotic plaque would likely cause arterial thrombus elsewhere, resulting in detrimental consequences. For one, the LPS-TLR pathway is a m4ajor contributor in thrombosis formation. Both TLR2 and TLR4 were found expressed on endothelial cells and platelets. Activation of TLR2 and TLR4 pathway would facilitate the release of VWF and factor VIII expression, contributing to platelet-proinflammatory cell aggregation [80]. For another, gut microbiota metabolites take part in arterial thrombosis as well. Feces transplantation of TMAO-rich gut microbiota into germ-free mice would promote platelet function and arterial thrombosis [81]. Recently, another gut microbial metabolite, Phenylacetylglutamine (PAGln), was shown to induce hyperreactivity of platelet via adrenergic receptors [82].
3.3. Myocardial Infarction
The connection between intestinal flora and myocardial infarction (MI) has been supported by a growing body of literature. In a rat model of acute myocardial infarction (AMI), enrichment of the Synergistetes phylum, Lachnospiraceae family, Spirochaetes phylum, Syntrophomonadaceae family, and Tissierella and Soehngenia genera was observed compared with the sham group, which is in parallel with gut barrier impairment [83]. In patients with ST-elevation myocardial infarction (STEMI), systemic microbiome alteration was also observed. Over 12% of plasma bacteria were identified to originate from the gut after STEMI, which is partially associated with the inflammatory response [84]. Accordingly, reduced cardiac damage and decreased inflammation were noticed following the abrogation of bacterial translocation [84]. Of clinical value, plasma TMAO levels may be potential markers to predict the risks of incident cardiovascular events in patients presenting with chest pain [85]. Such potency may in part be explained by TMAO-related proinflammatory monocyte augmentation [85]. Moreover, Tang et al. demonstrated that gut microbiota-derived SCFAs would benefit cardiac repair and improve post-MI outcome though modulation of immune composition [86]. With the administration of the probiotic Lactobacillus plantarum 299v, the leptin level in blood was reduced, leading to enhancement of ischemic tolerance in the myocardium and alleviation of acute cardiac injury after MI [87].
3.4. Heart Failure
As an irreversible end-stage disease, heart failure (HF) is characterized by oedema and dyspnoea, with a five-year mortality rate of over 50% [88]. At present, a growing body of research has confirmed the “gut hypothesis of heart failure” [89, 90]. That is, decreased cardiac output in HF leads to intestinal mucosa barrier damage and dysbacteriosis, with elevated levels of pathogenic bacteria such as Candida [91] and reduced levels of anti-inflammatory bacteria such as Faecalibacterium prausnitzii [3]. Reciprocally, intestinal flora promotes HF development by participating in mucosal immunity modulation [3]. Segmented filamentous bacteria can stimulate the secretion of IL-6 and IL-23 and then promote the differentiation of Th17 cells. Bacteroides fragilis increases the abundance of Foxp3+ Treg cells and induces the secretion of anti-inflammatory cytokines, which have been found to reduce ventricular remodelling in MI mice [92].
Not surprisingly, metabolites of intestinal flora are also important for HF. Although studies concerning SCFAs and HF are limited, it has been proven that SCFAs are beneficial for the intestinal mucosa [3]. The depletion of SCFAs would result in intestinal barrier destruction, which then facilitates the translocation of endotoxin into blood circulation and finally leads to HF [93, 94].
However, the level of TMAO has long been recognized as a risk factor. Savi et al. found that TMAO promotes the release of calcium ions in cardiac muscle cells of healthy mice and thus alters their contractility [95, 96]. Recently, the in-depth work carried out by Jin et al. showed that TMAO confers detrimental effects on adult cardiomyocytes by inducing T-tubule network damage and Ca handling dysfunction [97]. When TMAO was administered to HF mice, Organ et al. found that mouse cardiac function deteriorated significantly, characterized by pulmonary oedema, cardiac enlargement, and decreased ejection fraction [98]. Schuett et al. proved that TMAO could enhance patient susceptibility to HF by increasing myocardial fibrosis [99]. Likewise, Wang and his team proved that 3,3-dimethyl-1-butanol (DMB) ameliorates adverse cardiac structural remodelling in overload-induced HF mice by downregulating TMAO levels [100]. Given the critical role of TMAO in HF, it may serve as a potential therapeutic target.
3.5. Arrhythmia
Arrhythmia, including atrial fibrillation (AF), ventricular arrhythmia (VA), and atrioventricular block, is emerging as intractable CVD that contributes to heart failure or sudden cardiac death. Up-to-date studies have shown that anticancer therapies may induce cardiotoxicities, such as corrected QT interval prolongation and arrhythmia [101]. Additionally, Vahdatpour et al. found that atrial arrhythmia can be secondary to chronic lung disease-associated pulmonary hypertension [102]. Due to its prevalence and accompanying adverse events, investigation about arrhythmia has deepened, and we are now looking at the implications between gut microbiota and arrhythmia.
Zuo et al. previously identified variable metabolic patterns as well as imbalanced gut microbiota composition in patients with AF in which Ruminococcus, Streptococcus, and Enterococcus significantly increased while Faecalibacterium, Alistipes, Oscillibacter, and Bilophila obviously reduced [103]. Later, they found that patients with persistent AF (psAF) shared a great proportion of common features of gut microbiota dysbiosis [104]. In their latest study, the fecal microbiota from patients with psAF and those with paroxysmal AF were investigated, verifying a similar pattern of gut microbiota, with similar ratios of Firmicutes to Bacteroidetes [105].
Svingen et al. conducted a study in thousands of patients with suspected stable angina and proposed that plasma TMAO levels are definitely related to AF [106]. It is well known that thrombi can easily take place in the left atrial appendage of patients with AF, which then leads to embolism. Gong et al. found that in patients with AF, elevated TMAO levels are related to thrombus formation, manifested as platelet hyperreactivity [107]. It has been confirmed that the cardiac autonomic nervous system (CANS) can regulate the pathophysiology of AF or VA [108]. Meng et al. first proposed that preserving dysbacteriosis or modulating metabolites such as TMAO may be a target to treat arrhythmia due to the ability of TMAO to stimulate CANS and deteriorate ischaemia-induced VA by releasing proinflammatory markers such as IL-1β and TNF-α [109]. Similarly, according to the experiment of Yu et al., gut microbes have the ability to counteract AF progression by producing TMAO and can thus activate CANS in a rapid atrial pacing-induced canine AF model [110]. Likewise, in a propionate-treated hypertensive mouse model, the susceptibility to cardiac ventricular arrhythmias was significantly reduced, indicating possible links between SCFAs and arrhythmia development [22]. Although the connection between gut microbiota and arrhythmia has been established, the precise underlying mechanisms still await further investigation (Table 2).
4. Microorganism-Targeted Therapies
4.1. Fecal Microbiota Transplantation
As an effective approach to directly introduce intestinal flora, fecal microbiota transplantation (FMT) has gained much attention. The therapeutic value of FMT in gastrointestinal diseases, neurological and psychiatric disorders, and immunology regulation has been extensively examined [22, 111, 112]. However, studies concerning its application in CVDs are limited. Although oral supplementation of resveratrol has been proven to improve glucose homeostasis by altering gut microbiota, in the work of Kim and his colleagues [113], FMT from resveratrol-fed mice to obese mice was found to yield better results than oral administration of resveratrol alone, indicating that FMT is more straightforward and direct. Moreover, Hu et al. showed that FMT could abolish the increased proportion of Firmicutes/Bacteroidetes, diminish inflammatory infiltration in cardiomyocytes, and thereby attenuate myocarditis in mice [5]. However, in a double-blind trial involving 20 patients, the composition of intestinal flora was altered in the recipients after FMT from vegetarians, whereas the vasculitis indicators presented no improvement [114]. There are also disadvantages to FMT. For instance, endotoxins are transferred along with the donor microbiome. How to weigh the pros and cons of actual practice is still an issue to be addressed. To guarantee the reliable and smooth application of FMT in clinical use, the establishment of stool banks is on its way.
4.2. Probiotic Administration
Among the numerous bacteria residing in the host intestine, some are beneficial. An extra boost of these bacteria would probably bring positive results, thus leading to the application of probiotics. In a meta-analysis involving 846 individuals with hypertension, mild reductions in blood pressure, body mass index (BMI), and blood glucose levels were observed after probiotic administration, supporting the beneficial role of probiotics in blood pressure control [115]. Similarly, in other studies with spontaneously hypertensive rats, the probiotics Bifidobacterium breve and Lactobacillus fermentum were found to elicit antihypertensive effects by restoring gut microbiota balance and preventing endothelial dysfunction [116], whereas long-term supplementation with kefir ameliorated high blood pressure via improvement in intestinal integrity [117]. Moreover, in apoE-/- mice fed with HFD, supplementation with Lactobacillus rhamnosus GR-1 markedly reduced atherosclerotic lesion size by alleviating oxidative stress and inflammation [118]. Likewise, Lactobacillus plantarum ZDY04 has been shown to downregulate serum TMAO levels, which is a critical factor contributing to atherosclerosis development [119].
4.3. Herbal Medicine
Traditional Chinese medicine (TCM), which mainly utilizes herbs and their extracts, has recently been demonstrated to treat CVDs via intestinal microbial modulation. Ou et al. reviewed and summarized the mechanisms of gut flora in TCM's theory of “stasis of intermingled phlegm and blood stasis” [120]. For example, the fact that TMAO promotes thrombosis might be one of the major causes of CVDs [121]. Anlu et al. showed that berberine originating from the Chinese herb Coptis chinensis has the ability to regulate the “microbiota-metabolism-immunity” axis [122]. Moreover, resveratrol derived from Polygonum cuspidatum was demonstrated to attenuate TMAO-induced atherosclerosis in apoE-/- mice by remodelling microbiota as well as decreasing TMAO and BA levels [123]. In addition, Ghosh et al. found that curcumin, a phytochemical component of Curcuma longa, attenuates atherosclerosis in LDLR-/- mice by regulating intestinal barrier function [124]. Anwar et al. showed that Trigonelline, which is purified from the seeds of Trigonella foenum-graecum, can inhibit the growth of Citrobacter freundii and subsequently decrease the production of TMAO in mice [125].
5. Conclusion
Evidence from a compilation of studies of animals and humans indicates that the implications of gut microbiota and their metabolites in CVDs are well established. With high-throughput technologies, verification of the intestinal flora composition and in-depth mechanistic exploration are accessible. However, the links between gut microbiota and disease development are so complex that they involve immune regulation, the inflammatory response, gut barrier integrity, metabolic homeostasis, etc. Further investigations into the specific mechanisms are needed, which then share the possibility of being transferred into clinical practice.
Acknowledgments
This research was funded by the National Key Research and Development Program of China (2018YFA0108700), the NSFC Projects of International Cooperation and Exchanges (81720102004), the National Natural Science Foundation of China (81974019, 81970248), and the National Training Program of Innovation and Entrepreneurship for Undergraduates (2020105330125). AJE edited the manuscript for grammar, punctuation and spelling.
Conflicts of Interest
The authors declare no conflicts of interest, financial or otherwise.
Authors' Contributions
MZ conceived of and designed the study and revised the manuscript for important intellectual content; XZ performed the literature search. YC generated the figures and tables; MN performed the background research. MZ and PZ edited the manuscript. WZ and YC drafted the manuscript. All authors have read and approved the content of the manuscript. Wenyi Zhou, Yiyu Cheng, and Ping Zhu contributed equally to this work.
References
- 1.Brown J. M., Hazen S. L. Microbial modulation of cardiovascular disease. Nature Reviews Microbiology. 2018;16(3):171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang W. H., Kitai T., Hazen S. L. Gut microbiota in cardiovascular health and disease. Circulation Research. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang W., Li D. Y., Hazen S. L. Dietary metabolism, the gut microbiome, and heart failure. Nature Reviews Cardiology. 2019;16(3):137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiouptsi K., Reinhardt C. Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. British journal of pharmacology. 2018;175(24):4439–4449. doi: 10.1111/bph.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim T. T., Parajuli N., Sung M. M., et al. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. American Journal of Physiology-Endocrinology and Metabolism. 2018;315(4):E511–E519. doi: 10.1152/ajpendo.00471.2017. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Zhang T., Wang Y., et al. Baicalin ameliorates neuropathology in repeated cerebral ischemia-reperfusion injury model mice by remodeling the gut microbiota. Aging (Albany NY) 2020;12(4):3791–3806. doi: 10.18632/aging.102846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan S., Nie Y., Zhang Y., Huang C., Zhu X. Gut microbial dysbiosis is associated with profibrotic factors in liver fibrosis mice. Frontiers in cellular and infection microbiology. 2020;10:p. 18. doi: 10.3389/fcimb.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin M., Qian Z., Yin J., Xu W., Zhou X. The role of intestinal microbiota in cardiovascular disease. Journal of Cellular and Molecular Medicine. 2019;23(4):2343–2350. doi: 10.1111/jcmm.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques F. Z., Mackay C. R., Kaye D. M. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nature Reviews Cardiology. 2018;15(1):20–32. doi: 10.1038/nrcardio.2017.120. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Faden H. S., Zhu L. The response of the gut microbiota to dietary changes in the first two years of life. Frontiers in Pharmacology. 2020;11:p. 334. doi: 10.3389/fphar.2020.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fava F., Rizzetto L., Tuohy K. M. Gut microbiota and health: connecting actors across the metabolic system. Proceedings of the Nutrition Society. 2019;78(2):177–188. doi: 10.1017/S0029665118002719. [DOI] [PubMed] [Google Scholar]
- 12.Tang W. H. W., Bäckhed F., Landmesser U., Hazen S. L. Intestinal microbiota in cardiovascular health and disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2019;73(16):2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckburg P. B., Bik E. M., Bernstein C. N., et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill S. R., Pop M., DeBoy R. T., et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koliada A., Syzenko G., Moseiko V., et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiology. 2017;17(1):1–6. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indiani C., Rizzardi K. F., Castelo P. M., Ferraz L. F. C., Darrieux M., Parisotto T. M. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Childhood Obesity. 2018;14(8):501–509. doi: 10.1089/chi.2018.0040. [DOI] [PubMed] [Google Scholar]
- 17.Pascale A., Marchesi N., Govoni S., Coppola A., Gazzaruso C. The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Current Opinion in Pharmacology. 2019;49:1–5. doi: 10.1016/j.coph.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Chang P. V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences. 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macia L., Tan J., Vieira A. T., et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature communications. 2015;6(1):p. 6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 20.Tan J., McKenzie C., Potamitis M., Thorburn A. N., Mackay C. R., Macia L. The role of short-chain fatty acids in health and disease. Advances in Immunology. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 21.Smith P. M., Howitt M. R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartolomaeus H., Balogh A., Yakoub M., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim M. H., Kang S. G., Park J. H., Yanagisawa M., Kim C. H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406.e10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Schiattarella G. G., Sannino A., Toscano E., et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. European Heart Journal. 2017;38(39):2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang R., Ge X., Han L., et al. Gut microbe-generated metabolite trimethylamineN‐oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obesity Reviews. 2019;20(6):883–894. doi: 10.1111/obr.12843. [DOI] [PubMed] [Google Scholar]
- 26.Manor O., Zubair N., Conomos M. P., et al. A multi-omic association study of trimethylamine N-oxide. Cell Reports. 2018;24(4):935–946. doi: 10.1016/j.celrep.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 27.Sun X., Jiao X., Ma Y., et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochemical and Biophysical Research Communications. 2016;481(1-2):63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Yue C., Yang X., Li J., et al. Trimethylamine N-oxide prime NLRP3 inflammasome via inhibiting ATG16L1-induced autophagy in colonic epithelial cells. Biochemical and Biophysical Research Communications. 2017;490(2):541–551. doi: 10.1016/j.bbrc.2017.06.075. [DOI] [PubMed] [Google Scholar]
- 29.Seldin M. M., Meng Y., Qi H., et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear Factor-κB. Journal of the American Heart Association. 2016;5(2) doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriaa A., Bourgin M., Potiron A., et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. Journal of Lipid Research. 2019;60(2):323–332. doi: 10.1194/jlr.R088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villette R., Kc P., Beliard S., et al. Unraveling host-gut microbiota dialogue and its impact on cholesterol levels. Frontiers in Pharmacology. 2020;11 doi: 10.3389/fphar.2020.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny D. J., Plichta D. R., Shungin D., et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host & Microbe. 2020;28(2):245–257.e6. doi: 10.1016/j.chom.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridlon J. M., Harris S. C., Bhowmik S., Kang D. J., Hylemon P. B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7(1):22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyce S. A., Gahan C. G. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Digestive Diseases. 2017;35(3):169–177. doi: 10.1159/000450907. [DOI] [PubMed] [Google Scholar]
- 35.Wang H., Latorre J. D., Bansal M., et al. Microbial metabolite deoxycholic acid controls Clostridium perfringens-induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Scientific Reports. 2019;9(1, article 14541) doi: 10.1038/s41598-019-51104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen K. S., Wong M. H., Shah P. K., et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proceedings of the National Academy of Sciences. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullick A. E., Tobias P. S., Curtiss L. K. Modulation of atherosclerosis in mice by Toll-like receptor 2. The Journal of Clinical Investigation. 2005;115(11):3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Besten G., Bleeker A., Gerding A., et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-Dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez M., Canfora E. E., Jocken J., Blaak E. E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11(8):p. 1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan X., Liu Y., Long J., et al. Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Molecular Nutrition & Food Research. 2019;63(17, article e1900257) doi: 10.1002/mnfr.201900257. [DOI] [PubMed] [Google Scholar]
- 41.Tirosh A., Calay E. S., Tuncman G., et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Science Translational Medicine. 2019;11(489):p. eaav0120. doi: 10.1126/scitranslmed.aav0120. [DOI] [PubMed] [Google Scholar]
- 42.Bronden A., Knop F. K. Gluco-metabolic effects of pharmacotherapy-induced modulation of bile acid physiology. The Journal of Clinical Endocrinology & Metabolism. 2020;105(1):362–373. doi: 10.1210/clinem/dgz025. [DOI] [PubMed] [Google Scholar]
- 43.Ding L., Chang M., Guo Y., et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids in Health and Disease. 2018;17(1):p. 286. doi: 10.1186/s12944-018-0939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng L., Jena P. K., Hu Y., et al. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. The Journal of pathology. 2017;243(4):431–441. doi: 10.1002/path.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito K., Suzuki R., Koyanagi Y., Isogai H., Yoneyama H., Isogai E. Inhibition of enterohemorrhagic Escherichia coli O157:H7 infection in a gnotobiotic mouse model with pre-colonization by Bacteroides strains. Biomedical Reports. 2019;10(3):175–182. doi: 10.3892/br.2019.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathew O. P., Ranganna K., Mathew J., et al. Cellular effects of butyrate on vascular smooth muscle cells are mediated through disparate actions on dual targets, histone deacetylase (HDAC) Activity and PI3K/Akt Signaling Network. International Journal of Molecular Sciences. 2019;20(12):p. 2902. doi: 10.3390/ijms20122902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim K., Kwon O., Ryu T. Y., et al. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Molecular Medicine Reports. 2019;20(2):1569–1574. doi: 10.3892/mmr.2019.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nie N., Bai C., Song S., Zhang Y., Wang B., Li Z. Bifidobacterium plays a protective role in TNF-α-induced inflammatory response in Caco-2 cell through NF-κB and p38MAPK pathways. Molecular and Cellular Biochemistry. 2020;464(1-2):83–91. doi: 10.1007/s11010-019-03651-3. [DOI] [PubMed] [Google Scholar]
- 49.Li C. J., Elsasser T. H. Butyrate-induced apoptosis and cell cycle arrest in bovine kidney epithelial cells: involvement of caspase and proteasome pathways1. Journal of Animal Science. 2005;83(1):89–97. doi: 10.2527/2005.83189x. [DOI] [PubMed] [Google Scholar]
- 50.Iannucci L. F., Sun J., Singh B. K., et al. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochemical and biophysical research communications. 2016;480(3):461–467. doi: 10.1016/j.bbrc.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 51.Qiao C. M., Sun M. F., Jia X. B., et al. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Experimental Cell Research. 2020;387(1, article 111772):p. 111772. doi: 10.1016/j.yexcr.2019.111772. [DOI] [PubMed] [Google Scholar]
- 52.Liu J., Wang Y., Meng H., et al. Butyrate rather than LPS subverts gingival epithelial homeostasis by downregulation of intercellular junctions and triggering pyroptosis. Journal of Clinical Periodontology. 2019;46(9):894–907. doi: 10.1111/jcpe.13162. [DOI] [PubMed] [Google Scholar]
- 53.Wu P., Chen J., Chen J., et al. Trimethylamine N-oxide promotes apoE(-/-) mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. Journal of Cellular Physiology. 2020;235(10):6582–6591. doi: 10.1002/jcp.29518. [DOI] [PubMed] [Google Scholar]
- 54.Gu J., Huang W., Zhang W., et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. International Immunopharmacology. 2019;75, article 105832 doi: 10.1016/j.intimp.2019.105832. [DOI] [PubMed] [Google Scholar]
- 55.Cohen H., Baram N., Edry-Botzer L., Munitz A., Salomon D., Gerlic M. Vibriopore-forming leukocidin activates pyroptotic cell death via the NLRP3 inflammasome. Emerging Microbes & Infections. 2020;9(1):278–290. doi: 10.1080/22221751.2020.1720526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapkin R. S., Navarro S. L., Hullar M., Lampe J. W. Diet and gut microbes act coordinately to enhance programmed cell death and reduce colorectal cancer risk. Digestive Diseases and Sciences. 2020;65(3):840–851. doi: 10.1007/s10620-020-06106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lavtar P., Rudolf G., Maver A., et al. Association of circadian rhythm genes ARNTL/BMAL1 and CLOCK with multiple sclerosis. PloS One. 2018;13(1, article e0190601) doi: 10.1371/journal.pone.0190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Wei Z. Y., Chen J., et al. Acute sleep-wake cycle shift results in community alteration of human gut microbiome. Msphere. 2020;5(1) doi: 10.1128/mSphere.00914-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segers A., Desmet L., Thijs T., Verbeke K., Tack J., Depoortere I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiologica. 2019;225(3, article e13193) doi: 10.1111/apha.13193. [DOI] [PubMed] [Google Scholar]
- 60.Marques F. Z., Nelson E., Chu P. Y., et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 61.Wu X., Chen L., Zeb F., et al. Clock-Bmal1 mediates MMP9 induction in acrolein-promoted atherosclerosis associated with gut microbiota regulation. Environmental Pollution. 2019;252(Part B):1455–1463. doi: 10.1016/j.envpol.2019.06.042. [DOI] [PubMed] [Google Scholar]
- 62.Page I. H. The mosaic theory of arterial hypertension--its interpretation. Perspectives in Biology and Medicine. 1967;10(3):325–333. doi: 10.1353/pbm.1967.0031. [DOI] [PubMed] [Google Scholar]
- 63.Li J., Zhao F., Wang Y., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):p. 14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huart J., Leenders J., Taminiau B., et al. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension. 2019;74(4):1005–1013. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 65.Kim S., Goel R., Kumar A., et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clinical Science. 2018;132(6):701–718. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pluznick J. L., Protzko R. J., Gevorgyan H., et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proceedings of the National Academy of Sciences. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrows I. R., Ramezani A., Raj D. S. Inflammation, immunity, and oxidative stress in hypertension-partners in crime? Advances in Chronic Kidney Disease. 2019;26(2):122–130. doi: 10.1053/j.ackd.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Man A. W. C., Li H., Xia N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients. 2020;12(1):p. 119. doi: 10.3390/nu12010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ge X., Zheng L., Zhuang R., et al. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Advances in Nutrition. 2020;11(1):66–76. doi: 10.1093/advances/nmz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J., Li T., Wu H., et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea-induced hypertension in a high salt diet via regulating TMAO level and CD4(+) T cell induced-type I inflammation. Biomedicine & Pharmacotherapy. 2019;112, article 108580 doi: 10.1016/j.biopha.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 71.Ufnal M., Jazwiec R., Dadlez M., Drapala A., Sikora M., Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Canadian Journal of Cardiology. 2014;30(12):1700–1705. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 72.Yan X., Jin J., Su X., et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circulation Research. 2020;126(7):839–853. doi: 10.1161/CIRCRESAHA.119.316394. [DOI] [PubMed] [Google Scholar]
- 73.Koren O., Spor A., Felin J., et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences. 2011;108(Supplement_1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jie Z., Xia H., Zhong S. L., et al. The gut microbiome in atherosclerotic cardiovascular disease. Nature Communications. 2017;8(1):p. 845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright S. D., Burton C., Hernandez M., et al. Infectious agents are not necessary for murine atherogenesis. The Journal of Experimental Medicine. 2000;191(8):1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Z., Klipfell E., Bennett B. J., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kasahara K., Krautkramer K. A., Org E., et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nature Microbiology. 2018;3(12):1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du Y., Li X., Su C., et al. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. British Journal of Pharmacology. 2020;177(8):1754–1772. doi: 10.1111/bph.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He Z., Hao W., Kwek E., et al. Fish oil is more potent than flaxseed oil in modulating gut microbiota and reducing trimethylamine-N-oxide-exacerbated atherogenesis. Journal of Agricultural and Food Chemistry. 2019;67(49):13635–13647. doi: 10.1021/acs.jafc.9b06753. [DOI] [PubMed] [Google Scholar]
- 80.Hasan R. A., Koh A. Y., Zia A. The gut microbiome and thromboembolism. Thrombosis Research. 2020;189:77–87. doi: 10.1016/j.thromres.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huynh K. Novel gut microbiota-derived metabolite promotes platelet thrombosis via adrenergic receptor signalling. Nature Reviews Cardiology. 2020;17(5):p. 265. doi: 10.1038/s41569-020-0367-y. [DOI] [PubMed] [Google Scholar]
- 82.Lassiger-Herfurth A., Pontarollo G., Grill A., Reinhardt C. The gut microbiota in cardiovascular disease and arterial thrombosis. Microorganisms. 2019;7(12):p. 691. doi: 10.3390/microorganisms7120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Z. X., Li S. F., Chen H., et al. The changes of gut microbiota after acute myocardial infarction in rats. PLoS One. 2017;12(7, article e0180717) doi: 10.1371/journal.pone.0180717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X., Li J., Guo J., et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6(1):p. 66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haghikia A., Li X. S., Liman T. G., et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(9):2225–2235. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang T., Chen H. C., Chen C. Y., et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139(5):647–659. doi: 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 87.Lam V., Su J., Koprowski S., et al. Intestinal microbiota determine severity of myocardial infarction in rats. The FASEB journal. 2011;26(4):1727–1735. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponikowski P., Voors A. A., Anker S. D., et al. 016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 89.Heianza Y., Ma W., Manson J. E., Rexrode K. M., Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. Journal of the American Heart Association. 2017;6(7) doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peng J., Xiao X., Hu M., Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sciences. 2018;214:153–157. doi: 10.1016/j.lfs.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 91.Pasini E., Aquilani R., Testa C., et al. Pathogenic gut flora in patients with chronic heart failure. JACC: Heart Failure. 2016;4(3):220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Jia Q., Li H., Zhou H., et al. Role and effective therapeutic target of gut microbiota in heart failure. Cardiovascular Therapeutics. 2019;2019:10. doi: 10.1155/2019/5164298.5164298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang T. T., Yuan J., Zhu Z. F., et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Research in Cardiology. 2012;107(1):232–232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 94.Nagatomo Y., Tang W. H. Intersections between microbiome and heart failure: revisiting the gut hypothesis. Journal of Cardiac Failure. 2015;21(12):973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zabell A., Tang W. H. Targeting the microbiome in heart failure. Current Treatment Options in Cardiovascular Medicine. 2017;19(4):p. 27. doi: 10.1007/s11936-017-0528-4. [DOI] [PubMed] [Google Scholar]
- 96.Savi M., Bocchi L., Bresciani L., et al. Trimethylamine-N-oxide (TMAO)-induced impairment of cardiomyocyte function and the protective role of urolithin B-glucuronide. Molecules. 2018;23(3):p. 549. doi: 10.3390/molecules23030549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin B., Ji F., Zuo A., et al. Destructive role of TMAO in T-tubule and excitation-contraction coupling in the adult cardiomyocytes. International Heart Journal. 2020;61(2):355–363. doi: 10.1536/ihj.19-372. [DOI] [PubMed] [Google Scholar]
- 98.Organ C. L., Otsuka H., Bhushan S., et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circulation: Heart Failure. 2016;9(1, article e002314) doi: 10.1161/CIRCHEARTFAILURE.115.002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schuett K., Kleber M. E., Scharnagl H., et al. Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. Journal of the American College of Cardiology. 2017;70(25):3202–3204. doi: 10.1016/j.jacc.2017.10.064. [DOI] [PubMed] [Google Scholar]
- 100.Wang G., Kong B., Shuai W., Fu H., Jiang X., Huang H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. The Journal of Nutritional Biochemistry. 2020;78:p. 108341. doi: 10.1016/j.jnutbio.2020.108341. [DOI] [PubMed] [Google Scholar]
- 101.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nature Reviews Cardiology. 2020;17(8):474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vahdatpour C. A., Luebbert J. J., Palevsky H. I. Atrial arrhythmias in chronic lung disease-associated pulmonary hypertension. Pulmonary Circulation. 2020;10(1, article 204589402091068) doi: 10.1177/2045894020910685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zuo K., Li J., Li K., et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. GigaScience. 2019;8(6) doi: 10.1093/gigascience/giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zuo K., Li J., Wang P., et al. Duration of persistent atrial fibrillation is associated with alterations in human gut microbiota and metabolic phenotypes. Msystems. 2019;4(6) doi: 10.1128/mSystems.00422-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuo K., Yin X., Li K., et al. Different types of atrial fibrillation share patterns of gut microbiota dysbiosis. Msphere. 2020;5(2) doi: 10.1128/mSphere.00071-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Svingen G., Zuo H., Ueland P. M., et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. International Journal of Cardiology. 2018;267:100–106. doi: 10.1016/j.ijcard.2018.04.128. [DOI] [PubMed] [Google Scholar]
- 107.Gong D., Zhang L., Zhang Y., Wang F., Zhao Z., Zhou X. Gut microbial metabolite trimethylamine N-oxide is related to thrombus formation in atrial fibrillation patients. The American Journal of the Medical Sciences. 2019;358(6):422–428. doi: 10.1016/j.amjms.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Shivkumar K., Ajijola O. A., Anand I., et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. The Journal of Physiology. 2016;594(14):3911–3954. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meng G., Zhou X., Wang M., et al. Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. EBioMedicine. 2019;44:656–664. doi: 10.1016/j.ebiom.2019.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu L., Meng G., Huang B., et al. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. International Journal of Cardiology. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 111.Antushevich H. Fecal microbiota transplantation in disease therapy. Clinica Chimica Acta. 2020;503:90–98. doi: 10.1016/j.cca.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F., Zhang T., Zhu H., Borody T. J. Evolution of fecal microbiota transplantation in methodology and ethical issues. Current Opinion in Pharmacology. 2019;49:11–16. doi: 10.1016/j.coph.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 113.de Groot P. F., Frissen M. N., de Clercq N. C., Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes. 2017;8(3):253–267. doi: 10.1080/19490976.2017.1293224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu X. F., Zhang W. Y., Wen Q., et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacological Research. 2019;139:412–421. doi: 10.1016/j.phrs.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 115.Smits L. P., Kootte R. S., Levin E., et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. Journal of the American Heart Association. 2018;7(7) doi: 10.1161/JAHA.117.008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chi C., Li C., Wu D., et al. Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Current Hypertension Reports. 2020;22(5) doi: 10.1007/s11906-020-01041-5. [DOI] [PubMed] [Google Scholar]
- 117.Robles-Vera I., Toral M., la Visitación N., et al. Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: role of short-chain fatty acids. Molecular Nutrition & Food Research. 2020;64(6, article 1900616) doi: 10.1002/mnfr.201900616. [DOI] [PubMed] [Google Scholar]
- 118.de Almeida S. M., Mowry F. E., Peaden S. C., Andrade T. U., Biancardi V. C. Kefir ameliorates hypertension via gut-brain mechanisms in spontaneously hypertensive rats. The Journal of Nutritional Biochemistry. 2020;77 doi: 10.1016/j.jnutbio.2019.10831. [DOI] [PubMed] [Google Scholar]
- 119.Fang Y., Chen H. Q., Zhang X., et al. Probiotic administration of lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE-/- mice fed with a high-fat diet. European Review for Medical and Pharmacological Sciences. 2019;23(8):3533–3541. doi: 10.26355/eurrev_201904_17722. [DOI] [PubMed] [Google Scholar]
- 120.Qiu L., Tao X., Xiong H., Yu J., Wei H. Lactobacillus plantarumZDY04 exhibits a strain-specific property of lowering TMAOviathe modulation of gut microbiota in mice. Food & Function. 2018;9(8):4299–4309. doi: 10.1039/C8FO00349A. [DOI] [PubMed] [Google Scholar]
- 121.Ou Y., Zhang C., Yao M., Wang L. Gut Flora: Novel therapeutic target of Chinese medicine for the treatment of cardiovascular diseases. Evidence-Based Complementary and Alternative Medicine. 2019;2019:7. doi: 10.1155/2019/3719596.3719596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anlu W., Dongcheng C., He Z., et al. Using herbal medicine to target the "microbiota-metabolism-immunity" axis as possible therapy for cardiovascular disease. Pharmacological Research. 2019;142:205–222. doi: 10.1016/j.phrs.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 123.Chen M. L., Yi L., Zhang Y., et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7(2):e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghosh S. S., Bie J., Wang J., Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice--role of intestinal permeability and macrophage activation. PloS One. 2014;9(9, article e108577) doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anwar S., Bhandari U., Panda B. P., Dubey K., Khan W., Ahmad S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. Journal of Pharmaceutical and Biomedical Analysis. 2018;159:100–112. doi: 10.1016/j.jpba.2018.06.027. [DOI] [PubMed] [Google Scholar]


