Significance
Models of future climate change have tended to focus on changes in average temperature, which in physiological terms are relatively trivial. Of more physiological importance is the future elevated risk of heat waves. We show here that during peak lactation in two species of small rodent the upper lethal temperature is reduced by around 3 to 6 °C, making this time a critically sensitive period to hot ambient temperatures. If these effects are confirmed in studies of wild rodents, they would point to large potential population impacts that are ignored in current models. Studies of the impact of high temperatures on wild small rodents are urgently required to fill this knowledge gap.
Keywords: lactation, energetics, climate change, lethal temperature, heat waves
Abstract
Predicted increases in global average temperature are physiologically trivial for most endotherms. However, heat waves will also increase in both frequency and severity, and these will be physiologically more important. Lactating small mammals are hypothesized to be limited by heat dissipation capacity, suggesting high temperatures may adversely impact lactation performance. We measured reproductive performance of mice and striped hamsters (Cricetulus barabensis), including milk energy output (MEO), at temperatures between 21 and 36 °C. In both species, there was a decline in MEO between 21 and 33 °C. In mice, milk production at 33 °C was only 18% of that at 21 °C. This led to reductions in pup growth by 20% but limited pup mortality (0.8%), because of a threefold increase in growth efficiency. In contrast, in hamsters, MEO at 33 °C was reduced to 78.1% of that at 21 °C, yet this led to significant pup mortality (possibly infanticide) and reduced pup growth by 12.7%. Hamster females were more able to sustain milk production as ambient temperature increased, but they and their pups were less capable of adjusting to the lower supply. In both species, exposure to 36 °C resulted in rapid catastrophic lactation failure and maternal mortality. Upper lethal temperature was lowered by 3 to 6 °C in late lactation, making it a critically sensitive window to high ambient temperatures. Our data suggest future heat wave events will impact breeding success of small rodents, but this is based on animals with a long history in captivity. More work should be performed on wild rodents to confirm these impacts.
Life on Earth is facing increasing temperatures and increasing variability in the climate (1). Global temperatures are predicted to rise by between 1.5 and 5.5 °C by 2100, depending on different scenarios (2). These changes are physiologically trivial for most endotherms, which often experience much larger swings in ambient temperature on a daily basis. However, as the average temperature increases, the risk of periods with extreme high temperatures (so-called heat waves) also increases (3). Heat waves are defined as periods where the temperature exceeds the previously established 95th temperature percentile for a given period (4). Their increased likelihood comes about because of changes in the temperature probability density function as the climate warms and occurs under all scenarios where the distribution is fixed but shifted upward, shows increased variability as well as an upward shift, or shows elevated right skew (4, 5). Climate models suggest that heat waves will not only increase in frequency but also in severity (maximum temperature) and duration (6, 7).
There has been a steady rise in the number of days per year where the temperature–humidity index (THI) is >70, the threshold causing heat stress in cattle. Between 1963 and 2003 in southeast England there was on average only 1 d/y when the THI exceeded the threshold. This increased to on average 5 d/y between 2003 and 2006. It is predicted this will increase to 30 d/y by the end of this century (8). The present-day average duration of heat waves is from 8.3 to 12.7 d, but this is predicted to increase to 11.4 to 17.0 d in the future (6). Urban heat wave days per year are predicted to increase from 6 between 1981 and 2005 to 92 in the future in the southeastern United States (9).
These heat wave events are physiologically more significant and their increased frequency, severity, and duration are rapidly emerging as an important threat to a variety of animals and humans (10). In humans, heat waves cause significantly increased mortality and are possibly the most important health aspect of climate change (11–13). For example, the heat wave event in France in 2003 killed 15,000 people (14).
In small mammals, high temperature has direct effects on many aspects of physiology, behavior, and life history (15–20). High temperatures adversely impact the body by reducing the ability to dissipate heat and thermoregulate (21). This issue is in theory more significant during periods of high energy requirements (22, 23). Lactation is the most energy-demanding period for female mammals, during which both food intake and energy expenditure are maximized to meet the energy requirements of their offspring (22–25). For example, the energy expenditure components including resting metabolic rate (RMR), daily energy expenditure (DEE), and milk energy output (MEO) are considerably increased during lactation, and accordingly energy intake is also increased to offset the energy expended (26–32). Although nonshivering thermogenesis mediated by uncoupling protein 1 (UCP1) in brown adipose tissue is considerably reduced (33), heat production as a by-product of digestion and milk synthesis is inevitably increased, elevating the risk of hyperthermia. This is because lactating females are hypothesized to be limited by the capacity to dissipate body heat, namely the heat dissipation limitation hypothesis (22, 23, 34, 35). It is widely acknowledged that large domesticated animals such as cattle and pigs, with a low surface-to-volume ratio, are susceptible to heat wave events (21, 36, 37). However, the susceptibility to elevated ambient temperatures of small mammals with high surface-to-volume ratios has received far less attention.
Although lactation in small mammals is marked by extreme hyperphagia, food intake reaches an asymptote in the second half of the period. Studies in mice indicate this asymptote depends on the capacity of the mother to dissipate body heat (23, 28, 38–41). These observations have since been extended to numerous other small mammals including three vole species (29, 42, 43), gerbils (44), and two species of hamster (45, 46). Because heat dissipation is affected by ambient temperature, hotter temperatures should lead to reduced lactation performance (milk production). This has been confirmed by exposing small mammals to temperatures up to 30 °C (38, 42–44, 46, 47). However, the impact is lower than expected. This may be because elevated temperatures are physiologically more favorable for offspring development (43), and/or because offspring modulate their behavior, mitigating the impact of reduced milk delivery by improving growth efficiency (32). It is unclear whether exposure to higher temperatures will have more significant negative effects or if the offspring will exhibit further responses to compensate. As a first step toward understanding these effects, we have studied here the responses of two captive lactating small rodent species to temperatures exceeding 30 °C.
Results
Body Mass.
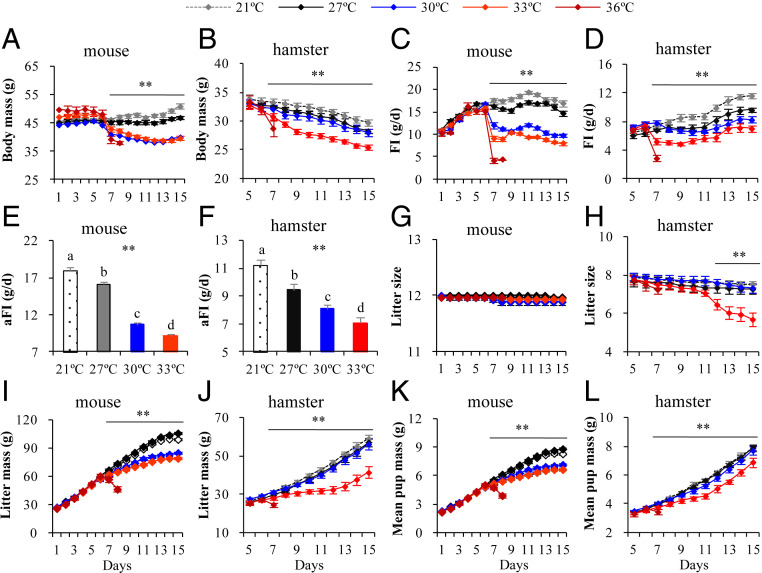
Using the mouse and striped hamster as model systems, we exposed lactating females in late lactation (from day 6 onward) to ambient temperatures of 21, 27, 30, 33, and 36 °C. Ambient temperature had an immediate significant negative effect on body mass (mice, day 7, F4,121 = 13.42, P < 0.01, Fig. 1A; hamsters, day 7, F4,122 = 4.03, P < 0.01, Fig. 1B). Body mass of the mice at 27 °C was similar to that of mice at 21 °C over the period of lactation, whereas body masses of mice in the 30 and 33 °C groups were lower by 21.3 and 22.0% on day 15 compared with those at 21 °C (day 15, F3,116 = 54.82, P < 0.01; Fig. 1A). Female mice at 36 °C lost on average 18.3% of body weight over the 24 h following temperature manipulation. At 36 °C, four out of six exposed lactating mothers died within 24 h of temperature exposure. Mass loss was greater in those that died (20 ± 0.4%) compared with those that survived (14.6 ± 2.1%). The experiment at 36 °C was terminated at this point. Female hamsters lactating at 33 °C showed significantly lower body mass than the groups kept at 21, 27, and 30 °C in late lactation (day 15, F3,115 = 11.92, P < 0.01; Fig. 1B). Consistent with the mice, two out of eight exposed lactating hamsters appeared to be in a dehydrated state and on the point of death within 24 h of temperature exposure to 36 °C and the whole group had lost on average 14.0% of their body weight the previous day. The hamster experiment at 36 °C was also terminated at this point. The two hamsters subsequently died.
Fig. 1.
Body mass (A and B), food intake (C and D), asymptotic food intake (E and F), litter size (G and H), litter mass (I and J), and mean pup mass (K and L) in female rodents lactating at different temperatures. Swiss mice: A, C, E, G, I, and K; striped hamsters: B, D, F, H, J, and L. Data are means ± SEM; asterisks indicate a significant effect of temperature (P < 0.05), **P < 0.01. Different letters above the columns indicate a significant difference among the four groups (P < 0.05).
Food Intake.
Ambient temperature had a significant effect on food intake from day 7 onward in both species (mice, day 7, F4,121 = 54.68, P < 0.01, Fig. 1C; hamsters, day 7, F4,60 = 19.65, P < 0.01, Fig. 1D). Female mice and hamsters lactating at 30 and 33 °C significantly decreased their food intake relative to females at 27 and 21 °C. The female mice and hamsters at 36 °C consumed only 4.1 ± 0.5 and 2.9 ± 0.4 g food, respectively, on day 7. The asymptotic food intake (aFI) was decreased by 10.5% at 27 °C, 40.6% at 30 °C, and 49.2% at 33 °C relative to the aFI at 21 °C in female mice (F3,117 = 208.13, P < 0.01; Fig. 1E), and decreased by 12.5, 24.8, and 36.9% in female hamsters (F3,53 = 24.00, P < 001; Fig. 1F).
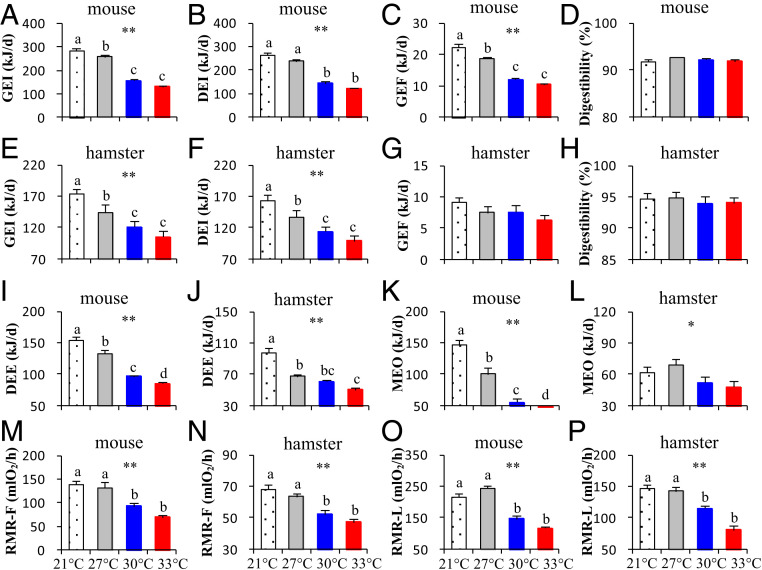
The female mice lactating at 30 and 33 °C showed significantly less energy intake (gross energy intake [GEI], Fig. 2A; digestible energy intake [DEI], Fig. 2B) and produced less feces compared with those at 21 and 27 °C (gross energy of feces [GEF], Fig. 2C). There was no effect of ambient temperature on digestibility (Fig. 2D). GEI and DEI of female hamsters were also significantly decreased at 30 and 33 °C compared with 21 °C (GEI, Fig. 2E; DEI, Fig. 2F). Temperature had no significant effect on GEF (Fig. 2G) and digestibility (Fig. 2H).
Fig. 2.
Gross and digestive energy intake, GE of feces, digestibility, daily energy expenditure, and milk energy expenditure, as well as resting metabolic rate of females and litters (RMR-M and RMR-L) during lactation at different temperatures. Swiss mice: A–D, I, K, M, and O; striped hamsters: E–H, J, L, N, and P. Data are means ± SEM; asterisks indicate a significant effect of temperature (P < 0.05), *P < 0.05, **P < 0.01. Different letters above the columns indicate a significant difference between the groups (P < 0.05).
Daily Energy Expenditure and Milk Energy Output.
DEE of female mice measured by doubly labeled water was inversely related to the ambient temperature and was reduced by 14.5% at 27 °C, 37.7% at 30 °C, and 45.1% at 33 °C compared with that at 21 °C (F3,39 = 68.29, P < 0.01; Fig. 2I). The calculated MEO from the difference between DEI and DEE of mice decreased strongly with increasing ambient temperature (F3,69 = 56.87, P < 0.01; Fig. 2K). In detail, the MEO averaged 146.6 ± 7.2 kJ/d in the females lactating at 21 °C, and was decreased by 31.8% at 27 °C, 61.9% at 30 °C, and 82.1% at 33 °C relative to that at 21 °C. In the hamsters, the DEE also declined significantly as the temperature increased (Fig. 2 J and L). In detail, the DEE was reduced by 31.5% at 27 °C, 37.6% at 30 °C, and 48.5% at 33 °C compared with that at 21 °C (F3,53 = 36.35, P < 0.01; Fig. 2J). However, the calculated MEO from the difference between metabolizable energy intake (MEI) and DEE of hamsters did not decline as much with increasing ambient temperature as it did in the mice (F3,53 = 2.96, P < 0.05; Fig. 2L). In detail, the MEO averaging 61.7 ± 5.4 kJ/d in the females lactating at 21 °C was not significantly different at 27 °C but was decreased by 15.2% at 30 °C and 21.9% at 33 °C relative to that at 21 °C.
Litter Mass.
Despite the large reduction in milk production, the litter size was not affected by ambient temperature in mice (Fig. 1G). However, female mice exposed to 30 and 33 °C raised significantly lighter litters than those at 21 and 27 °C on day 8 and onward (day 8, F4,121 = 3.69, P < 0.01; day 15, F3,117 = 61.48, P < 0.01; Fig. 1I). On average, pups weaned at 33 °C were 20.0% lighter than those at 21 °C (Fig. 1K). This was possible because of a large increase in growth efficiency, which was 22.0% at 21 °C and increased to 38.6% at 27 °C, 50.3% at 30 °C, and 63.3% at 33 °C (F3,38 = 3.59, P < 0.05). Although the hamster females managed to sustain their milk output much better than the mice, they and their pups seemed much less capable of dealing with the reduction. At 33 °C, litter size declined significantly in hamsters compared with that at 21, 27, and 30 °C (Fig. 1H), possibly due to infanticide. The female hamsters at 33 °C raised only 5.7 pups on day 15 of lactation, and it decreased by 24.5% compared with that at 21 °C. Female hamsters exposed to 33 °C raised a significantly lighter litter mass than the other three temperatures on day 7 and onward (day 7, F4,122 = 4. 94, P < 0.01; Fig. 1J). Litter mass was lower by 3.2% at 27 °C, 4.9% at 30 °C, and 30.0% at 33 °C compared with that at 21 °C. Pup mass was also significantly affected by temperature on day 7 and onward (day 7, F4,122 = 3. 27, P < 0.01; Fig. 1L), and the pups weaned at 33 °C were 12.7% lighter than those at 21 °C. On average, growth efficiency was 22.3% at 21 °C, and increased to 31.8% at 27 °C, 31.9% at 30 °C, and 36.7% at 33 °C (F3,53 = 2.52, P = 0.07).
Resting Metabolic Rate of Females and Litters.
RMR of female mice at 30 and 33 °C was decreased by 33.6 and 53.2%, respectively, compared with those at 21 and 27 °C (F3,74 = 6.35, P < 0.01; Fig. 2M). Consistent with these changes, RMR of female hamsters was also significantly decreased at 30 and 33 °C relative to 21 and 27 °C (F3,42 = 10.16, P < 0.01; Fig. 2N). RMR of litters was also significantly affected by temperature in both rodents, and the litters weaned at 30 and 33 °C showed significantly lower RMR than those weaned at 21 and 27 °C (mice, F3,74 = 12.67, P < 0.01, Fig. 2O; hamsters, F3,42 = 29.52, P < 0.01, Fig. 2P).
Body Mass and Body Temperature in Response to Extreme Hot Temperature.
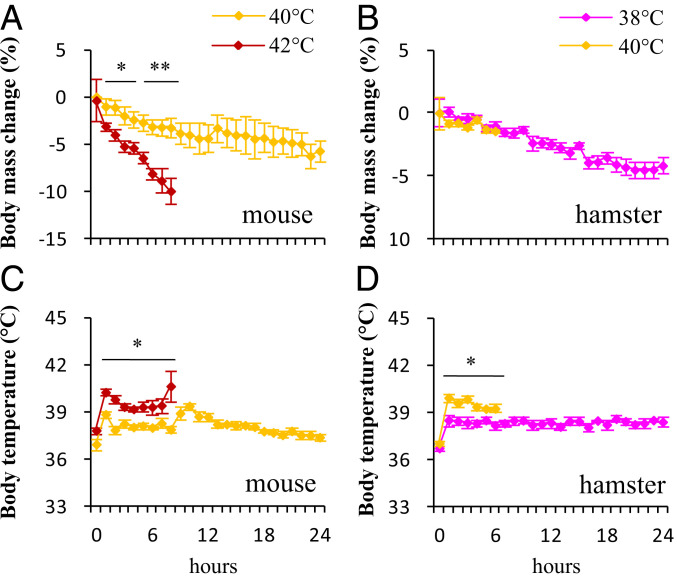
Nonlactating female mice survived at 40 °C for 24 h without difficulty (Fig. 3A). At 42 °C, two out of seven individuals looked like they were going to die after a 7-h exposure because they were moribund and unresponsive to stimulation. Therefore, the exposure experiment was terminated. Upper lethal ambient temperature for Swiss mice is likely between 40 and 42 °C. Body mass dropped considerably following the hot exposure in both groups, and it decreased by 5.7% over 24 h at 40 °C and by 10.0% over 8 h at 42 °C (40 °C, F23,115 = 4.63, P < 0.01; 42 °C, F4,42 = 21.84, P < 0.01; Fig. 3A). Nonlactating female hamsters survived at 38 °C for 24 h, but only survived at 40 °C for about 6 h before we terminated the experiment because several individuals appeared about to die (Fig. 3B), suggesting that upper lethal ambient temperature for striped hamsters may be between 38 and 40 °C. Body temperature (Tb) of female mice increased during the first hour of exposure, on average to 38.8 ± 0.2 °C at 40 °C and 40.2 ± 0.2 °C at 42 °C (hour 1, t11 = 5.44, P < 0.05; hour 8, t11 = 2.47, P < 0.05; Fig. 3C). The Tb of female hamsters increased significantly following hot exposure, and averaged 38.5 ± 0.4 °C at 38 °C and 39.9 ± 0.2 °C at 40 °C at 1 h of hot exposure (hour 1, t12 = 3.11, P < 0.05; hour 6, t12 = 2.95, P < 0.05; Fig. 3D).
Fig. 3.
Body mass change (A and B) and body temperature (C and D) in nonlactating females exposed to hot temperatures over 24 h. Swiss mice: 40 °C, n = 6; 42 °C, n = 7; striped hamsters: 38 °C, n = 7; 40 °C, n = 7. Data are means ± SEM; asterisks indicate a significant difference between the two groups (P < 0.05), *P < 0.05, **P < 0.01.
Discussion
Many previous studies have concerned the likely impacts of future climate change on reproduction in endotherms (birds and mammals) (48–52). The primary focus of such studies has been the potential problem of appropriate timing of reproductive effort, because the timing of maximal resource availability may change with temperature but photoperiodic triggers for breeding seasonality will not (53–58). Other studies have concerned the impact on survival of hibernating mammals (59). Here we present an issue that has not been previously addressed, which is the direct impact that elevated ambient temperatures, such as might be experienced in heat waves, will have on reproductive performance of small mammals. We emphasize this problem is independent of any impact on resource availability. Notably, the mice in the studies described here had access to ad libitum food supplies. Yet, as predicted from the heat dissipation limit theory, their lactation performance declined as temperatures rose. At 33 °C, the mice were only able to produce 18% of the amount of milk they did at 21 °C. In striped hamsters, we observed a similar but attenuated effect, with hamsters at 33 °C producing 78% of the milk they did at 21 °C.
This reduction in milk production, however, had a much lower impact on the offspring in mice. Total weaned litter mass at 33 °C was 70% of that at 21 °C, and there was only minimal pup mortality. This was possible because pups mitigated the impact of reduced milk delivery by increasing their growth efficiency by a factor of 3 between 21 and 33 °C. This was in part because of the reduced resting metabolic rates of the offspring, but probably also involved suspending physical activity (32). In contrast, in the hamsters at 33 °C there was significant pup mortality (possibly infanticide by the mother to reduce demand) and the growth efficiency was less responsive to the lowered milk supply. Hence, the hamster offspring at 33 °C weaned 12.7% lighter.
Although the two species responded somewhat differently to the increasing temperature, in both of them there seemed to be a catastrophic tipping point between 33 and 36 °C. At 36 °C, four out of six exposed lactating mouse mothers died within 24 h and two out of eight for the hamsters. They likely died because of hyperthermia brought on by attempting to sustain lactation. Excessive mass loss suggested the mortality was probably due to dehydration as the animals attempted to regulate body temperature via evaporation. This would be exacerbated by water loss in milk. This mortality was unexpected. Since we also showed the same strain of nonlactating mice exposed to 40 °C survives without problems for at least 24 h but struggles at 42 °C, and nonlactating hamsters survive at 38 °C but struggle at 40 °C, this suggests the upper lethal ambient temperature was reduced in both species by 3 to 6 °C by the process of lactation (60).
Impacts of high temperature on mortality during lactation are well-known for large domesticated mammals such as cattle and pigs (61, 62). This is expected because of their low surface-to-volume ratio, and hence their reduced heat dissipation abilities. Our data indicate that small lactating mammals with high surface-to-volume ratios may also be severely impacted by high ambient temperatures. Although the findings in captive mice and hamsters were clear, we should be cautious when expanding these observations to a prediction of what will happen in wild small mammals during future heat wave events. Swiss mice have been in captivity for about 200 to 300 generations. During this time, spent at a constant temperature of 20 to 23 °C, these animals may have lost any natural abilities to respond to extreme environmental conditions. Concern over this possibility was in part why we expanded the work to include striped hamsters. Our hamster colony was established from wild-caught animals in 2013 and had therefore only been in captivity for around 12 generations when the current measurements were made. Nevertheless, this period of captive breeding may also have blunted their response to the high-temperature conditions. There is a clear need therefore for this work to be repeated on wild mammals.
A second issue is that climate change will be a slow process that gets gradually worse. Small rodents in the wild will experience an exposure to gradually worsening events and this may allow a degree of adaptation and acclimation to occur. Hence, the final impacts of heat wave events at the turn of the century may be much lower than reported when contemporary individuals are suddenly exposed to similar experimental events. Hence, studies of the responses of acclimated animals would also be useful to obtain a deeper understanding of the likely impacts. Finally, animals in a cage have limited options compared with their wild counterparts in terms of things like the ability to find cooler microclimates as refugia or cooling themselves down by immersion in water. Thus, studies allowing animals to exhibit a range of mitigation strategies will also be a useful future step forward.
Methods
Ethical Review.
All of the experiments were reviewed and approved by the Institutional Review Board of the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (approval nos. AP20150005 and AP20150006). This study was not designed with death as an end point but rather particular temperature treatments were terminated when animals died unexpectedly.
Animals and Experimental Protocol.
Virgin female Swiss mice, 10 to 11 wk old, were obtained from a laboratory colony from the Experimental Animal Centre of Shandong Province, China. Striped hamsters 3.0 to 3.5 mo of age were obtained from our laboratory-breeding colony, which started with animals that were initially trapped from farmland at the center of Hebei Province (115°13′E, 38°12′S), North China Plain. They were housed individually in plastic cages (29 × 18 × 16 cm) with fresh sawdust bedding in the animal house located at Wenzhou University, where temperature was kept constant at 21 ± 1 °C with a 12:12-h light:dark cycle (lights on at 0800 hours). Food (D12450B; Research Diets) and water were available ad libitum.
Experiment 1 was designed to examine energy intake and reproductive performance in female mice and hamsters exposed to ambient temperatures ranging from 21 to 36 °C. The primary outcome variable was milk production. Based on the quantified variation from our previous measures of milk production at 21 °C in this mouse strain using the same procedures planned here (SD, 35 kJ/d), we performed a power analysis to define the sample size. This suggested that a sample size of 25 per group would be necessary to detect an effect size in milk production of 35 kJ/d (i.e., effect size, 1 SD) using a one-way ANOVA with five levels, with a power of 80% at alpha = 0.05. Consequently, 146 female mice and 185 female hamsters were paired with males for 11 d, after which the males were removed; 127 female mice and 127 female hamsters became pregnant and gave birth. On day 0 of lactation (parturition day), litters were manipulated to make the litter size 12 for all female mice and 8 for female hamsters. Females were randomly assigned to one of five temperature manipulation groups following parturition: 21, 27, 30, 33, and 36 °C groups. Mice and hamsters were kept at 21 °C until day 6 of lactation and then transferred to their allocated temperature. Among the first six female mice and eight female hamsters assigned to 36 °C, four female mice and two hamsters died within 24 h of temperature exposure, and the other two female mice and one hamster were close to death at the end of day 7. These two mice and one hamster were transferred back to 21 °C but did not contribute data to any analysis. Based on ethical considerations, we did not continue to expose further females to the 36 °C treatment. The remaining individuals that had been previously assigned to the 36 °C treatment were reallocated among the other groups. This resulted in final sample sizes for each group as follows: mice: 21 °C, n = 29; 27 °C, n = 34; 30 °C, n = 27; and 33 °C, n = 31; hamsters: 21 °C, n = 38; 27 °C, n = 26; 30 °C, n = 24; and 33 °C, n = 31. The females in the 21 °C group were maintained at 21 °C throughout lactation. All pups were weaned on day 16 of lactation. Body mass of females and litter mass were measured on a daily basis (to 0.1 g; Sartorius balance), and litter size and litter mass were also measured daily.
Food Intake, Gross Energy Intake, and Digestibility.
Food intake of females was measured on a daily basis throughout lactation. As described previously, food intake was calculated as the mass of food missing from the hopper every day, subtracting food residues mixed with the bedding (orts) (63). aFI was calculated based on the food intake between days 9 and 15. GEI and digestibility were measured between days 13 and 14 of lactation (64). Briefly, food was provided quantitatively at the start of day 13 of lactation, and uneaten food, orts, and feces were collected 24 h later. The spillage of food and feces was sorted and separated manually after they were dried at 60 °C for 10 d to constant mass. Gross energy contents of the diet and feces were determined using a bomb calorimeter (C2000). GEI, DEI, and digestibility were calculated using the equations GEI (kJ/d) = dry matter intake × energy content of food; GE of feces (kJ/d) = dry mass of feces × energy content of feces; DEI (kJ/d) = GEI − GE of feces; and digestibility (%) = DEI/GEI × 100% (65, 66).
RMR of Females and Litters.
RMR was quantified at weaning as the rate of oxygen consumption with an O2-measuring module high-speed sensor unit (994620-CS-HSP-01) for calorimetric measurements in an open-flow respirometry system (TSE PhenoMaster System). Air was pumped at a rate of 1 L/min through a cylindrical sealed Perspex chamber (1.5 L). Gases leaving the chamber were directed through the oxygen analyzer at a flow rate of 380 mL/min, and the data were collected every 1 min (TSE PhenoMaster System). The metabolic chamber temperature was controlled to within ±0.5 °C in an incubator. Both females and litters were measured at the temperature of 30.5 ± 0.5 °C (within the thermal neutral zone of laboratory mouse and striped hamsters) (60, 67). Neither females nor pups were deprived of food before the measurements started. After a 1-h adaptation to the chamber, oxygen consumption was recorded for 2.5 h at 1-min intervals, and for 1 h for the litters. Metabolic rate was calculated from the consecutively lowest readings over 10 min, using the following equation: VO2 = FR (FiO2 − FeO2)/(1 − FiO2 × (1 − RQ)), where FR is flow rate, FiO2 is input fractional concentration of O2 to the chamber, FeO2 is excurrent fractional concentration of O2 from the chamber, and RQ is respiratory quotient (68, 69). Here, RQ was assumed to be 0.85 (70). Metabolic rate was corrected to standard temperature and air pressure conditions and expressed as mL O2/h. Body mass was recorded before and after the measurements. All measurements were made between 1000 and 1700 hours.
Daily Energy Expenditure and Milk Energy Output.
Daily energy expenditure (kJ/d) was measured using the doubly labeled water (DLW) technique (71, 72). This method has been previously validated by comparison with indirect calorimetry in a range of small mammals (22). Briefly, on day 13 of lactation, females were weighed and injected intraperitoneally with ∼0.2 mL of DLW containing enriched 2H and 18O. The syringe was weighed (to 0.1 mg) using a Sartorius balance before and immediately after the injection. Females were re-placed in the home cages, and initial blood samples to estimate initial isotope enrichments were taken after 1 h of isotope equilibration (73). After exactly 24 h (to avoid circadian effects) (74, 75), final blood samples were taken to estimate isotope elimination rates. Baseline, initial, and final blood samples were collected by tail tipping for mice and from the orbital cavity for hamsters, and immediately sealed in two 60-µL glass capillaries using a handheld butane torch. Both ends of the capillaries were additionally sealed with sealing wax. The females and their offspring were also weighed at the time of DLW injection and final blood collection. Analysis of the isotopic enrichment of blood was performed blind to the experimental manipulations, using a liquid isotope water analyzer (Los Gatos Research) (76). Initially, the blood encapsulated in the capillaries was vacuum-distilled (77), and the resulting distillate was used for analysis. Samples were run alongside five laboratory standards for each isotope and international standards to correct delta values to parts per million. A single-pool model was used to calculate rates of CO2 production as recommended for use in animals less than 5 kg in body mass (78). There are several approaches for the treatment of evaporative water loss in the calculation (79). We assumed evaporation was 25% of the water flux (equation 7.17 in ref. 80), which minimizes error in a range of conditions (81, 82).
MEO was calculated from the difference between MEI and DEE, during which MEI was calculated from DEI × (100% − 3%), since urinary energy loss was assumed to be 3% of DEI (35). The growth efficiency of pups was calculated as the kilojoules of MEO over days 9 to 14 of lactation, assuming constant milk production per day over this period, divided by the kilojoules of growth over the same asymptote period (days 9 to 14 of lactation); kilojoules of growth was calculated as litter mass gain between days 9 and 14 multiplied by gross energy content of pups (35, 83).
Experiment 2 was designed to define the maximum temperature at which nonreproductive females could survive. Maximal survival temperature will be slightly lower than the upper lethal temperature, but has the benefit that it can be measured without killing individuals via hyperthermia, which is necessary to establish upper lethal temperature. Thirteen female mice and 14 female hamsters maintained at 21 °C, age 9 to 10 wk, were randomly assigned to two hot-temperature groups. Based on the lethal core Tb of laboratory mice described by Gordon (60), 40 and 42 °C were used as the two levels of hot ambient temperatures for mice and 38 and 40 °C for hamsters. The two groups of mice were exposed to 40 °C (n = 6) or 42 °C (n = 7), and hamsters were exposed to 38 °C (n = 7) and 40 °C (n = 7) for 24 h. The animals were watched continuously, and body mass and Tb were measured at hourly intervals. Body mass was measured to 0.1 g (Sartorius balance). Tb was measured using an encapsulated thermosensitive passive transponder (diameter 2 mm and length 14 mm; Destron Fearing). The transponder was implanted abdominally 1 wk before the hot exposure, and a pocket reader was then used to receive and collect Tb data. The female mice were not obviously uncomfortable at 40 °C and female hamsters were not too hot at 38 °C, whereas after 8 h of exposure to 42 °C for mice and 6 h of exposure to 40 °C for hamsters, two out of seven mice and one out of seven hamsters were stationary and unresponsive and appeared likely to die. In this situation, the experiment was terminated and all of the female mice at 42 °C and the hamsters at 40 °C were transferred back to 21 °C. Hence, no animals died as a result of the manipulations.
Statistics.
Data were analyzed using SPSS 21.0 statistical software. Distributions of all variables were tested for normality using the Kolmogorov–Smirnov test. In experiment 1, effects of temperature on the measurements, including body mass, GEI, DEI, DEE, MEO, and litter size and litter mass, were analyzed using one-way ANOVA, followed by Tukey’s honestly significant difference post hoc tests where appropriate. In experiment 2, the changes of body mass and Tb over the period of hot exposure were examined using repeated-measures ANOVA. The effects of temperature on body mass change and Tb were examined using an independent t test. Data are reported as means ± SEM. Statistical significance was determined at P < 0.05.
Acknowledgments
This work was partly supported by grants (31670417, 31870388) from the National Natural Science Foundation of China and the Chinese Academy of Sciences Strategic Program (XDB13030100). We thank Song Tan and Jing Wen from Wenzhou University for their assistance with animal care, and Peter Thomson and Marina Stamatiou from the University of Aberdeen for technical assistance with isotope analysis.
Footnotes
The authors declare no competing interest.
Data Availability.
All relevant data have be placed on the Open Science framework (identifier: DOI 10.17605/OSF.IO/WBNG9).
References
- 1.Intergovernmental Panel on Climate Change (IPCC) , “Climate change” in Impacts, Adaptation, and Vulnerability, Field C. B., Ed. (Cambridge University Press, Cambridge, UK, 2014), pp. 1–32. [Google Scholar]
- 2.Stocker T., et al. , Eds., “IPCC, 2013: Climate change 2013: The physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change” (Cambridge University Press, Cambridge, UK, 2013).
- 3.Christidis N., Jones G. S., Stott P. A., Dramatically increasing chance of extremely hot summers since the 2003 European heatwave. Nat. Clim. Chang. 5, 46–50 (2014). [Google Scholar]
- 4.Guirguis K., Gershunov A., Cayan D. R., Pierce D. W., Heat wave probability in the changing climate of the southwest US. Clim. Dyn. 50, 3853–3864 (2018). [Google Scholar]
- 5.Schär C. et al., The role of increasing temperature variability in European summer heatwaves. Nature 427, 332–336 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Meehl G. A., Tebaldi C., More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Diffenbaugh N. S., Scherer M., Observational and model evidence of global emergence of permanent, unprecedented heat in the 20(th) and 21(st) centuries. Clim. Change 107, 615–624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn R. J. H., Mead N. E., Willett K. M., Parker D. E., Analysis of heat stress in UK dairy cattle and impact on milk yields. Environ. Res. Lett. 9, 064006 (2014). [Google Scholar]
- 9.Oleson K. W., Anderson G. B., Jones B., McGinnis S. A., Sanderson B., Avoided climate impacts of urban and rural heat and cold waves over the U.S. using large climate model ensembles for RCP8.5 and RCP4.5. Clim. Change 146, 377–392 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin J. M., Mead J. I., Barboza P. S., Bison body size and climate change. Ecol. Evol. 8, 4564–4574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehn L., McCormick S., Heat exposure and maternal health in the face of climate change. Int. J. Environ. Res. Public Health 14, 853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazdiyasni O. et al., Increasing probability of mortality during Indian heat waves. Sci. Adv. 3, e1700066 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo Y. T. E. et al., Increasing mitigation ambition to meet the Paris Agreement’s temperature goal avoids substantial heat-related mortality in U.S. cities. Sci. Adv. 5, eaau4373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poumadère M., Mays C., Le Mer S., Blong R., The 2003 heat wave in France: Dangerous climate change here and now. Risk Anal. 25, 1483–1494 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Mifsud K. R. et al., Epigenetic mechanisms in stress and adaptation. Brain Behav. Immun. 25, 1305–1315 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Stawski C., Geiser F., Will temperature effects or phenotypic plasticity determine the thermal response of a heterothermic tropical bat to climate change? PLoS One 7, e40278 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovegrove B. G. et al., Are tropical small mammals physiologically vulnerable to Arrhenius effects and climate change? Physiol. Biochem. Zool. 87, 30–45 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Godde C. et al., Climate change and variability impacts on grazing herds: Insights from a system dynamics approach for semi-arid Australian rangelands. Glob. Change Biol. 25, 3091–3109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radchuk V. et al., Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 10, 3109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiniou N., Noblet J., Influence of high ambient temperatures on performance of multiparous lactating sows. J. Anim. Sci. 77, 2124–2134 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Speakman J. R., Krol E., Comparison of different approaches for the calculation of energy expenditure using doubly labeled water in a small mammal. Physiol. Biochem. Zool. 78, 650–667 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Speakman J. R., Król E., Limits to sustained energy intake. XIII. Recent progress and future perspectives. J. Exp. Biol. 214, 230–241 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Hammond K. A., Diamond J., Maximal sustained energy budgets in humans and animals. Nature 386, 457–462 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Ohrnberger S. A., Hambly C., Speakman J. R., Valencak T. G., Limits to sustained energy intake. XXIX. The case of the golden hamster (Mesocricetus auratus). J. Exp. Biol. 221, jeb183749 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Hammond K. A., Konarzewski M., Torres R. M., Diamond J., Metabolic ceilings under a combination of peak energy demands. Physiol. Zool. 67, 1479–1506 (1994). [Google Scholar]
- 27.Hammond K. A., Kristan D. M., Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus). Physiol. Biochem. Zool. 73, 547–556 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Johnson M. S., Thomson S. C., Speakman J. R., Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 204, 1925–1935 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Sadowska E. T. et al., Limits to sustained energy intake. XXIII. Does heat dissipation capacity limit the energy budget of lactating bank voles? J. Exp. Biol. 219, 805–815 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Sadowska J., Gębczyński A. K., Lewoc M., Konarzewski M., Not that hot after all: No limits to heat dissipation in lactating mice selected for high or low BMR. J. Exp. Biol. 222, jeb204669 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Valencak T. G., Hackländer K., Ruf T., Peak energy turnover in lactating European hares: A test of the heat dissipation limitation hypothesis. J. Exp. Biol. 213, 2832–2839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valencak T. G. et al., Limits to sustained energy intake. XXI. Effect of exposing the mother, but not her pups, to a cold environment during lactation in mice. J. Exp. Biol. 216, 4326–4333 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Król E., Speakman J. R., Switching off the furnace: Brown adipose tissue and lactation. Mol. Aspects Med. 68, 18–41 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Król E., Speakman J. R., Limits to sustained energy intake. VI. Energetics of lactation in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4255–4266 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Król E., Speakman J. R., Limits to sustained energy intake. VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4267–4281 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Vilas Boas Ribeiro B. P. et al., Heat negatively affects lactating swine: A meta-analysis. J. Therm. Biol. 74, 325–330 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Koch F. et al., Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. U.S.A. 116, 10333–10338 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Król E., Johnson M. S., Speakman J. R., Limits to sustained energy intake. VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 206, 4283–4291 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Król E., Murphy M., Speakman J. R., Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 210, 4233–4243 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Speakman J. R., Król E., Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z. J. et al., Limits to sustained energy intake XXV: Milk energy output and thermogenesis in Swiss mice lactating at thermoneutrality. Sci. Rep. 6, 31626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S. H., Zhang L. N., Speakman J. R., Wang D. H., Limits to sustained energy intake. XI. A test of the heat dissipation limitation hypothesis in lactating Brandt’s voles (Lasiopodomys brandtii). J. Exp. Biol. 212, 3455–3465 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Simons M. J. P. et al., Ambient temperature shapes reproductive output during pregnancy and lactation in the common vole (Microtus arvalis): A test of the heat dissipation limit theory. J. Exp. Biol. 214, 38–49 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Yang D. B. et al., Limits to sustained energy intake. XIX. A test of the heat dissipation limitation hypothesis in Mongolian gerbils (Meriones unguiculatus). J. Exp. Biol. 216, 3358–3368 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Zhao Z. J., Chi Q. S., Cao J., Limits to sustainable energy budget during lactation in the striped hamster (Cricetulus barabensis) raising litters of different size. Zoology (Jena) 113, 235–242 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Ohrnberger S. A., Monclús R., Rödel H. G., Valencak T. G., Ambient temperature affects postnatal litter size reduction in golden hamsters. Front. Zool. 13, 51 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Z. J., Energy budget during lactation in striped hamsters at different ambient temperatures. J. Exp. Biol. 214, 988–995 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Molnár P. K., Derocher A. E., Klanjscek T., Lewis M. A., Predicting climate change impacts on polar bear litter size. Nat. Commun. 2, 186 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milligan S. R., Holt W. V., Lloyd R., Impacts of climate change and environmental factors on reproduction and development in wildlife. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3313–3319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rezende E. L., Bacigalupe L. D., Thermoregulation in endotherms: Physiological principles and ecological consequences. J. Comp. Physiol. B 185, 709–727 (2015). [DOI] [PubMed] [Google Scholar]
- 51.McKinnon L., Nol E., Juillet C., Arctic-nesting birds find physiological relief in the face of trophic constraints. Sci. Rep. 3, 1816 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson I. R., Bryant D. M., Climate change and constraints on breeding. Nature 406, 366–367 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Thomas D. W., Blondel J., Perret P., Lambrechts M. M., Speakman J. R., Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291, 2598–2600 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Bronson F. H., Climate change and seasonal reproduction in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3331–3340 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Post E., Forchhammer M. C., Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2369–2375 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNab B. K., Geographic and temporal correlations of mammalian size reconsidered: A resource rule. Oecologia 164, 13–23 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Morellet N. et al., Seasonality, weather and climate affect home range size in roe deer across a wide latitudinal gradient within Europe. J. Anim. Ecol. 82, 1326–1339 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Patz J. A., Campbell-Lendrum D., Holloway T., Foley J. A., Impact of regional climate change on human health. Nature 438, 310–317 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Humphries M. M., Thomas D. W., Speakman J. R., Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418, 313–316 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Gordon C. J., Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 37, 654–685 (2012). [Google Scholar]
- 61.Crescio M. I., Forastiere F., Maurella C., Ingravalle F., Ru G., Heat-related mortality in dairy cattle: A case crossover study. Prev. Vet. Med. 97, 191–197 (2010). [DOI] [PubMed] [Google Scholar]
- 62.D’Allaire S., Drolet R., Brodeur D., Sow mortality associated with high ambient temperatures. Can. Vet. J. 37, 237–239 (1996). [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao Z. J. et al., Limits to sustained energy intake. XVIII. Energy intake and reproductive output during lactation in Swiss mice raising small litters. J. Exp. Biol. 216, 2349–2358 (2013). [DOI] [PubMed] [Google Scholar]
- 64.Zhang J. Y., Zhao X. Y., Wen J., Tan S., Zhao Z. J., Plasticity in gastrointestinal morphology and enzyme activity in lactating striped hamsters (Cricetulus barabensis). J. Exp. Biol. 219, 1327–1336 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Grodzinski W., Wunder B. A., “Ecological energetics of small mammals” in Small Mammals: Their Productivity and Copulation Dynamics, Golley E. B., Petrusewiez K., Ryszkowski L., Eds. (Cambridge University Press, Cambridge, UK, 1975), pp. 173–204. [Google Scholar]
- 66.Zhao Z. J. et al., Effect of food restriction on energy budget in warm-acclimated striped hamsters. Physiol. Behav. 147, 220–226 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Speakman J. R., Rossi F. P., No support for socio-physiological suppression effect on metabolism of paired white mice (Mus sp.). Funct. Ecol. 13, 373–382 (1999). [Google Scholar]
- 68.Arch J. R., Hislop D., Wang S. J., Speakman J. R., Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. 30, 1322–1331 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Chi Q. S., Wang D. H., Thermal physiology and energetics in male desert hamsters (Phodopus roborovskii) during cold acclimation. J. Comp. Physiol. B 181, 91–103 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Withers P. C., Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J. Appl. Physiol. 42, 120–123 (1977). [DOI] [PubMed] [Google Scholar]
- 71.Lifson N., McClintock R., Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 12, 46–74 (1966). [DOI] [PubMed] [Google Scholar]
- 72.Butler P. J., Green J. A., Boyd I. L., Speakman J. R., Measuring metabolic rate in the field: The pros and cons of the doubly-labelled water and heart rate methods. Funct. Ecol. 18, 168–183 (2004). [Google Scholar]
- 73.Król E., Speakman J. R., Isotope dilution spaces of mice injected simultaneously with deuterium, tritium and oxygen-18. J. Exp. Biol. 202, 2839–2849 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Speakman J. R., Racey P. A., The equilibrium concentration of O-18 in body-water—Implications for the accuracy of the doubly-labeled water technique and a potential new method of measuring RQ in free-living animals. J. Theor. Biol. 127, 79–95 (1987). [Google Scholar]
- 75.Speakman J. R., Racey P. A., Consequences of non-steady state CO2 production for accuracy of the doubly-labeled water technique—The importance of recapture interval. Comp. Biochem. Physiol. 90, 337–340 (1988). [Google Scholar]
- 76.Berman E. S. et al., Direct analysis of δ2H and δ18O in natural and enriched human urine using laser-based, off-axis integrated cavity output spectroscopy. Anal. Chem. 84, 9768–9773 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagy K. A., “The doubly labeled water (3HH18O) method: A guide to its use” (Publication No. 12-1417, University of California Los Angeles, 1983).
- 78.Speakman J. R., How should we calculate CO2 production in doubly labeled water studies of animals? Funct. Ecol. 7, 746–750 (1993). [Google Scholar]
- 79.Visser G. H., Schekkerman H., Validation of the doubly labeled water method in growing precocial birds: The importance of assumptions concerning evaporative water loss. Physiol. Biochem. Zool. 72, 740–749 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Speakman J. R., Ed., Doubly Labelled Water. Theory and Practice, (Chapman & Hall, London, 1997). [Google Scholar]
- 81.Van Trigt R. et al., Validation of the DLW method in Japanese quail at different water fluxes using laser and IRMS. J. Appl. Physiol. 93, 2147–2154 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Drożdż A., “Metabolic cages for small mammals” in Methods for Ecological Bioenergetics, International Biological Programme Handbook, No. 24, Grodzinski W., Klekowski R. Z., Duncan A., Eds. (Blackwell Scientific, Oxford, 1975), pp. 346–351. [Google Scholar]
- 83.Zhao Z. J., Chi Q. S., Cao J., Milk energy output during peak lactation in shaved Swiss mice. Physiol. Behav. 101, 59–66 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data have be placed on the Open Science framework (identifier: DOI 10.17605/OSF.IO/WBNG9).