Abstract
Background
The use of medicated mouthwashes and gels in the home care maintenance of dental implants is controversial due to the possibility of residue deposition on the implant collar. The aim of this in vitro study was to analyse, by means of scanning electron microscopy (SEM), the amount of residues on dental implant collars treated with various commercial home dental care products.
Methods
Gel and mouthwash products were tested on 10 implants. The gels included sodium fluoride, amine fluoride, and sodium hyaluronate products. The mouthwashes tested contained triclosan, nimesulide, stannous fluoride, amine fluoride, and hexetidine-chlorobutanol. The SEM observations were performed at different magnifications in double modality SE (secondary electrons) and BSE (backscattered electrons) to qualitatively assess any residual products. The image quantitative analysis was performed by Image J® software to assess areas occupied by residuals. All results were analysed by the same researcher with experience in electron microscopy.
Results
The fluoride-based gel products left wider areas occupied by residuals than the mouthwash products. In particular the fluoride-based and hyaluronate products left the highest amount of residues. Among mouthwashes, fluoride-based and triclosan products showed the highest amount of residuals deposition.
Discussion
Oral hygiene procedures and related professional products are fundamental to the prevention, treatment, and control of microorganisms. In the case of implants, mechanical and chemical plaque control strategies are even more important since the potentially harmful biofilm covers abiotic titanium surfaces. In situ fixture maintenance is crucial for dental implant therapy success. Correct recommendation of home care products for bacterial control is fundamental to the health of implants and their surrounding tissues.
Conclusions
Data from this experimental study showed that home care commercial products in gel formulation, especially those containing fluoride, leave more residuals on titanium smooth surfaces than mouthwash products. The longer permanence of the products may lead to a more effective plaque control than other products.
Keywords: chemical agents, dental devices home care, dental implants, mouthwashes, oral health, oral hygiene
Abstract
Contexte
L’utilisation de bains de bouche et de gels médicamentés lors des soins d’entretien à domicile des implants dentaires porte à controverse en raison de la possibilité de dépôts de résidus sur le collet de l’implant. La présente étude in vitro visait à analyser par microscopie électronique à balayage (MÉB) la quantité de résidus sur les collets d’implants dentaires traités in vitro avec divers produits commerciaux de soins dentaires à domicile.
Méthodologie
Les produits de gels et de bains de bouche ont été testés sur 10 implants. Les gels comprenaient les produits de fluorure de sodium, de fluorure d’amine et de hyaluronate de sodium. Les bains de bouche évalués contenaient du triclosan, de la nimésulide, du fluorure d’étain et du hexétidine-chlorobutanol. Les observations par MÉB ont été effectuées à diverses amplifications en modalité double SE (électrons secondaires) et BSE (électrons rétrodiffusés) pour évaluer de manière qualitative tout produit résiduel. L’analyse quantitative de l’image a été effectuée à l’aide du logiciel Image J® pour évaluer les zones ayant des résidus. Tous les résultats ont été analysés par le même chercheur expérimenté dans le domaine de la microscopie électronique.
Résultats
Les produits de gels à base de fluorure ont laissé des résidus sur de plus vastes zones que les produits de bains de bouche. En particulier, les produits à base de fluorure et le hyaluronate ont laissé la plus grande quantité de résidus. Parmi les bains de bouche, les produits à base de fluorure et de triclosan ont révélé la plus grande quantité de dépôts de résidus.
Discussion
Les procédures d’hygiène buccodentaire et les produits professionnels qui y sont liés sont essentiels à la prévention, au traitement et au contrôle des microorganismes. En matière d’implants, les stratégies de contrôle mécanique et chimique de la plaque sont encore plus importantes puisque le biofilm potentiellement nocif couvre les surfaces abiotiques du titane. L’entretien in situ du montage est primordial au succès de la thérapie de l’implant dentaire. La bonne recommandation de produits de soins à domicile pour le contrôle des bactéries est essentielle à la santé des implants et des tissus environnants.
Conclusions
Les données de cette étude expérimentale ont montré que les produits commerciaux de soins à domicile en formule de gels, surtout ceux contenant du fluorure, laissent une plus grande quantité de résidus sur les surfaces lisses du titane que les produits de bains de bouche. La permanence plus longue des produits peut mener à un contrôle plus efficace de la plaque que d’autres produits.
PRACTICAL IMPLICATIONS OF THIS RESEARCH .
Dental implants are now widely considered a valid intervention for the replacement of missing teeth.
Harmful biofilm can quickly cover the abiotic titanium surfaces and surrounding tissues, making mechanical and chemical home care plaque control strategies for clients critical.
Commercial oral care products in gel formulation, especially those containing fluoride, leave more residuals on titanium smooth surfaces than mouthwash products, potentially leading to more effective plaque control.
INTRODUCTION
Oral hygiene products and mechanical plaque control are fundamental to maintaining the health of the oral cavity and, in particular, th,e dental and periodontal tissues.1 Toothpastes containing fluorides have decreased tooth decay on the natural dentition for decades.2 In their meta-analysis, Walsh et al.2 confirmed the preventive action of fluoride toothpastes on caries when used in children and adolescents. Fluoride toothpastes bond to the tooth surface enamel, making it stronger and less susceptible to demineralization.3- 4 However, this is, a slow process that requires the presence of fluoride in the mouth for extended periods of time,5 and the low concentra,tions that are found after brushing with fluoride toothpastes may not be enough to significantly reduce the dissolution of tooth minerals.6
Mouthwashes and gels containing chemicals, such as chlorhexidine, help the daily battle against microorganisms responsible for periodonti,tis. The American Dental Association (ADA) has accepted chlorhexidine gluconate as an effective treatment for gingivitis.3 Chlorhexidine possesses the property of substantivity that adsorbs to tooth surfaces and disrupts the cytoplasmic membranes of the microorganisms.7
Periodontitis and dental caries often lead to tooth loss if microorganisms are not kept under control.8-11 Since dental implants are now widely considered a valid intervention for the replacement of missing teeth, there are many who believe that dental implants, along with their prosthodontic rehabilitation components, provide a solution to end all periodontal problems; unfortunately, they have not fulfilled such expectations.12-13 Indeed, if in selected clients the success of implants is reported to be high, the implant placement intervention may fail for several reasons.14 In addition, the placed dental fixture is a unique rehabilitation connecting the oral environment to bone tissue. Therefore, it is exposed to microbial colonization in the same manner as natural teeth, making the surrounding host tissues susceptible to infection.15
All biological and non-biological surfaces in the oral cavity are covered by a microbial biofilm,16 thus the control of the equilibrium between the host and these microorganisms is vital for the maintenance of both healthy teeth and healthy implants. Hence, home care efforts are just as critical for individuals with dental implants as they are for individuals with natural teeth. However, scant evidence exists about which home care products—particularly mouthwashes and gels—are safe to use on dental implants. The use of medicated mouthwashes and topical gel applications is still controversial since it has been, proposed that salts or other residuals can remain on the implant collar.17
The aim of this in vitro study was to determine, by means of visual examination using scanning electron microscopy (SEM), whether some commercial products designed for oral health maintenance leave residues on the neck portion of implants.
MATERIALS AND METHODS
This study was conducted on 10 implants provided by Sweden and Martina® S.P.A. Five of the implants were Sweden and Martina® “Khono” implants, with the fixture covered in titanium-plasma-spray (TPS); 5 other implants were Sweden and Martina® “Premium,” with the surface made in nano-Pore. In addition to the different type of fixture surfaces, the considered portion for the production adhesion was the collar, which is overlapping, in order to make the area comparable.
Commercial oral hygiene products
Overall, 4 fluoride-based gel products were tested and 4 mouthwash products were tested. Fluorine® Gel and Mentadent® Gel were sodium fluoride-based, Elmex® Gel was amine fluoride-based, and Aminogam® Gel contained sodium hyaluronate. The 4 mouthwashes tested contained triclosan (DentoOral® mouthwash), hexetidine and chlorobutanol (Buccagel® mouthwash), nimesulide (Erefflog® mouthwash), and amine fluoride plus stannous fluoride (Meridol® ).
Three gel products were tested on the “Khono” implants; a mouthwash was used as positive control and saline solution was used as negative control. The mouthwash products were tested on the “Premium” implants; a gel product was used as positive control and saline solution was used as negative control.
Study protocol
The study steps were as follows:
Implant handling always by sterile pliers (to fasten it on an appropriate support)
Implant labelling and product assignment
Product application for 30 minutes, in order to simulate the manufacturer indications
Water rinsing
Final SEM observation of the pre-established surface
One single product was tested on one single fixture.
SEM observations
The SEM (Philips XL30CP, The Netherlands) observations were performed as previously described18-21 at different magnifications in double modality SE and BSE to qualitatively assess the presence or absence of residual products. The observations were performed at 20.0 kV, at a working distance ranging from 14.5 mm to 17.5 mm, capturing images at 15x magnification. In order to homogenize the observations, all the implants were observed at the same reference points.
Image analysis
The image analysis was performed by the Image J® software, using the thresholding process22 to assess the occupied area of the residuals. In particular, the area was measured and the grey tones associated with the residuals were isolated. Then the occupied area was assessed by the software and the percentage area was calculated. Given the small sample size, only a descriptive comparison of the residuals could be performed.
RESULTS
The SEM observations were easily identified as all of the products left some residue on the smooth implant surfaces, including the negative control constituted by the saline solutions (Figures 1 and 2).
In particular, the areas occupied by the sodium fluoride-based gels (Fluorine® Gel and Mentadent® Gel) were smaller than those of the amine fluoride gel (Elmex® Gel) and the gel with an amino acid and hyaluronic acid composition (Aminogam® Gel). In addition, the SEM observation revealed the presence of tiny particles presenting a very peculiar shape (Figure 1D) in the sample exposed to the amine fluoride gel, while the sample exposed to the hyaluronic acid gel was covered completely (Aminogam® Gel) (Figure 2E).
Among the mouthwash products, the highest assessed values were found with the DentoOral® product, followed by the mouthwash containing fluoride (Meridol® ). In the sample exposed to the triclosan, the residuals observed by SEM were distributed more widely along the smooth surface (Figure 2B).
In addition, the image analysis showed that some of the gel products left more residues, occupying a larger area than those left by the mouthwash formulations (Table 1). It is worth noting, moreover, that the products containing amine fluoride, both gel and mouthwash, left high percentages of residues, and the hyaluronated gel remained on the entire surface.
DISCUSSION
Microbial biofilm, in general, represents a complex bacterial community living under peculiar conditions protected from UV light, dehydration, host immune cells, and killing molecules.23 Hence, when an infection is biofilm mediated, the adhesive bacteria are extremely dangerous and difficult for the immune system to eliminate.24 In the oral cavity, in the presence of saliva and direct contact with the external environment, biofilm formation is physiological.25 The initial biofilm formation on cleaned teeth is estimated to occur in 6 hours, while the biofilm formation on the implant takes more time, but with similar stages.26
When an imbalance or dysbiosis of the microbial population within the biofilm covering the biotic and abiotic surfaces of the oral cavity occurs, it leads to the development ,of oral pathologies, such as caries, periodontitis, and peri-implantitis.27 Oral hygiene procedures and related professional home care products are fundamental to the control of this dysbiosis of oral biofilm in order to prevent such infective oral pathologies.
In the case of dental implants, mechanical and chemical plaque control strategies are even more important since the potentially harmful biofilm covers the abiotic titanium surfaces.28 Our morphological data have demonstrated how commercial home care products in 3 different gel formulations, especially those containing fluoride, leave residues on titanium smooth surfaces, while 3 tested mouthwash formulations left fewer residuals on titanium smooth surfaces.
Numerous inconsistencies in the literature regarding this topic have been identified.
Table1.
Numerical data of the quantitative image analysis
| Type of product | Area occupied in % |
|---|---|
| Sodium-fluoride gel | 4.48 |
| Sodium-fluoride gel | 1.21 |
| Amine fluoride gel | 36.59 |
| Stannous and amine fluoride mouthwash | 24.28 |
| Triclosan-based mouthwash | 34.12 |
| Hexetidine and chlorobutanol mouthwash | 14.29 |
| Nimesulide mouthwashes | 28.92 |
| Hyaluronated gel | 100 |
| Saline solution | 0.05 |
Huang and Lee29 in 2005 reported that the use of fluoride ions on titanium alloy surfaces was harmful. Indeed, in their study, the use of fluoride ions in artificial saliva with an acid pH caused the loss of the superficial oxide titanium film. Conversely, a 2009 study by Muguruma and colleagues30 observed that mouthwashes containing fluoride left residuals on titanium surfaces, but they concluded that these residuals did not adversely alter the mechanical properties of the titanium alloy. Quaranta et al.31 also highly recommended the use of amine fluoride mouthwash as a home care product for routine oral hygiene.
One commonality among all cited authors was their recognition of the potential damage derived from the combination of high levels of fluoride ion concentrations and low levels of salivary pH, such as 3.5. Interestingly, Joska et al.32 in a 2010 study demonstrated the high resistance of TiN alloy, which is used for the fabrication of endodontic instruments and orthodontic wires, in a simulated environment with high concentrations of fluoride ions in a strong acid pH. In 2013, Licausi et al.33 , building on their study results, demonstrated how the contact between fluoride ions and titanium alloys in artificial saliva leads to the formation of a salt incorporated into the examined surfaces. They assumed that the salt formation made the superficial layer of the titanium porous. However, more investigations using the profilometer are required to support these assumptions.
Within the limits of this current study, including the small sample size, the decision not to test each product against a control on each type of implant surface, and the lack of numerical analysis for comparison of results of the morphological evaluation of the examined titanium surfaces, the observations showed the presence of residuals of different molecules in all tested formulations, but no significant superficial damages were observed. In particular, the highest percentage of occupied residual area resulted from gel formulations of amine fluoride. This result cou,ld be due to the particular formulation of the gel and the nature of the amine fluoride. Specifically, gels and mouthwashes belong to colloidal systems, using water as the main solvent. Gels are lipophilic systems that are highly concentrated.34 The cohesion between the elementary particles within gels is higher in comparison to those of mouthwashes, allowing the formation of a 3-dimensional reticular structure that incorporates the solvent (gel).
Even if both formulations (gels and mouthwashes) have a high capillarity, which enables them to fill into very small spaces or fissures, they have a difference in viscosity. The mouthwashes are liquids in state; the gels have a more complex structure that promotes the internal friction between cross-linked molecules, opposing the outflow of the formulation. 35
Figure 1.
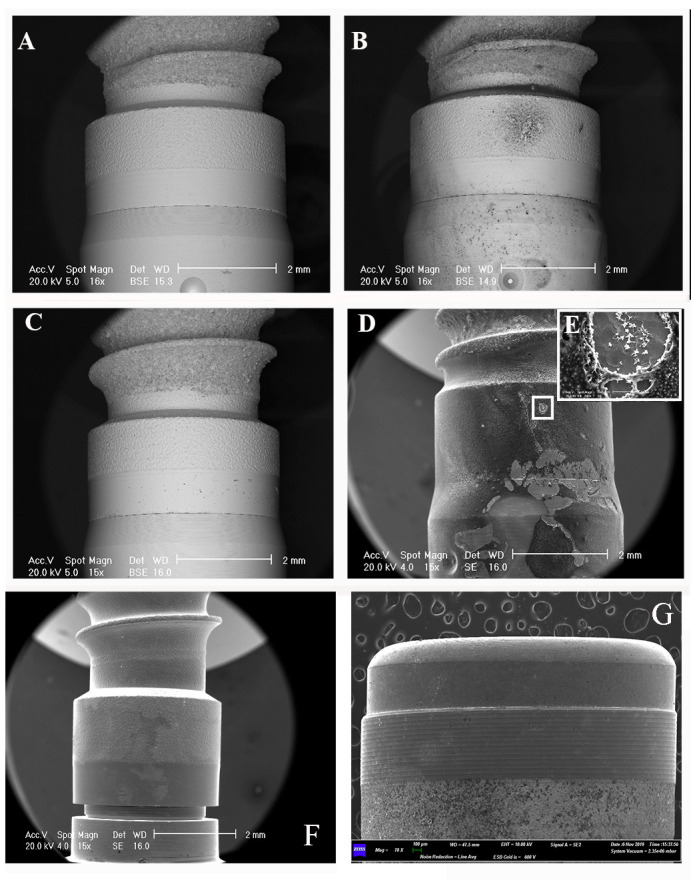
SEM observations of the “Khono” implant surface, before and after product applications A: “Khono” implant surface with no application of product. B: SEM observation after the application of fluoride gel. The observation presented in backscattered secondary electron shows punctiform residuals of the product along the considered smooth surface. C: SEM observation after the application of Mentadent® Gel. D: SEM observation after the application of Elmex® Gel. The observation presented in secondary electrons shows how the residual of the product occupies a very large area. E: Magnification of the salt of the product on the surface. No particular damage is detectable. F: SEM observation of the positive control after the application of the fluoride-based mouthwashes, which leaves a quite important area of residuals. G: SEM observation of the negative control (saline solution): the black arrows point to the small residuals.
Figure 2.
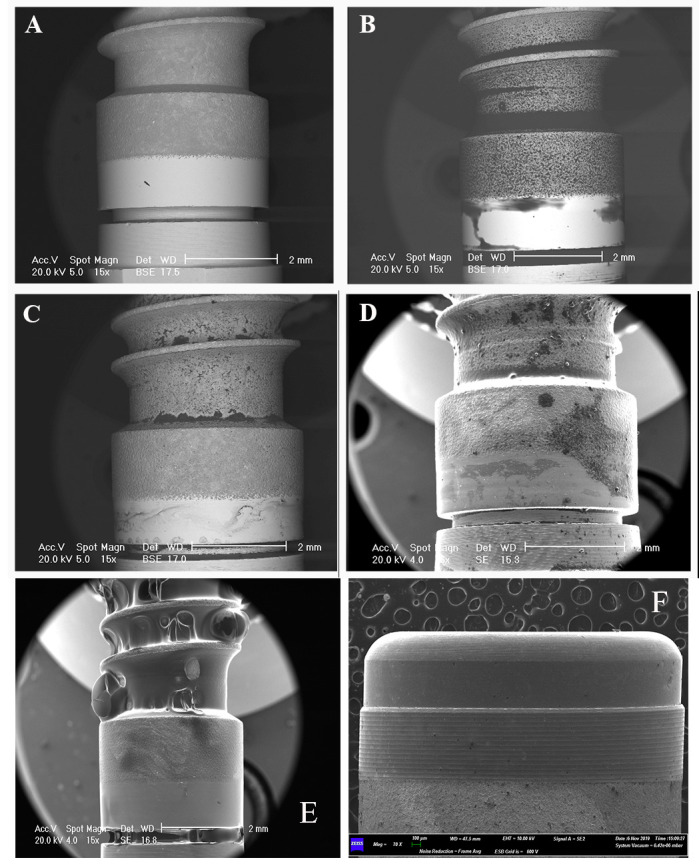
SEM observations of the “Premium” implant surface, before and after product applications A: “Premium” implant surface with no application of product. B: SEM observation after the application of DentoOral® mouthwash. The observation presented in backscattered secondary electron shows few areas occupied by the product’s residuals along the considered smooth surface. C: SEM observation after the application of Erreflog® mouthwash. The observation presented in backscattered secondary electron shows areas occupied by the product’s residuals along all of the considered smooth surface. D: SEM observation after the application of Buccagel® mouthwash. The observation presented in secondary electrons shows few areas occupied by the product’s residuals along the considered smooth surface. E: SEM observation of the positive control exposed to the Aminogam®, which occupies not only the considered area of the study (the collar) but all of the surface of the implant. F: SEM observation of the negative control (saline solution): the black arrows point to the small residuals.
In situ fixture maintenance is crucial for the success of dental implants. Given the complexity of the microbial environment of the oral cavity, recommendations for the safe use of home care oral hygiene products targeted for bacterial control are fundamental to the health of implants and their surrounding tissues.
CONCLUSIONS
The investigated dental home care products left residues on the smooth collar part of the implants. Future studies should investigate if amine fluoride product residue on the titanium surfaces of dental implants can demonstrate a positive bacteriostatic action for long-term fixture maintenance without harming the implant surface.
CONFLICT OF INTEREST
The authors are not aware of any existing or potential conflicts of interest.
Footnotes
CDHA Research Agenda category: risk assessment and management
REFERENCES
- 1. Wu CD, Savitt ED. Evaluation of the safety and efficacy of over‐the‐counter oral hygiene products for the reduction and control of plaque and gingivitis. Periodontology 2000 2002;28:91–105 [DOI] [PubMed] [Google Scholar]
- 2. Walsh T, Worthington HV, Glenny A-M, Appelbe P, Marinho VC, Shi X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev.. 2010:CD007868. [DOI] [PubMed]
- 3. Choo A, Delac DM, Messer LB. Oral hygiene measures and promotion: review and considerations. Aust Dent J 2001;46:166–173 [DOI] [PubMed] [Google Scholar]
- 4. Caruso S, Bernardi S, Pasini M, Giuca MR, Docimo R, Continenza MA, et al. The process of mineralisation in the development of human tooth. Eur J Paediatr Dent 2016;17:322–326 [PubMed] [Google Scholar]
- 5. Rølla G, Saxegaard E. Critical evaluation of the composition and use of topical fluorides, with emphasis on the role of calcium fluoride in caries inhibition. J Dent Res 1990;69:S780–785 [DOI] [PubMed] [Google Scholar]
- 6. Ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand 1999;57:325–329 [DOI] [PubMed] [Google Scholar]
- 7. Fischman SL. A clinician’s perspective on antimicrobial mouthrinses. J Am Dent Assoc 1994;12:20S–22S [DOI] [PubMed] [Google Scholar]
- 8. Larsen T, Fiehn NE. Dental biofilm infections—an update. APMIS 2017;125:376–384 [DOI] [PubMed] [Google Scholar]
- 9. Bernardi S, Bianchi S, Fantozzi G, Leuter C, Continenza MA, Macchiarelli G. Morphometric study on single-root premolars in a European population sample: An update on lengths and diameters. Eur J Anat 2019;23:17–25 [Google Scholar]
- 10. Nilsson H, Berglund JS, Renvert S. Longitudinal evaluation of periodontitis and tooth loss among older adults. J Clin Periodontol 2019;46:1041–1049 [DOI] [PubMed] [Google Scholar]
- 11. Silva Junior MF, Batista MJ, de Sousa MDLR. Risk factors for tooth loss in adults: A population-based prospective cohort study. PLoS One 2019;14(7):e0219240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Falisi G, Bernardi S, Rastelli C, Pietropaoli D, De Angelis F, Frascaria M, et al. “All on short” prosthetic-implant supported rehabilitations. Oral Implantol (Rome) 2017;10:477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernardi S, Gatto R, Severino M, Botticelli G, Caruso S, Rastelli C, et al. Short versus longer implants in mandibular alveolar ridge augmented using osteogenic distraction: One-year follow-up of a randomized split-mouth trial. J Oral Implantol 2018;44:184–191 [DOI] [PubMed] [Google Scholar]
- 14. Krisam J, Ott L, Schmitz S, Klotz AL, Seyidaliyeva A, Rammelsberg P, Zenthöfer A. Factors affecting the early failure of implants placed in a dental practice with a specialization in implantology—a retrospective study. BMC Oral Health 2019;19:208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernardi S, Bianchi S, Tomei AR, Continenza MA, Macchiarelli G. Microbiological and SEM-EDS evaluation of titanium surfaces exposed to periodontal gel: In vitro study. Materials (Basel) 2019;12:pii:E1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannig C, Hannig M, Rehmer O, Braun G, Hellwig E, Al-Ahmad A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch Oral Biol 2007;52:1048–1056 [DOI] [PubMed] [Google Scholar]
- 17. Ntrouka VI, Slot DE, Louropoulou A, Van der Weijden F. The effect of chemotherapeutic agents on contaminated titanium surfaces: A systematic review. Clin Oral Implants Res 2011;22:681–689 [DOI] [PubMed] [Google Scholar]
- 18. D’Ercole S, Tripodi D, Marzo G, Bernardi S, Continenza MA, Piattelli A, et al. Microleakage of bacteria in different implant-abutment assemblies: an in vitro study. J Appl Biomater Funct Mater 2015;13:e174–80 [DOI] [PubMed] [Google Scholar]
- 19. Bernardi S, Bianchi S, Botticelli G, Rastelli E, Tomei AR, Palmerini MG, et al. Scanning electron microscopy and microbiological approaches for the evaluation of salivary microorganisms behaviour on anatase titanium surfaces: In vitro study. Morphologie 2018;102:1–6 [DOI] [PubMed] [Google Scholar]
- 20. Palmerini MG, Belli M, Nottola SA, Miglietta S, Bianchi S, Bernardi S, et al. Mancozeb impairs the ultrastructure of mouse granulosa cells in a dose-dependent manner. J Reprod Dev 2018;64:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmerini MG, Nottola SA, Tunjung WAS, Kadowaki A, Bianchi S, Cecconi S, et al. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem Biophys Res Commun 2016;481:159–164 [DOI] [PubMed] [Google Scholar]
- 22. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: An open-source platform for biological-image analysis Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aparna MS, Yadav S. Biofilms: microbes and disease Braz J Infect Dis 2008;12:526–530 [DOI] [PubMed] [Google Scholar]
- 24. Busscher HJ, Bos R, van der Mei HC. Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol Lett 1995;128:229–234 [DOI] [PubMed] [Google Scholar]
- 25. Do T, Devine D, Marsh PD. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin Cosmet Investig Dent 2013;5:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berger D, Rakhamimova A, Pollack A, Loewy Z. Oral biofilms: Development, control, and analysis. High Throughput 2018;7:E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kistler JO, Booth V, Bradshaw DJ, Wade WG. Bacterial community development in experimental gingivitis. PLoS One 2013;8:e71227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salvi GE, Zitzmann NU. The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: a systematic review. Int J Oral Maxillofac Implants 2014;29 Suppl:292–307 [DOI] [PubMed] [Google Scholar]
- 29. Huang H-H, Lee T-H. Electrochemical impedance spectroscopy study of Ti–6Al–4V alloy in artificial saliva with fluoride and/or bovine albumin. Dent Mater 2005;21:749–755 [DOI] [PubMed] [Google Scholar]
- 30. Muguruma T, Iijima M, Brantley WA, Yuasa T, Kyung H-M, Mizoguchi I.Effects of sodium fluoride mouth rinses on the torsional properties of miniscrew implants. Am J Orthod Dentofacial. Orthop.2011 139 588 593 [DOI] [PubMed] [Google Scholar]
- 31. Quaranta A, Ronconi LF, Di Carlo F, Vozza I, Quaranta M. Electrochemical behaviour of titanium in ammine and stannous fluoride and chlorhexidine 0.2 percent mouthwashes. Int J Immunopathol Pharmacol 2010;23:335–343 [DOI] [PubMed] [Google Scholar]
- 32. Joska L, Fojt J, Hradilova M, Hnilica F, Cvrcek L. Corrosion behaviour of TiN and ZrN in the environment containing fluoride ions. Biomed Mater 2010;5:054108 [DOI] [PubMed] [Google Scholar]
- 33. Licausi MP, Igual Muñoz A, Amigó Borrás V. Influence of the fabrication process and fluoride content on the tribocorrosion behaviour of Ti6Al4V biomedical alloy in artificial saliva. J Mech Behav Biomed Mater 2013;20:137–148 [DOI] [PubMed] [Google Scholar]
- 34.Carretti E, Deia L, Weiss RG. Soft matter and art conservation. Rheoreversible gels and beyond. 2005 Soft Matter. 1:17–22. [Google Scholar]
- 35. Anusavice KJ, Shen C, Rawls HR.Phillips’ science of dental materials. 12th ed. St. Louis (MO):Saunders (an imprint of Elsevier Inc.);2013. [Google Scholar]


