Abstract
Oxidative stress plays a key role in the pathophysiology of post-cardiac arrest syndrome. Molecular hydrogen reduces oxidative stress and exerts anti-inflammatory effects in an animal model of cardiac arrest. However, its effect on human post-cardiac arrest syndrome is unclear. We consecutively enrolled five comatose post-cardiac arrest patients (three males; mean age, 65 ± 15 years; four cardiogenic, one septic cardiac arrest) and evaluated temporal changes in oxidative stress markers and cytokines with inhaled hydrogen. All patients were treated with target temperature management. Hydrogen gas inhalation (2% hydrogen with titrated oxygen) was initiated upon admission for 18 h. Blood hydrogen concentrations, plasma and urine oxidative stress markers (derivatives of reactive oxygen metabolites, biological antioxidant potential, 8-hydroxy-2'-deoxyguanosine, Nɛ-hexanoyl-lysine, lipid hydroperoxide), and cytokines (interleukin-6 and tumor necrosis factor-α) were measured before and 3, 9, 18, and 24 h after hydrogen gas inhalation. Arterial hydrogen concentration was measurable and it was equilibrated with inhaled hydrogen. Oxidative stress was reduced and cytokine levels were unchanged in cardiogenic patients, whereas oxidative stress was unchanged and cytokine levels were diminished in the septic patient. The effect of inhaled hydrogen on oxidative stress and cytokines in comatose post-cardiac arrest patients remains indefinite because of methodological weaknesses.
Keywords: molecular hydrogen gas, anti-oxidative effect, anti-inflammatory effect, target temperature management
Introduction
Despite improvements in cardiopulmonary resuscitation and comprehensive post-arrest care, out-of-hospital cardiac arrest (OHCA) remains a major source of mortality in developed countries.(1) Post-cardiac arrest syndrome (PCAS) comprises a complex combination of pathophysiological processes that are mainly characterized by brain and myocardial injury and a systemic ischemia/reperfusion (I/R) response.(2) A dramatic increase in reactive oxygen species (ROS) generation occurs with the return of spontaneous circulation (ROSC), and oxidative stress elicited by ROS is recognized as having a central role in the development, progression, and pathophysiology of PCAS.(2) Cardiac arrest (CA) and resuscitation results in an acute phase response that has been compared to sepsis, as PCAS is often referred to as a sepsis-like syndrome.(3) I/R is associated with the activation of several interleukins (ILs), tumor necrosis factor (TNF)-α, and other cytokines.(3–6) Of note, levels of inflammation are independently associated with increased mortality with OHCA.(7)
Target temperature management (TTM) is the only clinically-validated treatment option to improve neurological outcomes associated with PCAS.(8,9) The neuroprotective mechanisms of TTM include the attenuation of oxidative stress and anti-inflammatory effects.(10–13) However, recent studies reported that TTM does not exert beneficial effects on cytokine levels in PCAS.(6,14–17)
Molecular hydrogen (H2) is a mild antioxidant that selectively reduces highly active oxidants such as hydroxyl radicals and peroxynitrites.(18) A growing body of evidence has indicated the pleiotropic effects of H2 including anti-inflammatory and anti-apoptotic effects, in addition to anti-oxidative effects after I/R.(19–21) Inhaled H2 gas attenuates oxidative stress in the heart and brain, suppresses the activation of microglia and apoptosis, and improves neurological outcomes in a rodent cardiac arrest model.(15,22) Further, the neuroprotective effects of perioperatively inhaled H2 gas were reported using a large animal porcine model of cardiopulmonary bypass.(23) Recently, we also clinically translated the H2 gas inhalation method for humans with PCAS.(24) Although reported in animal studies, the effect of H2 on oxidative stress and inflammatory cytokines in human PCAS has not been studied. Thus, the aim of the present study was to evaluate these effects using human PCAS subjects.
Materials and Methods
Study design
This was a sub-study of a single-center, open-label, single-arm, prospective intervention study, with the primary interest being the feasibility and safety of inhaled H2 gas for patients with PCAS (Clinical trial identifier: UMIN000012381).(24) The study protocol was approved by the ethics committee of Keio University (Approval number: 20130075). Written informed consent was obtained from the patient’s family and from each patient who regained the necessary mental capacity.
Selection of patients
We used the same subjects from a previous study.(24) We consecutively enrolled five eligible patients between January 1, 2014 and January 19, 2015. The inclusion and exclusion criteria were reported previously.(24) Briefly, adult patients between 20 and 80 years of age who experienced non-traumatic OHCA and sustained a coma, in which the individual could not follow verbal commands (Glasgow Coma Scale, ≤8) after successful resuscitation, were included. Patients with pre-existing severe neurological dysfunction, oxygen saturation less than 95% with 50% oxygen inhalation, systolic blood pressure less than 90 mmHg after adequate fluids and the use of catecholamines, and those who could not initiate H2 gas inhalation within 12 h of ROSC were excluded.
Hydrogen gas inhalation
H2 gas inhalation (2% H2 with oxygen) was initiated using a ventilator system as previously described.(24) H2 gas inhalation was initiated upon ICU admission and was continued for 18 h in conjunction with general post-arrest care (Supplemental Fig. 1*).
General management of post-cardiac arrest patients
Multidisciplinary post-arrest care in accordance with the latest guidelines at the time was provided for each patient.(1,25) All patients were managed with target temperature management between 34 and 36°C (see Supplementary materials* for detailed treatment protocols).
Measurement of arterial hydrogen gas concentrations
Gas chromatography analysis was performed to determine the arterial H2 gas concentration as described previously.(18) Briefly, 1 ml of arterial blood was drawn before and 3, 9, 18, and 24 h after the initiation of H2 gas inhalation. Drawn arterial blood was immediately injected into a closed aluminum bag containing 25 ml of air and stored at room temperature until analysis. After complete transfer of the H2 gas from the blood to the air in the closed bag, 1 ml of air containing H2 gas was drawn from the bag and H2 gas concentration was measured by gas chromatography analysis (Breath Gas Analyzer, Model TGA2000; TERAMECS Co. Ltd., Kyoto, Japan). Measurements were performed within 5 days of collection. The arterial H2 gas concentration was calculated based on comparisons with a calibration curve of standardized concentrations of H2 gas [standard H2 gas concentrations: 0.43, 0.78, 1.3, 2.5, 5, and 10% v/v (Taiyo Nippon Sanso Corporation, Tokyo, Japan)].
Measurement of oxidative stress markers and inflammatory cytokines
Arterial blood was collected before and 3, 9, 18, and 24 h after the initiation of H2 gas inhalation. Immediately after collection, blood was centrifuged at 1,500 × g for 10 min at room temperature and collected plasma was snap frozen with liquid nitrogen and stored at –80°C until analysis. Urine was collected before and 24 h after the initiation of H2 gas inhalation through the urethral catheter and was frozen at –80°C. Plasma concentrations of oxidative stress markers and inflammatory cytokines were measured at the aforementioned five time points. Urine oxidative stress markers were measured before and 24 h after hydrogen gas inhalation. Oxidative stress markers and inflammatory cytokines were selected based on prior knowledge.(3,6,11,15,16,22)
Oxidative and anti-oxidative activities were assessed by measuring derivatives of reactive oxygen metabolites (dROMs) and biological antioxidant potential (BAP),(26–29) respectively, using FREE Carpe Diem (Diacron International, Grosseto, Italy) as per the manufacturer’s instructions. Plasma and urine 8-hydroxy-2'-deoxyguanosine (8-OHdG), an index of oxidative DNA damage, as well as Nɛ-hexanoyl-lysine (HEL) and lipid hydroperoxide (LPO), an index of lipid peroxidation, were measured with respective ELISA kits at the Japan Institute for the Control of Aging (JaICA), Shizuoka, Japan. Urine 8-OHdG, HEL, and 8-isoprostane, also an index of lipid peroxidation, were measured with respective ELISA kits at JaICA, Shizuoka, Japan. Urine oxidative stress marker levels were corrected based on urine creatinine values. Plasma IL-6 and TNF-α were measured with a chemiluminescent enzyme immunoassay (Quanti Glo ELISA Human IL-6 Immunoassay and Quanti Glo ELISA Human TNF-α Chemiluminescent 2nd generation, respectively; both from R&D Systems, Minneapolis, MN). Plasma samples were obtained from all subjects (n = 5), and urine samples were obtained from four subjects.
Statistical analysis
Variables were presented as the mean ± SD. A one-way repeated analysis of variance followed by a Tukey-Kramer multiple comparison test, paired t test, or Wilcoxon matched-pairs signed ranks-test was used when appropriate. Data are presented as the mean ± SD, unless otherwise indicated. A p value <0.05 was considered statistically significant. All statistical analyses were conducted using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA).
Results
Patient characteristics
Patient characteristics were reported previously.(24) Briefly, three patients (60%) were male, and the average age was 65 ± 15 years. One patient had a history of chronic kidney disease and was on maintenance hemodialysis, and other past medical histories are shown in Table 1. Four patients (80%) were diagnosed with cardiac etiology, and one patient experienced non-cardiogenic CA from hemodynamic deterioration due to sepsis with pneumonia. The estimated time from collapse to ROSC and ROSC to initiation of H2 gas inhalation was 16 ± 4.7 min and 4.9 ± 1.2 h, respectively. Four patients who experienced CA with a cardiac cause were treated with TTM at 34°C, and TTM at 36°C was selected for one patient with sepsis. The time interval from ROSC to reach target temperature was 6.4 ± 3.7 h. Respiratory conditions were stable up to 24 h after H2 gas inhalation (Table 2). The mean arterial pressure was well maintained in all patients during H2 gas inhalation and TTM with the continuous infusion of catecholamines (Table 3). Four patients with cardiac causes survived 90 days with a favorable neurological outcome (cerebral performance category of 1; Patients 2–5), whereas one patient with sepsis (Patient 1) died of worsened pneumonia 22 h after the discontinuation of H2 inhalation.(24)
Table 1.
Past medical history
| Patient | Active | Inactive |
|---|---|---|
| 1 | None | |
| 2 | AF, CHF, ESRD | Rectal cancer |
| 3 | None | |
| 4 | GERD, HOCM, hypertension | Psoriatic arthritis |
| 5 | Dyslipidemia, HOCM | ASD, IE |
AF, atrial fibrillation; ASD, atrial septum defect; CHF, chronic heart failure; ESRD, end stage renal disease; GERD, gastroesophageal reflux disease; HOCM, hypertrophic obstructive cardiomyopathy; IE, infectious endocarditis.
Table 2.
Arterial gas analysis in patients with post-cardiac arrest syndrome
| Admission | 3 h | 9 h | 18 h | 24 h | |
|---|---|---|---|---|---|
| FiO2 | 0.48 ± 0.04 | 0.48 ± 0.04 | 0.48 ± 0.04 | 0.48 ± 0.04 | 0.52 ± 0.2 |
| PEEP (cm H2O) | 6.0 ± 2.2 | 6.0 ± 2.2 | 6.0 ± 2.2 | 6.0 ± 2.2 | 7.0 ± 2.7 |
| pH | 7.366 ± 0.08 | 7.344 ± 0.1 | 7.409 ± 0.06 | 7.364 ± 0.05 | 7.391 ± 0.09 |
| PCO2 (mmHg) | 43.3 ± 7.9 | 42.4 ± 4.6 | 39.0 ± 6.0 | 42.7 ± 4.5 | 41.7 ± 9.5 |
| PO2 (mmHg) | 136 ± 14 | 147 ± 53 | 135 ± 31 | 150 ± 64 | 126 ± 26 |
| HCO3− (mmol/L) | 23.9 ± 1.0 | 22.2 ± 3.2 | 24.0 ± 1.4 | 23.7 ± 1.1 | 24.3 ± 0.6 |
| B.E. (mmol/L) | –0.8 ± 1.8 | –2.4 ± 3.6 | –0.1 ± 1.6 | –0.9 ± 1.5 | –0.6 ± 1.7 |
| Lactate (mmol/L) | 1.38 ± 0.4 | 5.08 ± 6.6 | 2.72 ± 2.0 | 2.5 ± 2.4 | 1.45 ± 0.5 |
Hours indicate time after the initiation of hydrogen gas inhalation. B.E., base excess; FiO2, fraction of oxygen; HCO3−, bicarbonate ion; PCO2, pressure of carbon dioxide; PEEP, positive end expiratory pressure; PO2, pressure of oxygen.
Table 3.
Mean arterial pressure and catecholamine dosage in patients with post-cardiac arrest syndrome
| Admission | 3 h | 9 h | 18 h | 24 h | |
|---|---|---|---|---|---|
| MAP (mmHg) | 88.0 ± 12.3 | 85.9 ± 22.8 | 91.1 ± 20.0 | 94.3 ± 11.4 | 88.0 ± 24.6 |
| Norepinephrine (µg/kg/min) | 0.06 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.05 ± 0.1 | 0.1 ± 0.1 |
| Dopamine (µg/kg/min) | 0 | 0 | 0 | 0.6 ± 1.3 | 1.6 ± 3.6 |
| Dobutamine (µg/kg/min) | 0.6 ± 1.3 | 0.6 ± 1.3 | 0.6 ± 1.3 | 0.5 ± 1.1 | 0 |
Hours indicate time after the initiation of hydrogen gas inhalation. MAP, mean arterial pressure.
Blood hydrogen gas concentrations
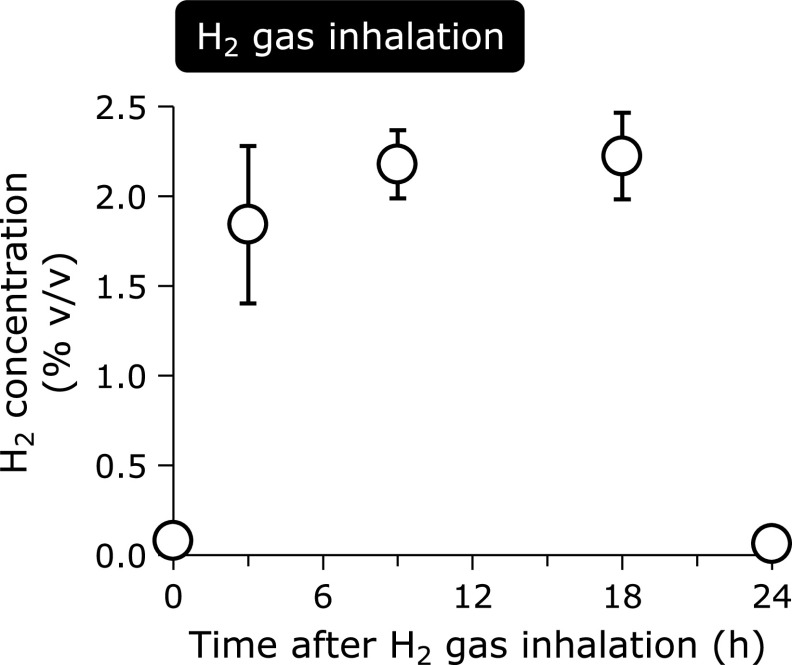
Arterial H2 concentrations reached approximately 2% during H2 gas inhalation, but it was washed out after the discontinuation of inhalation at 24 h (Fig. 1). Measurements of arterial H2 at low concentrations were imprecise since it was calculated from the calibration curve.
Fig. 1.
Arterial hydrogen concentrations in patients with post-cardiac arrest syndrome treated with H2 gas inhalation. Data are presented as the mean ± SD. H2 concentrations were measured by gas chromatography analysis based on comparisons with a calibration curve of standardized concentrations of H2 gas. Arterial H2 concentrations reached approximately 2% during inhalation. Arterial H2 concentrations decreased after discontinuing H2 gas inhalation. H2, molecular hydrogen; % v/v, volume percent.
Temporal trends in oxidative stress markers
dROM was significantly increased 3 h post-H2 gas inhalation and was maintained during H2 gas inhalation but decreased at 24 h after H2 gas inhalation (Fig. 2A). BAP significantly decreased during H2 gas inhalation. In contrast to that observed for dROM, a slight increase in BAP levels was observed after the discontinuation of H2 gas inhalation (Fig. 2B). The BAP over dROM ratio, which indicates a relative tolerance to oxidative stress, was decreased approximately linearly between 3 and 18 h of H2 gas inhalation and sharply increased after completing H2 gas inhalation (Fig. 2C). Of note, all patients were treated with TTM during H2 gas inhalation and body temperature was maintained at the target temperature (maintenance phase) between 6.4 and 24 h of the observation period (Supplemental Fig. 1*).
Fig. 2.
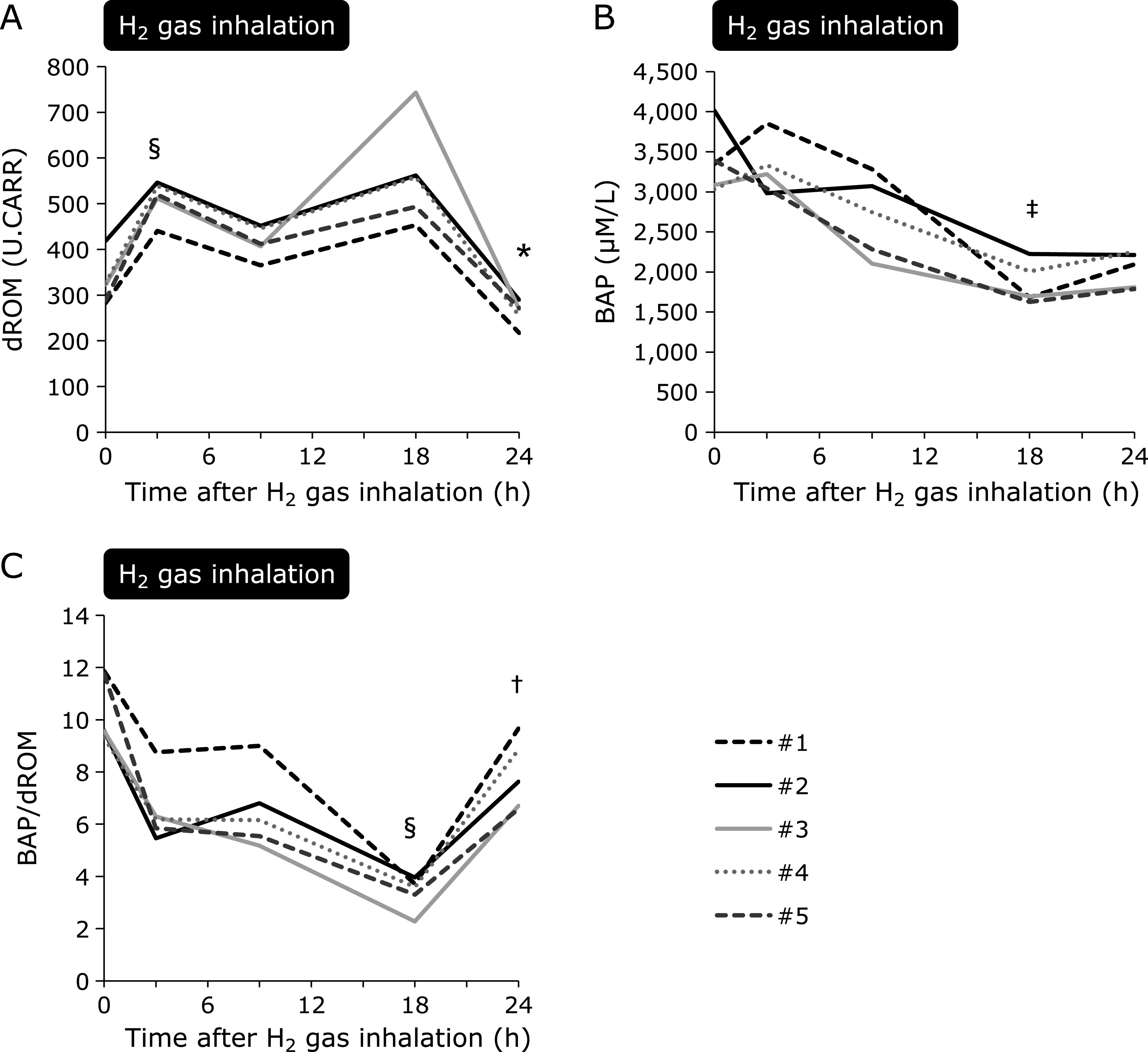
Changes in plasma dROMs and BAP with H2 gas inhalation. Each line indicates an individual patient. The black broken line indicates the patient with sepsis. (A) dROM values were 338.8 ± 55.8 U.CARR before H2 inhalation. After an increase to 511.4 ± 42.0 U.CARR at 3 h post-H2 gas inhalation (p<0.001, compared to 0 h), dROM levels were maintained during H2 gas inhalation but decreased to 271.4 ± 14.6 U.CARR 24 h after H2 gas inhalation (p = 0.01, compared with 18 h). (B) BAP was 3,379.6 ± 450.6 µM/L before H2 gas inhalation, and it decreased during H2 gas inhalation (p = 0.001, compared to 0 h). In contrast to that observed for dROMs, a slight increase in BAP levels was observed after the discontinuation of H2 gas inhalation (2,014.9 ± 251.4 µM/L at 24 h; p = 0.23, compared to 18 h). (C) The BAP to dROM ratio was reduced during H2 inhalation (p<0.001) and increased after completing H2 gas inhalation (p = 0.004, 18 h vs 24 h). All patients were still being treated with target temperature management (TTM) at 24 h. *p = 0.01, compared to 18 h; †p = 0.004, compared to 18 h; ‡p = 0.001, compared to 0 h; §p<0.001, compared to 0 h. dROMs, derivatives of reactive oxygen metabolites; BAP, biological antioxidant potential; H2, molecular hydrogen.
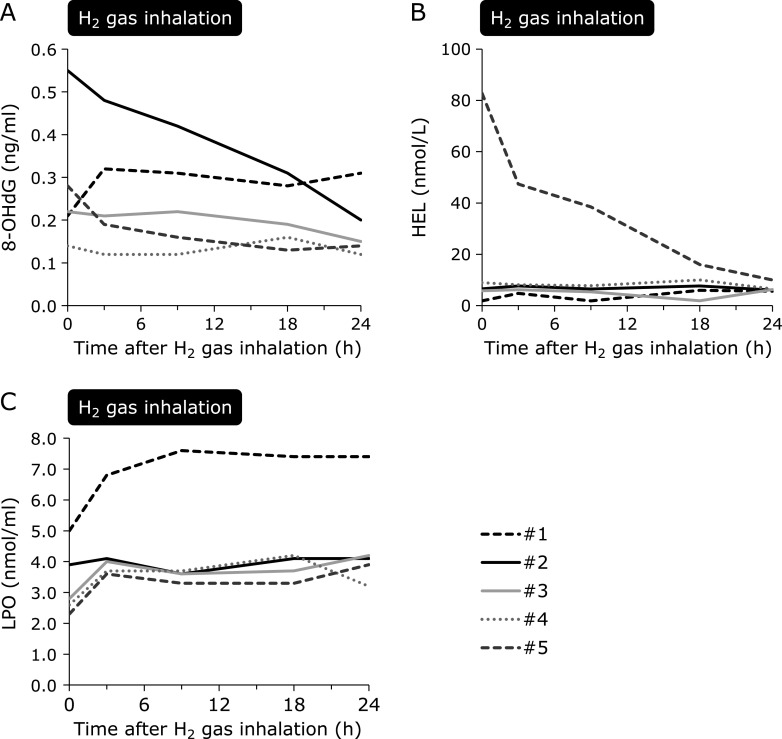
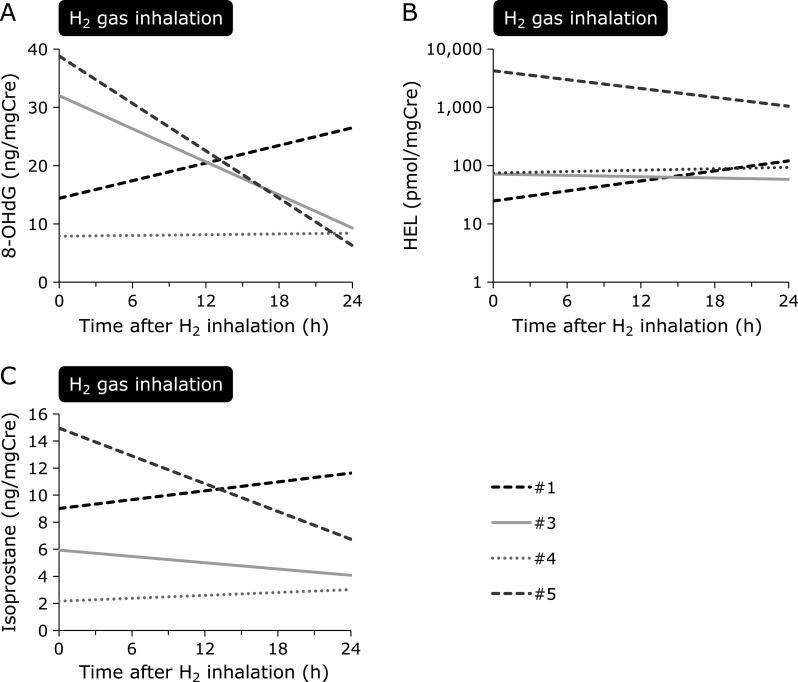
Except for those in one patient with sepsis, despite a difference in the degree, 8-OHdG levels decreased after H2 gas inhalation at 24 h (Fig. 3A). HEL decreased dramatically in one patient but was maintained at the same levels in other patients (Fig. 3B). LPO slightly increased at 3 h after the initiation of H2 gas inhalation but was then maintained over 24 h. Compared to levels of other measured oxidative stress markers, LPO was markedly high in the patient with sepsis (Fig. 3C). No apparent change in 8-OHdG, HEL, and LPO, as compared to that for dROM and BAP, was observed after discontinuing H2 gas inhalation. Urine samples were examined from four patients, excluding one anuric patient with chronic kidney disease who was on maintenance hemodialysis. Urine 8-OHdG, HEL, and isoprostane levels increased 24 h after H2 gas inhalation in one patient with sepsis (Fig. 4), whereas urine oxidative stress markers were reduced or maintained at the same level 24 h after H2 gas inhalation in the other three cardiogenic post-CA patients (Fig. 4).
Fig. 3.
Temporal changes in plasma oxidative stress markers in patients with post-cardiac arrest syndrome treated with H2 gas inhalation. Each line indicates an individual patient. (A) 8-OHdG, a marker of DNA damage, was decreased, except for in one patient with sepsis, from 0.30 ± 0.18 ng/ml to 0.15 ± 0.03 ng/ml before H2 gas inhalation and at 24 h, respectively (p = 0.31). (B) HEL, an early marker of lipid peroxidation, decreased dramatically in one patient, but remained the same in other patients (p = 0.39). (C) LPO, a marker of lipid peroxidation, slightly increased within 3 h after the initiation of H2 gas inhalation (p = 0.05, 0 h vs 3 h), but was then maintained for 24 h (p = 0.96, 3 h vs 24 h). LPO was markedly high in the patient with sepsis. 8-OHdG, 8-hydroxy-2'-deoxyguanosine; H2, molecular hydrogen; HEL, Nɛ-hexanoyl-lysine; LPO, lipid hydroperoxide.
Fig. 4.
Temporal changes in urine oxidative stress markers in patients with post-cardiac arrest syndrome treated with H2 gas inhalation. Each line indicates an individual patient. Urine was collected from the urinary catheter before and 24 h after H2 gas inhalation. Urine could not be assessed in one patient who was anuric and on maintenance hemodialysis. (A) Urine 8-OHdG, a marker of DNA damage, increased in the patient with sepsis, but decreased in the other patients (26.2 ± 16.2, 8.0 ± 1.5 ng/mgCre; 0 h and 24 h, respectively, p = 0.20). (B) Urine HEL, an early marker of lipid peroxidation, also increased in the patient with sepsis, but decreased in the other patients (1,463.7 ± 2,407.9, 401.0 ± 563.0 pmol/mgCre; 0 h and 24 h, respectively, p = 0.59). (C) Urine isoprostane, a marker of lipid peroxidation, was elevated in the patient with sustained sepsis, whereas it slightly decreased in the other patients (7.7 ± 6.6, 4.6 ± 1.9 ng/mgCre; 0 h and 24 h, respectively, p = 0.37). 8-OHdG, 8-hydroxy-2'-deoxyguanosine; Cre, creatinine; H2, molecular hydrogen; HEL, Nɛ-hexanoyl-lysine.
Temporal trends in inflammatory cytokines
Plasma IL-6 and TNF-α levels were markedly high in the patient with sepsis, as compared to those in the other cardiogenic post-CA patients (Fig. 5). In the patient with sepsis, IL-6 and TNF-α were significantly attenuated during H2 gas inhalation and increased after its discontinuation (Fig. 5A and B). Levels of IL-6 and TNF-α were low among patients with cardiogenic post-CA and were comparable during H2 gas inhalation. In contrast, IL-6 and TNF-α were higher in one cardiogenic post-CA patient undergoing maintenance hemodialysis and increased during the observational period in this individual (Fig. 5C and D).
Fig. 5.
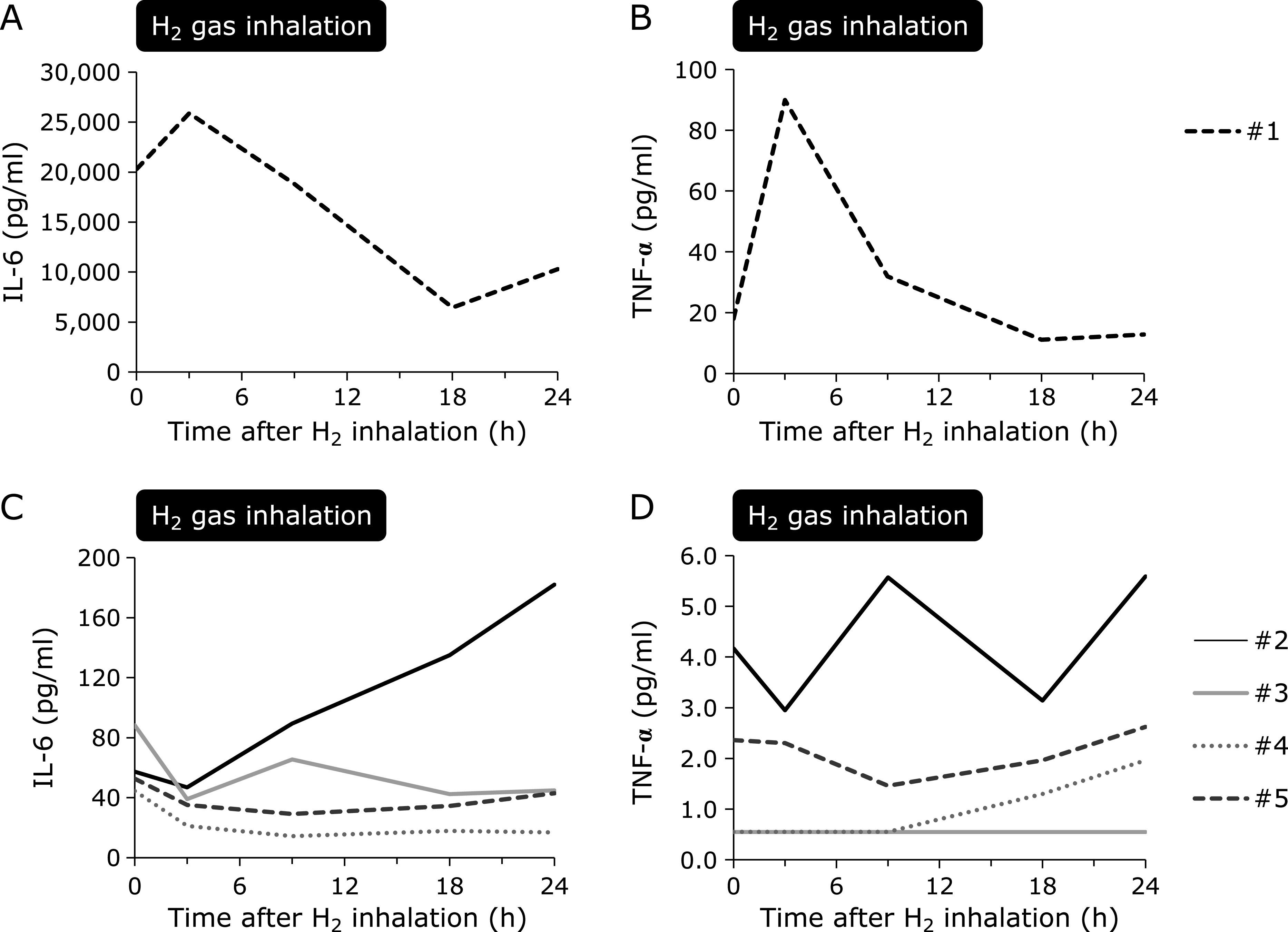
Temporal changes in inflammatory cytokines in patients with post-cardiac arrest syndrome treated with H2 gas inhalation. Each line indicates an individual patient. (A) IL-6, and (B) TNF-α levels were markedly high in one patient with sepsis. Although both IL-6 and TNF-α increased within 3 h after H2 gas inhalation, they were subsequently decreased drastically. After completing H2 inhalation, IL-6 and TNF-α levels increased again. (C) IL-6 and (D) TNF-α levels were low among the other four patients. Both IL-6 and TNF-α increased in one patient with a history of end-stage kidney disease treated with maintenance hemodialysis. IL-6 and TNF-α levels were maintained at similar levels over 24 h in the other three patients (p = 0.13, p = 0.28, respectively). H2, molecular hydrogen; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
Discussion
In the present study, the temporal changes in oxidative stress and inflammatory cytokines upon H2 gas inhalation were sequentially measured in patients with PCAS. The results revealed that oxidative stress markers were reduced in cardiogenic post-CA patients but were slightly elevated in the patient with sepsis with H2 gas inhalation. Inflammatory cytokine levels remained unchanged in cardiogenic post-CA patients, whereas a dramatic reduction was observed in one patient with sepsis. To the best of our knowledge, this is the first study to report changes in oxidative stress markers and inflammatory cytokines in PCAS patients undergoing H2 gas inhalation.
Although animal studies suggested the association between cardiac arrest and oxidative stress,(30,31) scarce data existed with regard to changes in oxidative stress in human PCAS. It was previously reported that TTM at 33°C reduces both dROM and BAP in PCAS patients.(11) This result is consistent with our findings; specifically, dROM and BAP were both reduced at 24 h after the initiation of H2 gas inhalation, where patients finished H2 gas inhalation at 18 h but were still treated at the target temperature at this point. However, opposite to our expectations, a slight increase in dROM, a decrease in BAP, and a decrease in the BAP to dROM ratio was also observed. It is noteworthy that all patients showed the same temporal change regardless of differences in target temperatures used and cardiac arrest etiologies. Since the patient with sepsis was treated with normothermia, these changes in dROM and BAP might not be solely due to hypothermia. Moreover, the observed sharp increase in the BAP to dROM ratio after finishing H2 gas inhalation, even upon treatment with the target temperature, might be attributed to the cessation of H2 gas inhalation. However, the reason for these findings needs to be elucidated in future studies.
It was previously reported that small differences can be observed in thiobarbituric acid reactive species among favorable and poor neurological outcome groups early after ROSC, but that other oxidative stress markers and antioxidant enzyme levels are not significantly different.(32) In another study, increased oxidative stress, indicated by increased plasma coenzyme Q10 and free fatty acids, was reported in post-cardiac arrest patients.(33) Time-course changes in several lipids and antioxidants were also assessed, but with a lack of comparisons using definitive endpoints, these results have limited clinical value. The optimal mode and timing for measuring oxidative stress markers in human PCAS is unclear. Therefore, we measured a panel of oxidative stress markers in an exploratory attempt. An association between elevated 8-OHdG levels and cardiovascular disease is known to occur in patients,(34,35) and H2 inhalation was previously reported to attenuate 8-OHdG production in the heart and brain based on animal models of I/R injury.(15,19) With H2 gas inhalation, our results showed a decrease in both plasma and urine 8-OHdG levels during the first 24 h after admission.
Lipid peroxidation is another important oxidative stress that occurs after CA.(36,37) Recently, HEL was identified as a novel lipid hydroperoxide-modified lysine residue that is produced in the earlier phase of lipid peroxidation, as compared to traditionally-studied malondi-aldehyde and 4-hydroxy-2-nonenal, which are generated at the later stage of this process.(38) Regarding this marker, our results revealed a drastic decrease in one patient, with no significant increases observed in the other patients. LPO, another marker of lipid peroxidation, was also maintained at a steady level. Of note, isoprostane, a product of the free radical-catalyzed peroxidation of arachidonic acid,(21,39) decreased with H2 gas inhalation; this was thought to be because arachidonic acid is a main component of phospholipids and H2 has been reported to suppress the free radical chain reaction-dependent peroxidation of phospholipids.(40) However, all patients underwent TTM, which is also known to alleviate lipid peroxidation,(36) and since there was no obvious change at 18 h, the net effect of inhaled H2 in addition to TTM requires further study.
The association between elevated cytokine levels and poor outcomes was reported previously for PCAS.(6,7) Interestingly, TTM failed to lower cytokine levels, and IL-6 levels were even reported to be elevated during this treatment.(6,14) H2 gas inhalation was reported to lower IL-6 levels in an animal cardiac arrest model.(15) Overall cytokine levels were low and remained unchanged in patients with cardiac etiologies, suggesting that inhaled hydrogen might have attenuated the elevation in cytokines at an early phase with TTM. IL-6 was slightly elevated in one patient who was undergoing maintenance hemodialysis. However, pro-inflammatory cytokines are known to be higher in patients undergoing hemodialysis.(41–43) The fact that hemodialysis was not performed during the 24 h after admission might have contributed to the increase in pro-inflammatory cytokines in this patient. Further, one patient who experienced CA from sepsis showed incommensurable high cytokine levels compared to those in patients with cardiac etiologies. However, cytokine levels were drastically decreased upon H2 gas inhalation in this patient and a rebound was observed after the discontinuation of this treatment. These observations suggest the anti-inflammatory effect of H2.
We acknowledge several limitations to this study. Since this was a sub-study comprising a first-in-human PCAS study to evaluate the feasibility and safety of H2 inhalation, it lacks a control group who did not inhale H2. Moreover, oxidative stress markers and inflammatory cytokines were observed only for the first 24 h, while patients were subjected to TTM, and data after rewarming are lacking. Moreover, outcomes were assessed at 90 days in this study, and therefore, future studies are needed to evaluate the longer-term effect of inhaled H2 on cognitive function or quality of life. Due to the small sample size and overall high percentage of favorable outcomes, levels of cytokines were low compared to those in previous reports.(7,44) To further enhance our understanding of this treatment modality, we are currently conducting a large, multicenter, double-blind, randomized control trial in Japan,(45) and the results of this study are expected to reveal the anti-oxidative and anti-inflammatory effect of inhaled H2.
In the present study, temporal changes in blood H2 concentration, oxidative stress, and inflammatory cytokine levels with H2 gas inhalation were assessed in human PCAS. This was the first human study to evaluate oxidative stress and cytokine levels in PCAS patients who inhaled H2. Further clinical studies are needed to evaluate the effect of H2 gas on oxidative stress and inflammatory cytokines in these individuals.
Author Contributions
Study concept and design, KH, MSuzuki, SH; acquisition of data, TT, KH, JY, TS; analysis and interpretation of data, TT, KH, MSuzuki; drafting of the manuscript, TT; critical revision of the manuscript for important intellectual content, KH, JY, TS, MSuzuki, MSano, JS; statistical analysis, TT; obtained funding, SH.
Acknowledgments
The authors thank Shuko Onuki for providing excellent assistance and coordinating the research. This work was supported by the Japan Society for the Promotion of Science under Grant (KAKENHI number 24390405).
Abbreviations
- BAP
biological antioxidant potential
- dROMs
derivatives of reactive oxygen metabolites
- H2
molecular hydrogen
- HEL
Nɛ-hexanoyl-lysine
- IL
interleukins
- I/R
ischemia/reperfusion
- LPO
lipid hydroperoxide
- OHCA
out-of-hospital cardiac arrest
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- PCAS
post-cardiac arrest syndrome
- ROS
reactive oxygen species
- ROSC
return of spontaneous circulation
- TNF-α
tumor necrosis factor-α
- TTM
target temperature management
Conflict of Interest
SH, TT, MSuzuki, KH, and MSano received research funding from Taiyo Nippon Sanso Corporation (no identifiable number). The other authors state no disclosures.
Supplementary Material
References
- 1.Callaway CW, Donnino MW, Fink EL, et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015; 132 (18 Suppl 2): S465–S482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: 2452–2483. [DOI] [PubMed] [Google Scholar]
- 3.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002; 106: 562–568. [DOI] [PubMed] [Google Scholar]
- 4.Shyu KG, Chang H, Lin CC, Huang FY, Hung CR. Concentrations of serum interleukin-8 after successful cardiopulmonary resuscitation in patients with cardiopulmonary arrest. Am Heart J 1997; 134: 551–556. [DOI] [PubMed] [Google Scholar]
- 5.Mussack T, Biberthaler P, Kanz KG, et al. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med 2002; 30: 2669–2674. [DOI] [PubMed] [Google Scholar]
- 6.Callaway CW, Rittenberger JC, Logue ES, McMichael MJ. Hypothermia after cardiac arrest does not alter serum inflammatory markers. Crit Care Med 2008; 36: 2607–2612. [DOI] [PubMed] [Google Scholar]
- 7.Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Crit Care Med 2015; 43: 1223–1232. [DOI] [PubMed] [Google Scholar]
- 8.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346: 557–563. [DOI] [PubMed] [Google Scholar]
- 9.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346: 549–556. [DOI] [PubMed] [Google Scholar]
- 10.Alva N, Palomeque J, Carbonell T. Oxidative stress and antioxidant activity in hypothermia and rewarming: can RONS modulate the beneficial effects of therapeutic hypothermia? Oxid Med Cell Longev 2013; 2013: 957054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohi K, Miyamoto K, Fukuda K, et al. Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxid Med Cell Longev 2013; 2013: 562429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hackenhaar FS, Medeiros TM, Heemann FM, et al. Therapeutic hypothermia reduces oxidative damage and alters antioxidant defenses after cardiac arrest. Oxid Med Cell Longev 2017; 2017: 8704352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt KR, Tong G, Berger F. Mechanisms of hypothermia-induced cell protection in the brain. Mol Cell Pediatr 2014; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. The inflammatory response after out-of-hospital cardiac arrest is not modified by targeted temperature management at 33°C or 36°C. Resuscitation 2014; 85: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida K, Sano M, Kamimura N, et al. H2 gas improves functional outcome after cardiac arrest to an extent comparable to therapeutic hypothermia in a rat model. J Am Heart Assoc 2012; 1: e003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisschops LL, Hoedemaekers CW, Mollnes TE, van der Hoeven JG. Rewarming after hypothermia after cardiac arrest shifts the inflammatory balance. Crit Care Med 2012; 40: 1136–1142. [DOI] [PubMed] [Google Scholar]
- 17.Fries M, Stoppe C, Brucken D, Rossaint R, Kuhlen R. Influence of mild therapeutic hypothermia on the inflammatory response after successful resuscitation from cardiac arrest. J Crit Care 2009; 24: 453–457. [DOI] [PubMed] [Google Scholar]
- 18.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 2007; 13: 688–694. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida K, Sano M, Ohsawa I, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun 2008; 373: 30–35. [DOI] [PubMed] [Google Scholar]
- 20.Sano M, Suzuki M, Homma K, et al. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med Surg 2018; 5: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohi K, Satoh K, Miyamoto K, et al. Molecular hydrogen in the treatment of acute and chronic neurological conditions: mechanisms of protection and routes of administration. J Clin Biochem Nutr 2017; 61: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashida K, Sano M, Kamimura N, et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest independently of targeted temperature management. Circulation 2014; 130: 2173–2180. [DOI] [PubMed] [Google Scholar]
- 23.Cole AR, Perry DA, Raza A, et al. Perioperatively inhaled hydrogen gas diminishes neurologic injury following experimental circulatory arrest in swine. JACC Basic Transl Sci 2019; 4: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura T, Hayashida K, Sano M, et al. Feasibility and safety of hydrogen gas inhalation for post-cardiac arrest syndrome—first-in-human pilot study. Circ J 2016; 80: 1870–1873. [DOI] [PubMed] [Google Scholar]
- 25.Peberdy MA, Callaway CW, Neumar RW, et al.; American Heart Association. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122 (18 Suppl 3): S768–S786. [DOI] [PubMed] [Google Scholar]
- 26.Cesarone MR, Belcaro G, Carratelli M, et al. A simple test to monitor oxidative stress. Int Angiol 1999; 18: 127–130. [PubMed] [Google Scholar]
- 27.Ito K, Yano T, Morodomi Y, et al. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res 2012; 32: 1063–1067. [PubMed] [Google Scholar]
- 28.Ridker PM, Brown NJ, Vaughan DE, Harrison DG, Mehta JL. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004; 109 (25 Suppl 1): IV6–IV19. [DOI] [PubMed] [Google Scholar]
- 29.Trotti R, Carratelli M, Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med 2002; 44: 37–40. [PubMed] [Google Scholar]
- 30.Hackenhaar FS, Fumagalli F, Li Volti G, et al. Relationship between post-cardiac arrest myocardial oxidative stress and myocardial dysfunction in the rat. J Biomed Sci 2014; 21: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Qian J, Wang J, et al. Effects of oxygen concentrations on postresuscitation myocardial oxidative stress and myocardial function in a rat model of cardiopulmonary resuscitation. Crit Care Med 2015; 43: e560–e566. [DOI] [PubMed] [Google Scholar]
- 32.Orban JC, Garrel C, Déroche D, et al. Assessment of oxidative stress after out-of-hospital cardiac arrest. Am J Emerg Med 2016; 34: 1561–1566. [DOI] [PubMed] [Google Scholar]
- 33.Nagase M, Sakurai A, Sugita A, et al. Oxidative stress and abnormal cholesterol metabolism in patients with post-cardiac arrest syndrome. J Clin Biochem Nutr 2017; 61: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono Y, Nakamura K, Kimura H, et al. Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure. Circ J 2006; 70: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 35.Kroese LJ, Scheffer PG. 8-hydroxy-2'-deoxyguanosine and cardiovascular disease: a systematic review. Curr Atheroscler Rep 2014; 16: 452. [DOI] [PubMed] [Google Scholar]
- 36.Lei B, Tan X, Cai H, Xu Q, Guo Q. Effect of moderate hypothermia on lipid peroxidation in canine brain tissue after cardiac arrest and resuscitation. Stroke 1994; 25: 147–152. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke 1998; 29: 1679–1686. [DOI] [PubMed] [Google Scholar]
- 38.Kato Y, Mori Y, Makino Y, et al. Formation of nepsilon-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem 1999; 274: 20406–20414. [DOI] [PubMed] [Google Scholar]
- 39.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ, 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 1990; 87: 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iuchi K, Imoto A, Kamimura N, et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci Rep 2016; 6: 18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int 1998; 54: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein SL, Leung JC, Silverstein DM. Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol 2006; 1: 979–986. [DOI] [PubMed] [Google Scholar]
- 43.Rysz J, Banach M, Cialkowska-Rysz A, et al. Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Cell Mol Immunol 2006; 3: 151–154. [PubMed] [Google Scholar]
- 44.Bro-Jeppesen J, Kjaergaard J, Stammet P, et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33°C or 36°C. Resuscitation 2016; 98: 1–8. [DOI] [PubMed] [Google Scholar]
- 45.Tamura T, Hayashida K, Sano M, Onuki S, Suzuki M. Efficacy of inhaled HYdrogen on neurological outcome following BRain Ischemia During post-cardiac arrest care (HYBRID II trial): study protocol for a randomized controlled trial. Trials 2017; 18: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.