Abstract
BACKGROUND:
Isolated limb infusion with melphalan is a well-tolerated treatment for patients with in-transit extremity melanoma with an approximately 30% complete response (CR) rate. ADH-1 is a cyclic pentapeptide that disrupts N-cadherin adhesion complexes and when given systemically in a preclinical model of regional melphalan therapy demonstrated synergistic antitumor activity. A phase 1 dose escalation study to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with in-transit extremity melanoma was performed.
METHODS:
Dose escalation cohorts of 3 patients each received 1000, 2000, and 4000 mg (10 patients) of ADH-1 administered intravenously on Days 1 and 8 with standard dose melphalan via isolated limb infusion on Day 1. N-cadherin immunohistochemistry staining and quantitative polymerase chain reaction analysis were performed on pretreatment tumor. Response was defined at 3 months using modified Response Evaluation Criteria in Solid Tumors.
RESULTS:
Sixteen patients have been treated with no observed dose-limiting toxicities. Common treatment-related grade 1 or 2 toxicities included skin/dermatologic (n = 14) and pain (n = 12). Grade 3 toxicities included shortness of breath (n = 1), hypertension (n = 1), serologic toxicities (n = 4), and 1 grade 4 creatine phosphokinase elevation. In-field responses included 8 CRs, 2 partial responses, 1 stable disease, and 5 progressive diseases. Pharmacokinetic analysis demonstrated increasing ADH-1 concentrations at each dose and minimal variability in melphalan drug levels.
CONCLUSIONS:
Systemic ADH-1 at a dose of 4000 mg on Days 1 and 8 in combination with melphalan via isolated limb infusion is a well-tolerated, novel targeted therapy approach to regionally advanced melanoma. The number of CRs exceeded expectations, suggesting that targeting N-cadherin may be a new strategy for overcoming melanoma chemoresistance.
Keywords: ADH-1, limb infusion, melanoma, regional chemotherapy
In 2% to 10% of extremity melanoma cases, lesions recur in a locoregional fashion, confined to the extremity and located between the site of the primary lesion and regional lymph nodes1,2 in a pattern called in-transit disease. The presence of in-transit metastases is associated with a poor prognosis, with 5-year survival rates ranging from 12% to 37%.3–5 Systemic chemotherapy with the best-studied single-agent chemotherapies is associated with low, short-lived response rates.6,7 Treatment of in-transit disease is unique in that isolation of the limb using the techniques of hyperthermic isolated limb perfusion (HILP) with melphalan (L-phenylalanine mustard [LPAM])8–12 and more recently isolated limb infusion13–15 with melphalan ± dactinomycin can enable delivery of regional chemotherapy several orders of magnitude higher than can be attained with systemic administration. We recently reported a complete response (CR) at 3 months of 30% in patients (n = 50) undergoing isolated limb infusion with melphalan and dactinomycin and found that patients experienced significantly less major morbidity than with HILP (P = .037).16 New strategies to improve response rates for melanoma have focused on targeted agents that can improve sensitivity to chemotherapy by modulating known resistance proteins or targeting signaling proteins in survival/apoptotic pathways.17,18
ADH-1 is a novel, pentapeptide drug that targets and disrupts N-cadherin adhesion complexes. ADH-1 has been shown in phase I and II single-agent studies to be well tolerated and to show evidence of antitumor activity restricted to patients with N-cadherin–positive tumors.19–21 N-cadherin is theoretically an ideal protein to target in melanoma, because it is expressed on the majority of melanoma lesions as they progress from a predominantly E-cadherin phenotype as melanocytes to a predominantly N-cadherin phenotype during their vertical growth phase.22,23 This switch from E- to N-cadherin expression is also associated with changes in intracellular signaling pathways leading to increased proliferation, increased survival, and decreased apoptosis24 that if antagonized may improve tumor responses.
We hypothesized that ADH-1–induced disruption of N-cadherin adhesion complexes could induce downstream alterations in intracellular signaling pathways that would sensitize tumor cells to melphalan. In preclinical studies using a rat xenograft model of extremity melanoma, tumors treated with systemic ADH-1 in combination with melphalan via isolated limb infusion demonstrated decreased growth and increased apoptosis when compared with tumors treated with melphalan via isolated limb infusion alone.25 Here, we report results from a multicenter phase 1 dose escalation study to evaluate the safety, tolerability, pharmacokinetics, and antitumor activity of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with locally advanced in-transit melanoma of the extremity.
MATERIALS AND METHODS
Patient Eligibility
Patients were eligible for this study if they were ≥ 18 years of age and had histologically confirmed recurrent American Joint Committee on Cancer (AJCC) stage IIIB or IIIC extremity melanoma,26 tissue available for N-cadherin staining by immunohistochemistry (IHC), directly measurable cutaneous disease distal to planned tourniquet placement, and Eastern Cooperative Oncology Group performance status of 0 or 1. The following laboratory parameters were required before treatment: aspartate aminotransferase and alanine aminotransferase ≤2.5 times the upper limit of normal (ULN), serum bilirubin ≤times ULN, absolute granulocyte count ≥1.5 × 109/L, platelet count ≥100 × 109/L, hemoglobin ≥10 g/dL, serum creatinine ≤1.5 × ULN, and a negative serum or urine pregnancy test before study entry for women of childbearing potential. Patients were excluded if they had received ADH-1 before this study, although previous melphalan via isolated limb infusion was allowed. All patients were required to give informed consent, and the institutional review boards of the participating institutions approved the study.
Study Design
This trial was an open-label, multicenter, phase 1 dose escalation study. Treatment consisted of intravenous ADH-1 administered on Days 1 and 8 in addition to isolated limb infusion with melphalan on Day 1. ADH-1 doses of 1000 mg, 2000 mg, and 4000 mg were evaluated in successive cohorts in combination with melphalan administered regionally via isolated limb infusion at a dose of 10 mg/L for the upper extremity and 7.5 mg/L for the lower extremity (Fig. 1). The 4000-mg dose of ADH-1 was previously demonstrated to be safe in patients and was the highest dose for which there was already extensive safety data.27 The dose of melphalan was calculated according to limb volume and corrected for ideal body weight as previously described.16 Isolated limb infusion was performed as described previously,16 using a rapid infusion of melphalan (2–5 minutes) in the arterial catheter after the extremity had been warmed to at least 37.0°C. Melphalan was administered a minimum of 4 hours after ADH-1 dose on Day 1.
FIGURE 1.
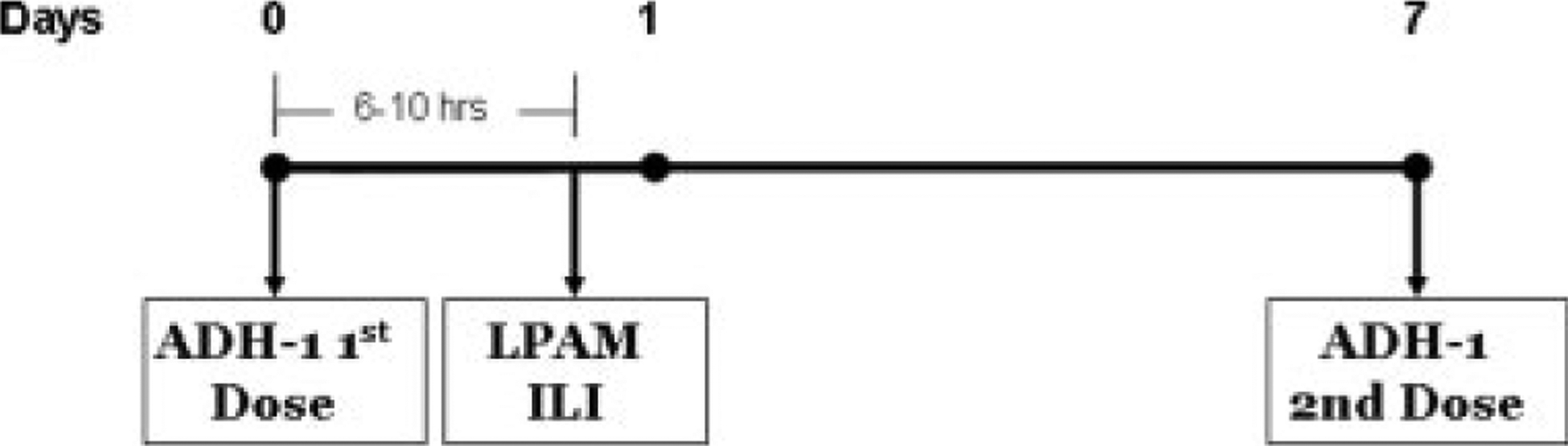
Trial design is depicted. LPAM indicates L-phenylalanine mustard; ILI, isolated limb infusion.
A minimum of 3 subjects and a maximum of 6 subjects were enrolled in each cohort, depending on the observed dose limiting toxicities (DLTs) using a standard phase 1 dose escalation design. A total of 10 subjects were enrolled in the maximum tolerated dose (MTD) cohort (including 3–6 subjects enrolled in the dose escalation phase). The MTD was determined by the highest dose at which <2 of 6 patients experienced a DLT. For cohorts 1–3, the ADH-1 dose escalated to the next dose level or expanded at the same dose level once the last subject in each cohort had been evaluated on Day 15 and a safety call had occurred between Adherex Technologies, Inc. (Durham, NC) and the site investigators to confirm the safety data. All subjects were followed for 12 weeks from the first dose of ADH-1 administration to collect safety and tolerability data.
Assessment of Tumor Response
Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors modified for cutaneous lesions at 1 time point 3 months after isolated limb infusion. Lesions were designated as target lesions and nontarget lesions.16 In-field response was documented at 3 months from isolated limb infusion as described,16 and patients were classified as having a CR, partial response (PR), stable disease (SD), or progression of disease (PD).
Toxicity Evaluation
Toxicity was assessed using the National Cancer Institute’s Common Toxicity Criteria for Adverse Events version 3.0. DLTs were drug-related toxicities defined as the following: absolute granulocyte count <0.5 × 109/L lasting ≥7 days, grade ≥3 neutropenic infection or febrile neutropenia, platelets <50 × 109/L lasting ≥7 days, symptomatic bleeding sufficient to require therapeutic intervention, nonhematological toxicity of grade ≥3 severity (except pain induced by study drug administration in the region of known tumor despite appropriate medical intervention or grade 3 fatigue), and grade ≥4 drug-related regional toxicities as defined by specified areas pertinent to limb toxicity (or inability to receive 2 weekly doses of ADH-1 in combination with regionally administered melphalan).
Pharmacokinetic Evaluations
During the 30-minute circulation of melphalan through the infusion circuit, blood samples (5 mL) from the venous stopcock of the circuit were obtained at 0 (preisolated limb infusion), 5, 10, 15, 20, 25, and 30 minutes to assess melphalan levels. The melphalan concentration in plasma was measured in each sample as published previously16 by an assay based on a published high-performance liquid chromatography (HPLC)-fluorescence method by Ehrsson et al.28
ADH-1 Pharmacokinetic Method
Just before the administration of melphalan during isolated limb infusion, a blood sample (5 mL) was obtained from the venous side of the circuit to assess ADH-1 concentration. ADH-1 in human plasma was detected using a validated HPLC method with single mass detection using MultiPROBE II automated extraction. The detection range was between 50 and 25,000 ng/mL.
IHC
N-cadherin IHC staining and scoring were conducted by Biotechnics (Hillsborough, NC). IHC slides produced to determine the presence of N-cadherin were processed from patient tumor samples embedded in paraffin wax blocks. After appropriate processing, sections were incubated overnight at 4°C with primary antibody (Invitrogen, Carlsbad, Calif, cat# 18–0224, 1:200 dilution) or mouse isotype negative control (Invitrogen cat# 08–6599) followed by incubation with secondary antibody (biotinylated goat antimouse, Jackson Immunore-search, West Grove, Pa, cat# 115-066-071, 1:500 dilution) for 30 minutes at room temperature. ABC reagent (Vectastain Elite ABC Kit, Vector Labs, Burlingame, Calif) was applied to sections and incubated for 30 minutes at 37°C. Sections were developed in diaminobenzidine for 7 minutes at room temperature and counterstained with hematoxylin.
Quantitative Polymerase Chain Reaction
Tumor biopsies from nontarget in-transit lesions were obtained before ADH-1 or isolated limb infusion with melphalan treatment and stored at −130°C until ready for use. The use of quantitative polymerase chain reaction (QPCR) was exploratory and not included as part of the initial trial protocol. Biopsies were homogenized using Lysing Matrix A (MP Biomedicals, Irvine, Calif) and a mini bead-beater (Biospec Products, Bartlesville, Okla). RNA was isolated, cDNA synthesized, and QPCR performed as described previously.29 See Table 1 legend for primer sequences. N-cadherin expression values were normalized to β-actin and expressed as fold difference from a human reference RNA sample (Stratagene, San Diego, Calif).
Table 1.
Patient Characteristics
| Sex | AJCC Stage | Disease Burden | Previous Regional Therapy | IT Lesion Biopsy Pretherapy IHC N-Cadherin Status | Archived Tissue IHC N-Cadherin Status | IT Lesion Biopsy Pretherapy QPCR N-Cadherin Expression |
|---|---|---|---|---|---|---|
| Woman | IIIC | Low | ILI-M | * | Negative | |
| Man | IIIC | High | ILI-M | Negative | Positive | |
| Woman | IIIC | Low | ILI-M | Negative | Positive | |
| Woman | IIIB | Low | * | Negative | ||
| Man | IIIC | Low | HILP-M | * | Negative | |
| Man | IIIC | Low | * | Positive | ||
| Man | IIIC | High | Positive | Positive | ||
| Woman | IIIC | Low | ILI-M | * | 1 negative/1 positive | |
| Man | IIIC | Low | ILI-M | * | Negative | |
| Woman | IIIB | High | Positive | |||
| Woman | IIIB | High | * | Positive | ||
| Woman | IIIC | High | Negative | Positive | ||
| Man | IIIB | High | HILP-M | Positive | Positive | |
| Woman | IIIB | Low | Positive | Positive | ||
| Man | IIIC | High | Positive | Positive | ||
| Woman | IIIC | Low | HILP-M | Negative | Positive |
IT indicates in-transit lesion; IHC, immunohistochemistry; QPCR, quantitative polymerase chain reaction; ILI-M, isolated limb infusion with melphalan; HILP-M, hyperthermic isolated limb perfusion with melphalan.
QPCR primer sequences:
N-cadherin, forward 5′-CCTGGAACGCAGTGTACAGA-3′, reverse 5′-CTAACCCGTCGTTGCTGTTT-3′; β-Actin, forward 5′-GGCATCCTCACCCTGAAGTA-3′, reverse 5′-AGGTGTGGTGCCAGATTTTC-3′.
Patient had single-target lesion, no biopsy allowed per protocol, and fine-needle aspiration insufficient for determination.
RESULTS
A total of 16 patients were treated according to Figure 1. Hypoxia and acidosis during isolated limb infusion were achieved with the following median values: pH at 30 minutes 7.11 (range, 6.99–7.23), base excess at 30 minutes −8.7 mmol/L (range, −14.1 to −3 mmol/L), pO2 at 30 minutes 6 mm Hg (range, 1–8 mm Hg), and median peak temperature 38.6°C (range, 37.8°C to 39.3°C). The median ischemic time was 65.5 minutes (range, 59–86 minutes), and the median volume recirculated over the 30-minute infusion was 1470 mL (range, 700 mL–1960 mL).
Safety Profile
There were no ADH-1 DLTs reported in any cohort. No clinical toxicities of severity higher than grade 3 by the National Cancer Institute’s Common Toxicity Criteria for Adverse Events version 3.0 were reported in any cohort. There was a grade 4 serologic toxicity in the form of an elevated creatine phosphokinase (CPK) in 1 patient in cohort 3. The patient was treated with dexamethasone; the CPK trended down and was normal at the 2-week follow-up visit. The grade 3 toxicities reported include elevated CPK (n = 2), anemia (n = 1), neutropenia (n = 1), shortness of breath (n = 1), and hypertension (n = 1). These toxicities resolved with appropriate treatment and were not likely to be related to ADH-1. The most common toxicities from all cohorts are listed in Table 2.
Table 2.
Toxicity Adverse Events Regardless of Relatedness Experienced Among Patients Receiving Systemic ADH-1 With Regional LPAM via Isolated Limb Infusion (n = 16)
| Adverse Event | Grade I & II, No. (%) | Grade III & IV, No. (%) |
|---|---|---|
| Cohort 1–2, n=6 | ||
| Rash/erythema/dermatitis | 6 (100) | – |
| Pain | 5 (83) | – |
| Edema | 3 (50) | – |
| Motor/sensory neuropathy | 3 (50) | – |
| Nausea | 2 (33) | – |
| Elevated LFTs | 2 (33) | – |
| Constipation | 1 (17) | |
| Vomiting | 1 (17) | |
| Hypotension | 1 (17) | |
| CPK elevation | 1 (17)* | |
| Cohort 3, n=10 | ||
| Rash/erythema | 8 (80) | – |
| Pain | 7 (70) | – |
| Fatigue | 4 (40) | – |
| Nausea | 4 (40) | – |
| Ulceration | 3 (30) | – |
| Pruritis | 3 (30) | – |
| Anemia/low hemoglobin | 3 (30) | 1 (10) |
| Vomiting | 2 (20) | – |
| Constipation | 2 (20) | |
| Cough | 2 (20) | |
| Thrombocytopenia | 2 (20) | – |
| Edema | 2 (20) | |
| Shortness of breath | – | 1 (10) |
| CPK elevation | 1 (10)* | 2 (20) |
| Blistering | 1 (10) | – |
| Motor/sensory neuropathy | 2 (20) | |
| Hypertension | 1 (10) | |
| Hypotension | 2 (20) | |
| Elevated LFTs | 1 (10) | – |
| Neutropenia | – | 1 (10) |
LPAM indicates L-phenylalanine mustard; LFT, liver function test; CPK, creatine phosphokinase.
Other grade I-II toxicities reported in single subjects include: alopecia, anorexia, anxiety, arthritic pain, atrial fibrillation, bladder spasm, bradycardia, chest pain/tightness, desquamation, dizziness, flatulence, headache, heart-burn, hematoma puncture site, hypocalcemia, infusion site pain, myositis, petechiae, procedural hypotension, sore throat, and ventricular arrhythmia.
These patients had elevation of CPK prior to undergoing isolated limb infusion.
Pharmacokinetic Analysis
The median time between the first dose of ADH-1 and isolated limb infusion was 8.1 hours (range, 4.4–11.5 hours) (n = 16). The half-life of ADH-1 in humans has been previously determined to be between 1.4 and 2.2 hours.30 Figure 2 (Top) depicts the ADH-1 plasma concentrations for each patient just before isolated limb infusion. These pharmacokinetic characteristics were similar to those reported in patients receiving ADH-1 as a single agent.20
FIGURE 2.
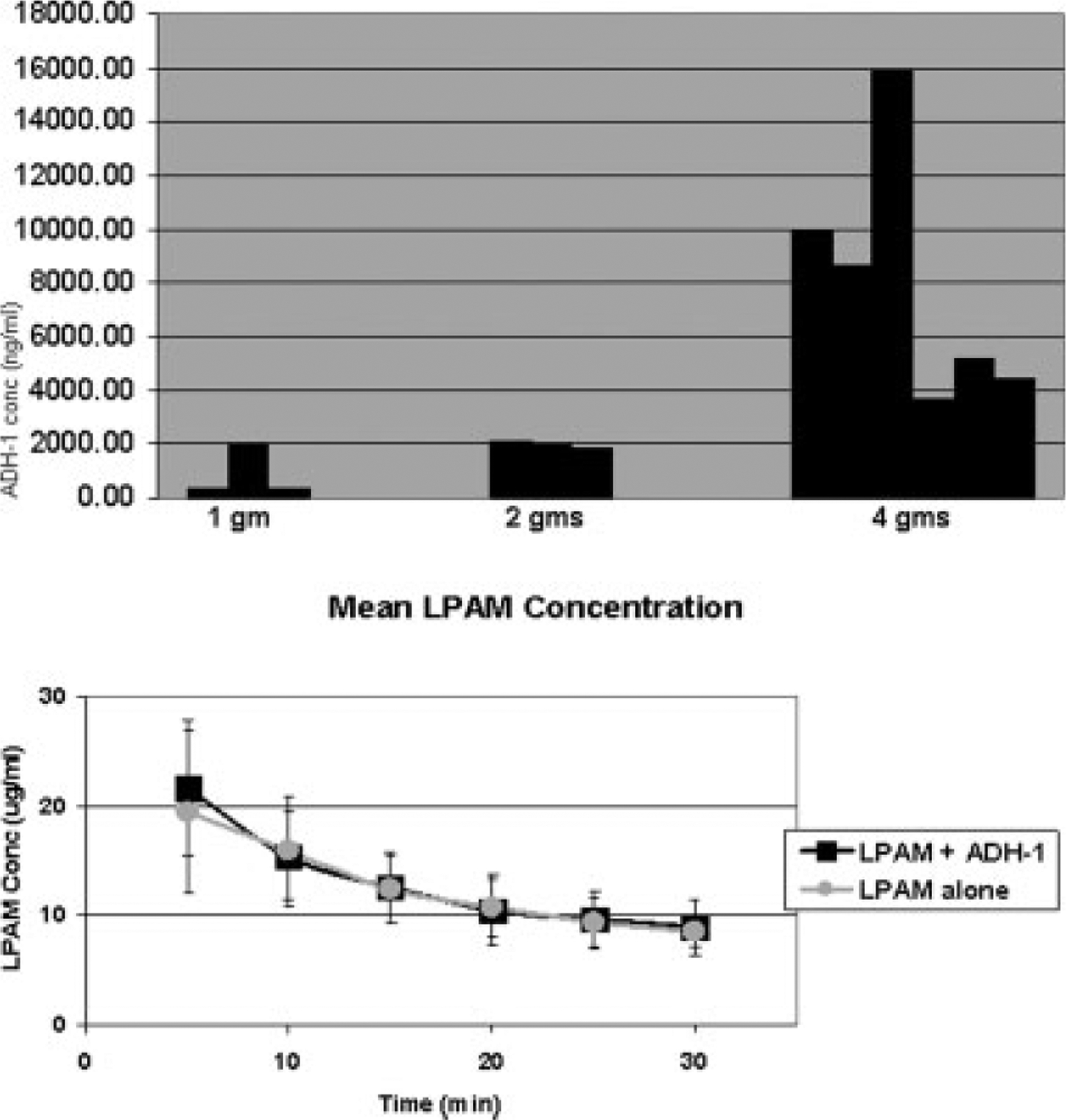
(Top) ADH-1 pharmacokinetic analysis revealed increasing ADH-1 concentrations at each dose escalation. (Bottom) Mean plasma L-phenylalanine mustard (LPAM) concentrations in patients in this study who had isolated limb infusion with LPAM plus systemic ADH-1 (n = 12) and mean LPAM concentrations from patients who had isolated limb infusion with LPAM alone (n = 29) are shown.
The median dose of melphalan for the upper extremity was 19.6 mg (range, 18.3–20.9 mg), whereas the median dose for the lower extremity was 46.6 mg (range, 33.6–70.3 mg). ADH-1 did not appear to alter LPAM pharmacokinetics, as shown in Figure 2 (Bottom). Mean plasma concentrations (±standard deviations) of LPAM obtained during isolated limb infusion were almost identical to mean LPAM values from patients who underwent isolated limb infusion with LPAM + dactinomycin.16
Clinical Response
All 16 patients were assessed for in-field tumor response at 3 months after isolated limb infusion. Eight (50%) patients achieved a CR, 2 (12.5%) patients achieved a PR, 1 (6.25%) patient had SD, and 5 (31.25%) patients had PD as depicted in Figure 3. This is surprisingly different from our previously published experience using isolated limb infusion with LPAM, in which the CR rate was 30%, PR was 7%, SD was 14%, and PD was 48%. Response data by treatment cohort and IHC N-cadherin expression are shown in Figure 4; patients were considered to be N-cadherin positive if any in-transit lesion or archived tissue was positive by IHC or QPCR. In the 8 patients who had previously been treated with melphalan via isolated limb infusion or isolated limb perfusion, there were 3 CRs, 1 patient had SD, and 4 patients had PD. Among the 3 CRs in this pretreated group, 1 patient had achieved a previous CR with melphalan via isolated limb infusion alone. There are 10 patients who are currently 6 months post-treatment. In this group at the 3-month post-isolated limb infusion evaluation, there were 6 CRs, 1 patient had SD, and 3 patients had PD. Of 6 CRs, 4 have maintained a CR at 6 months, whereas 2 had PD at 6 months. The patient with SD at 3 months underwent local excision and currently has no evidence of disease at 6 months.
FIGURE 3.
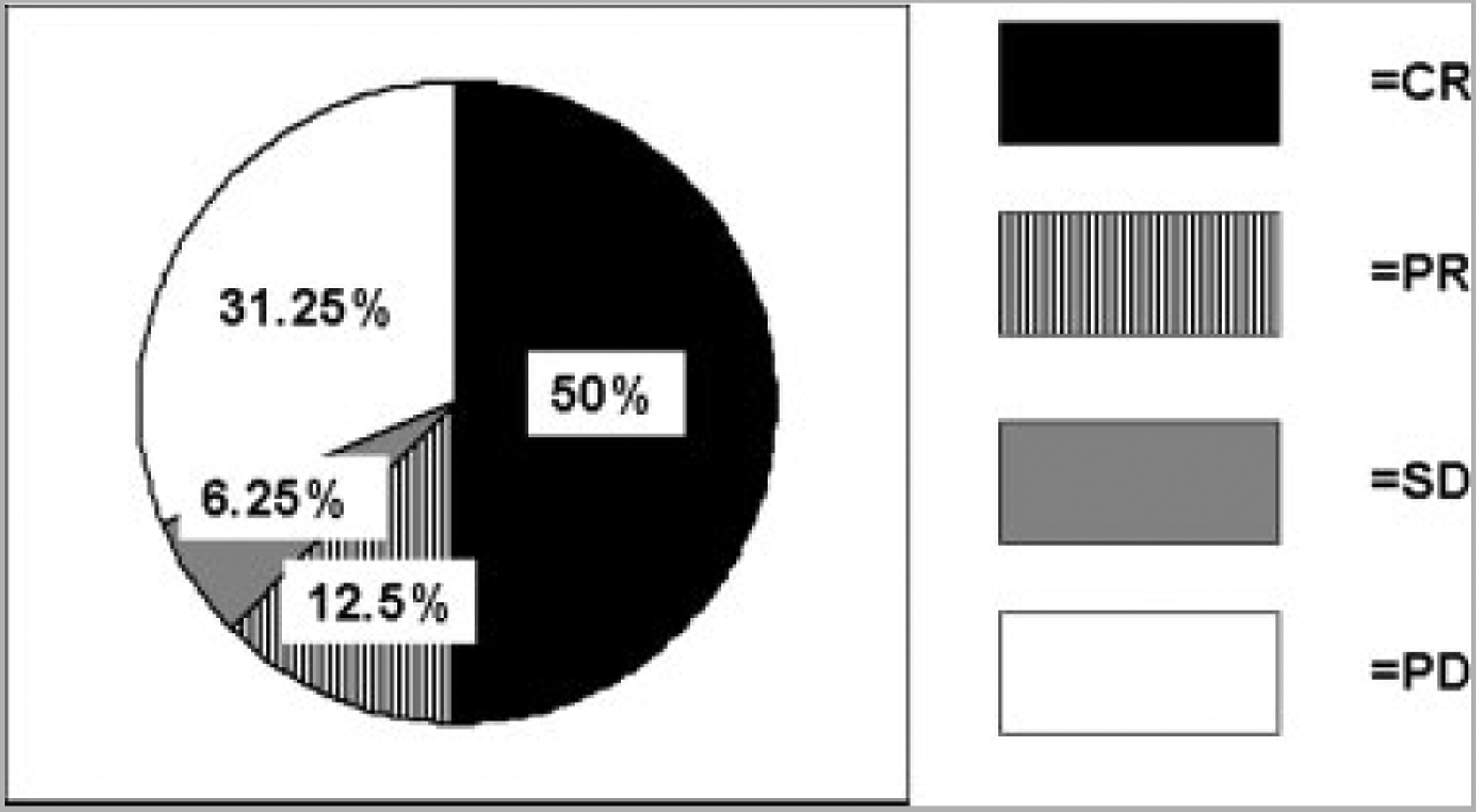
Response in 16 patients from this study treated with systemic ADH-1 plus isolated limb infusion with melphalan is shown. CR indicates complete response; PR, partial response; SD, stable disease; PD, progression of disease.
FIGURE 4.
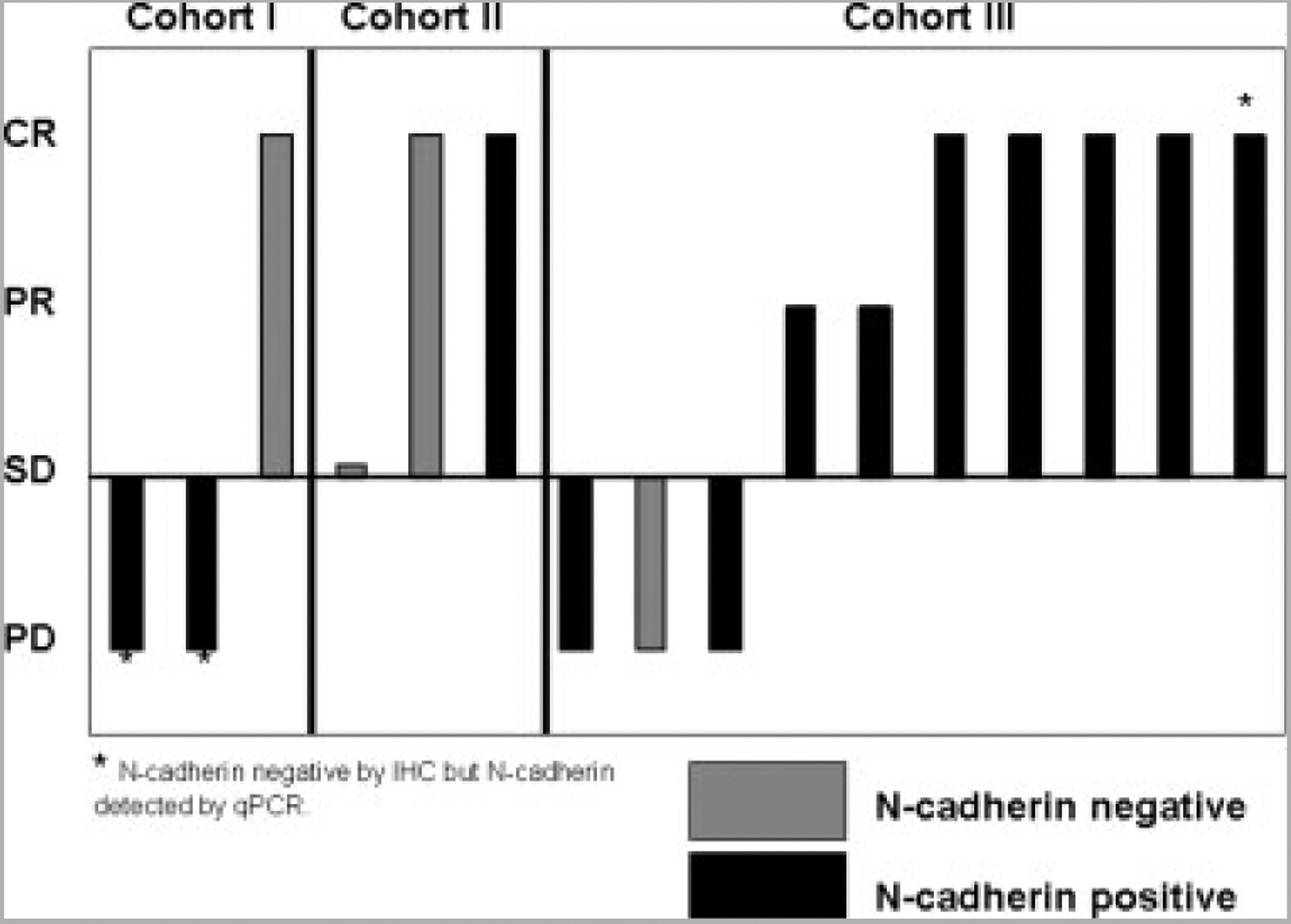
Clinical response at 3 months in trial patients according to N-cadherin status is shown. CR indicates complete response; PR, partial response; SD, stable disease; PD, progression of disease; IHC, immunohistochemistry; qPCR, quantitative polymerase chain reaction.
Four patients in this study had disease progression outside the region infused. All 4 of these patients had disease recurrence in locoregional lymph nodes or soft tissue just proximal to the tourniquet. There were 3 patients who achieved a CR in the field at 3 months, but 2 had progression out of field at 3 months, and 1 had progression out of field at 2 months. The other patient had PD in field at the 3-month assessment and had PD out of field. There was no radiographic or clinical evidence of other distant metastatic disease in any patient at 3 months post-isolated limb infusion.
DISCUSSION
The novel combination of systemic ADH-1 with melphalan via isolated limb infusion is the first to target N-cadherin as a method to augment the cytotoxic effects of chemotherapies. The rationale for this trial was built on a preclinical model that demonstrated dramatically augmented antitumor effects to chemotherapy using an N-cadherin antagonist.25 Isolated limb infusion and HILP with melphalan are widely accepted therapeutic approaches for patients with in-transit melanoma of the extremity.16 The combination of systemic ADH-1 and melphalan via isolated limb infusion was safe and well tolerated, with no DLTs seen in this group of 16 treated patients. Most of the toxicities experienced in these patients were known toxicities of melphalan via isolated limb infusion, as previously reported.16 None of these adverse events was considered medically significant, and all resolved without sequelae. As such, there appears to be no increase in toxicity for patients treated with the combination therapy in this study compared with patients treated with melphalan via isolated limb infusion alone.
Plasma concentrations of ADH-1 just before isolated limb infusion were similar among individuals in the same cohort, whereas the time between ADH-1 and melphalan administration was somewhat different between patients (median, 8.1 hours; range, 4.4–11.5 hours). Given the half-life of ADH-1 and based on the preclinical model, the goal of this study was to administer melphalan approximately 3 to 4 half-lives (6–8 hours) after the initial dose of ADH-1 to optimize potential therapeutic effects. Although the small set of patients in this study precludes any conclusions about the optimal time between ADH-1 and melphalan delivery, certainly this will be a factor to explore as the 4000-mg ADH-1 cohort is expanded in a phase 2 study.
Our hypothesis is that ADH-1 may sensitize melanoma cells to the cytotoxic effects of melphalan in part through ADH-1’s activity as an N-cadherin antagonist. In this study’s treated population of 16 patients, 9 (56%) had tissue that was positive by IHC for N-cadherin. In these 9 patients, there were 5 CRs, 2 PRs, and 2 PDs. The finding that 3 patients with N-cadherin–negative tissue obtained a CR is not surprising, given that we have previously demonstrated that N-cadherin expression is not correlated with drug resistance, and approximately 30% of patients respond completely to melphalan via isolated limb infusion alone. Whether ADH-1 helps tumors already sensitive to melphalan respond more completely or whether it sensitizes tumors that would not otherwise be sensitive to LPAM remains to be determined. In our preclinical studies, however, ADH-1 treatment increased LPAM-induced DNA adduct formation.25
In the first 6 patients, we explored the use of fine needle aspiration to make N-cadherin determination via IHC. We learned that these samples were not ideal for making this determination. As such, per trial protocol, subsequent patients had a punch biopsy of 1 in-transit lesion performed to obtain adequate tissue for N-cadherin determination. We have previously demonstrated using gene expression profiles that a single lesion is representative of all lesions in a patient with multiple nodules.31 Some patients (n = 7 of 16) only had 1 target lesion. These individuals had archived specimens assessed for N-cadherin determination. IHC may be a less sensitive method for detecting N-cadherin expression,25 and thus we explored the use of QPCR for detecting N-cadherin expression in clinical samples. N-cadherin expression was detected by QPCR in the 7 patients who had in-transit lesions amenable to punch biopsy. In 3 of these patients, N-cadherin expression was detected by QPCR, although not by IHC. As we expand cohort 3 in the phase 2 portion of this study, obtaining valid N-cadherin status on tissue will be important to determine whether any difference in efficacy of the treatment exists between N-cadherin– negative and N-cadherin–positive patients as well as to define what level of N-cadherin expression may be necessary to see ADH-1–induced augmentation of LPAM activity.
The CR rate in patients (n = 60) from the Sydney Melanoma Unit study with M. D. Anderson stage IIIab31 (corresponding to AJCC stage IIIC) was 33%, whereas the CR rate in patients (n = 74) with M. D. Anderson stage IIIa (corresponding to AJCC stage IIIB) was 43%. CR to melphalan via isolated limb infusion was shown by the Southern Methodist University study to be durable, with a limb recurrence-free interval of 22 months (inter-quartile range to 120 months) (unpublished data). In addition, for those patients who achieved a CR, survival was significantly longer (median, 53 months) than the survival in the group of patients in whom no CR occurred (median, 25 months) (P = .005) (unpublished data). Although regional therapy has not been proven to impact survival, results from Southern Methodist University suggest that achieving a CR may provide effective palliation. In the Duke study of isolated limb infusion with melphalan + dactinomycin (n = 50), overall response was 44%, with 30% being CRs and 14% being PRs. In this study, tumor responses, especially the CRs seen in this small subset of patients, were encouraging in comparison to reported response rates of melphalan via isolated limb infusion. The patient populations, technical details of isolated limb infusion, and other variables in the Duke study were similar to this study.16
Results from this study demonstrate that systemic ADH-1 on Days 1 and 8 in combination with melphalan via isolated limb infusion on Day 1 is a safe, well-tolerated, and novel targeted therapy approach for patients with in-transit extremity melanoma. The 50% CR rate achieved in the small number of patients in this study with no observed increases in toxicity warrants further investigation of this combination therapy. On the basis of this trial, the 4000-mg dose of ADH-1 on Days 1 and 8 in combination with melphalan dosed according to limb volume and corrected for ideal body weight on Day 1 will be the doses used in the phase 2 portion of this trial.
Footnotes
Presented in part at the American Society of Clinical Oncology’s 44st annual meeting, Chicago, Illinois, May 30-June 3, 2008, and at the Society of Surgical Oncology’s 2009 Annual Cancer Symposium, Phoenix, Arizona, March 2–4, 2008.
Conflict of Interest Disclosures
This trial was supported by a grant from Adherex Technologies, Inc.
References
- 1.Balch CM, Houghton AN, Peters LJ. Cutaneous Melanoma In: DeVita VT, Helbvlman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: JB Lippincott; 1993:1612–1661. [Google Scholar]
- 2.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymph-adenectomy. Ann Surg Oncol. 2005;12:587–596. [DOI] [PubMed] [Google Scholar]
- 3.Cascinelli N, Bufalino R, Marolda R, et al. Regional non-nodal metastasis of cutaneous melanoma. Eur J Surg Oncol. 1986;12:175–180. [PubMed] [Google Scholar]
- 4.Calabro A, Singletary SE, Balch CM. Patterns of relapse in 1001 consecutive patients with melanoma nodal metastases. Arch Surg. 1989;124:1051–1055. [DOI] [PubMed] [Google Scholar]
- 5.Zogakis TG, Bartlett DL, Libutti SK, et al. Factors affecting survival after complete response to isolated limb perfusion in patients with in-transit melanoma. Ann Surg Oncol. 2001;8:771–778. [DOI] [PubMed] [Google Scholar]
- 6.Sun W, Schuchter LM. Metastatic melanoma. Curr Treat Options Oncol. 2001;2:193–202. [DOI] [PubMed] [Google Scholar]
- 7.Hochster H, Strawderman MH, Harris JE, et al. Conventional dose melphalan is inactive in metastatic melanoma: results of an Eastern Cooperative Oncology Group Study (E1687). Anticancer Drugs. 1999;10:245–248. [DOI] [PubMed] [Google Scholar]
- 8.Skene AL, Bulman AS, Williams TF, et al. Hyperthermic isolated limb perfusion with melphalan in the treatment of advanced malignant melanoma of the lower Limb. Br J Surg. 1990;77:765–767. [DOI] [PubMed] [Google Scholar]
- 9.Grunhagen DJ, de Wilt JHW, van Geel AN, Eggermont AMM. Isolated limb perfusion for melanoma patients—a review of its indications and the role of tumour necrosis factor-alpha. Eur J Surg Oncol. 2006;32:371–380. [DOI] [PubMed] [Google Scholar]
- 10.Vrouenraets BC, Nieweg OE, Kroon BB. Thirty-five years of isolated limb perfusion for melanoma: indications and results. Br J Surg. 1996;83:1319–1328. [DOI] [PubMed] [Google Scholar]
- 11.Cornett WR, McCall LM, Petersen RP, et al. Prospective randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone versus melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;24:4196–4201. [DOI] [PubMed] [Google Scholar]
- 12.Taber SW, Polk HC Jr. Mortality, major amputation rates, and leukopenia after isolated limb perfusion with phenylalanine mustard for the treatment of melanoma. Ann Surg Oncol. 1997;4:440–445. [DOI] [PubMed] [Google Scholar]
- 13.Thompson JF, Kam PC, Waugh RC, Harman CR. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238–247. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JF, Waugh RC, Saw RPM, Kam PCA. Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat. 1994;7:188–192. [Google Scholar]
- 15.Lindner P, Doubrovsky A, Kam PCA, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9:127–136. [DOI] [PubMed] [Google Scholar]
- 16.Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;8:2195–2205. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12(7 suppl):2366s–2370s. [DOI] [PubMed] [Google Scholar]
- 18.McDermott DF, Sosman JA, Hodi FS, et al. Randomized phase II study of dacarbazine with or without sorafenib in patients with advanced melanoma. Paper presented at: 43rd Annual Meeting of the American Society of Clinical Oncology, June 1–5, 2007; Chicago, Ill. [Google Scholar]
- 19.Jonker DJ Stewart R, Goel L, et al. A phase I study of the novel molecularly targeted vascular targeting agent, ExherinTM (ADH-1), shows activity in some patients with refractory solid tumors stratified according to N-cadherin expression [abstract]. J Clin Oncol. 2005;23(suppl):16S Abstract 3038. [Google Scholar]
- 20.Sessa C, Perotti A, Maur M, et al. An enriched phase I, pharmacokinetic and pharmacodynamic study of the N-cadherin (NCAD) cyclic competitive binder exherin (ADH-1) in patients with solid tumors [abstract]. J Clin Oncol. 2006;24(suppl):18S Abstract 3042. [Google Scholar]
- 21.Stewart DJ, Jonker DJ, Goel R, et al. Final clinical and pharmacokinetic (PK) results from a phase 1 study of the novel N-cadherin (N-cad) antagonist, exherin (ADH-1), in patients with refractory solid tumors stratified according to N-cad expression [abstract]. J Clin Oncol. 2006;24(suppl): 18S Abstract 3016. [Google Scholar]
- 22.Matsuyoshi N, Tanaka T, Toda K, Imamura S. Identification of novel cadherins expressed in human melanoma cells. J Invest Dermatol. 1997;108:908–913. [DOI] [PubMed] [Google Scholar]
- 23.Hsu MY, Meier FE, Nesbit M, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi J, Chen N, Wang J, Siu CH. Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway. Mol Biol Cell. 2005;16:4386–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augustine CK, Yoshimoto Y, Gupta M, et al. Inhibition of N-cadherin significantly enhances anti-tumor activity of cytotoxic therapies in regional and systemic melanoma treatment. Cancer Res. 2008;68:3777–3784. [DOI] [PubMed] [Google Scholar]
- 26.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. [DOI] [PubMed] [Google Scholar]
- 27.Sessa A, Perotti M, Maur A. An enriched phase I, pharmacokinetic and pharmacodynamic study of the N-cadherin (NCAD) cyclic competitive binder exherin (ADH-1) in patients with solid tumors [abstract]. J Clin Oncol. 2006;24(suppl):18S Abstract 30242. [Google Scholar]
- 28.Ehrsson H, Eksborg S, Lindfors A. Quantitative determination of melphalan in plasma by liquid chromatography after derivatization with N-acetylcysteine. J Chromatogr Biomed Appl. 1986;380:220. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto Y, Augustine CK, Yoo JS, et al. Defining regional infusion treatment strategies for extremity melanoma: comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol Cancer Ther. 2007;6:1492–1500. [DOI] [PubMed] [Google Scholar]
- 30.ADH-1 Investigators Brochure, version 7.0 Durham, NC: Adherex Technologies; 2008. [Google Scholar]
- 31.Kroon HM, Moncrieff M, Kam PCA, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;11:3003–3013. [DOI] [PubMed] [Google Scholar]


