Significance
Our results suggest a rational way of designing and developing an improved typhoid conjugate vaccine and, by extension, to conjugate vaccines in general: first, modify a T-independent polysaccharide so that it no longer induces a T-independent response, then conjugate the polysaccharide to a suitable carrier protein restoring immunogenicity, thus creating a pure T-dependent antigen that induces a strongly boostable and long-lived response at an early age.
Keywords: T-independent response, T-dependent response, conjugate vaccine, polysaccharide, Salmonella Typhi
Abstract
Polysaccharide-protein conjugates have been developed to overcome the T-independent response, hyporesponsiveness to repeated vaccination, and poor immunogenicity in infants of polysaccharides. To address the impact of polysaccharide length, typhoid conjugates made with short- and long-chain fractions of Vi polysaccharide with average sizes of 9.5, 22.8, 42.7, 82.0, and 165 kDa were compared. Long-chain-conjugated Vi (165 kDa) induced a response in both wild-type and T cell-deficient mice, suggesting that it maintains a T-independent response. In marked contrast, short-chain Vi (9.5 to 42.7 kDa) conjugates induced a response in wild-type mice but not in T cell-deficient mice, suggesting that the response is dependent on T cell help. Mechanistically, this was explained in neonatal mice, in which long-chain, but not short-chain, Vi conjugate induced late apoptosis of Vi-specific B cells in spleen and early depletion of Vi-specific B cells in bone marrow, resulting in hyporesponsiveness and lack of long-term persistence of Vi-specific IgG in serum and IgG+ antibody-secreting cells in bone marrow. We conclude that while conjugation of long-chain Vi generates T-dependent antigens, the conjugates also retain T-independent properties, leading to detrimental effects on immune responses. The data reported here may explain some inconsistencies observed in clinical trials and help guide the design of effective conjugate vaccines.
Bacterial capsular polysaccharides (PSs) have been used for many decades as vaccines; however, their use has been limited due to the absence of immunogenicity in infants and young children, the lack of induction of immunologic memory, limited duration of antibody response and hyporesponsiveness to subsequent vaccination (1–4). These negative properties are likely due to the absence of T cell help in the antibody response induced by pure PS, which supports neither affinity maturation through somatic hypermutation in germinal centers nor class-switching (3, 5, 6). The mechanisms of hyporesponsiveness have been investigated in neonatal mouse models. Neonatal mice primed with meningococcal C (MenC) conjugate vaccine showed strong apoptosis of MenC-specific memory B cells when boosted with unconjugated MenC PS (7). Furthermore, mice boosted with unconjugated pneumococcal PS after neonatal priming with corresponding glycoconjugate exhibited significantly reduced germinal center formation, depletion of PS-specific antibody-secreting cells (ASCs), and reduced levels and avidity of PS-specific serum antibodies. Together, these events led to reduced protection against pneumococcal infection (8, 9). These studies suggest that hyporesponsiveness is not simply a passive lack of immune response, but is an active depletion of a relevant immune response. The weakness of PS as antigens can be overcome by conjugation to a carrier protein, effectively converting T-independent antigens to T-dependent antigens, enhancing memory induction, class-switching, and antibody production and affinity maturation at an early age, thereby facilitating the development of long-term protective immunity (5, 6).
Typhoid fever remains a major public health concern in low-income countries and affects millions of people each year in Asia and Africa (10). An effective vaccine based on Salmonella enterica serovar Typhi capsular Vi antigen is currently licensed, but only for children age >2 y. Furthermore, boosting of children with unconjugated Vi has resulted in hyporesponsiveness (11, 12). To overcome this, several glycoconjugate vaccines are currently in development (13, 14), while Vi-rEPA and Vi-TT are licensed in India and China (13, 15) and are undergoing Phase 4 effectiveness studies is several regions in Africa and Asia (16, 17).
A Vi-CRM197 conjugate vaccine (18–21) has been tested in Phase 1 and 2 trials in adults in Europe (22) and subsequently in Phase 2 trials in adults, children, and infants in India, Pakistan, and the Philippines (23). Vi-CRM197 was found to be considerably more immunogenic than unconjugated Vi, inducing much greater antibody responses after a single injection and at lower doses (22), and to induce specific antibody responses in infants (23). However, in children and infants age 9 to 12 mo, a second injection of Vi-CRM197 conjugate had no incremental effect on antibody levels, and in patients of all ages, anti-Vi antibody persistence was similar to that of the antibody response induced by unconjugated Vi (23).
We hypothesized that despite being conjugated to a carrier protein, long Vi chains cross-link B cell receptors, thereby inducing a partial T-independent response. To test this hypothesis, we undertook a series of experiments in mice. The objective was to identify the Vi size range for which unconjugated PS no longer induces a T-independent response but still induces a robust T-dependent antibody response when conjugated to a carrier protein. Furthermore, we also investigated how this translated into long-lived memory responses in early life.
Results
Long-Chain Vi-CRM197 Conjugate Elicits a T-Independent Response.
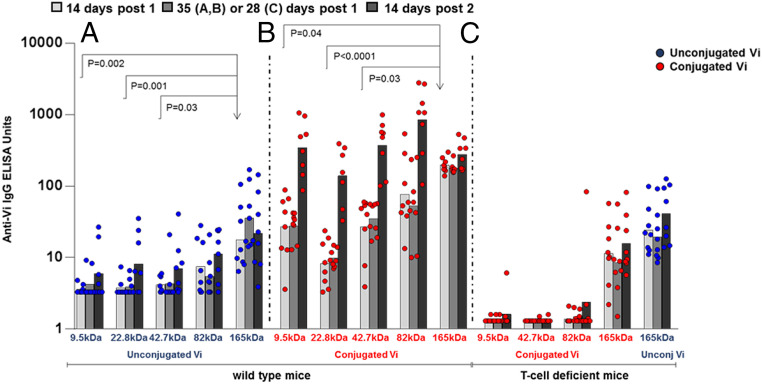
To investigate the role of the Vi polysaccharide length in eliciting T-independent and T-dependent antibody responses, five fractions of Vi with average sizes of 9.5, 22.8, 42.7, 82.0, and 165 kDa were produced and used in the following experiments either alone or after conjugation to CRM197 (Table 1 and SI Appendix, Figs. S1 and S2) (24). Fig. 1A shows that 10-wk-old adult mice immunized at day 0 and day 35 with the unconjugated Vi responded poorly to short-chain (9.5 to 42.7 kDa) Vi, while they had significant antibody response to the long-chain (165-kDa) Vi, which did not increase after the second dose. The 165-kDa Vi-CRM197 (Fig. 1B) induced a 12.8-fold greater Vi-specific IgG response than unconjugated 165-kDa Vi (Fig. 1A); the peak response occurred after one injection, with no increase following the second immunization, as for unconjugated Vi. For shorter Vi conjugates, despite a significantly lower Vi-specific IgG response at day 14 compared with the 165-kDa Vi-CRM197, there was a significant increase in Vi-specific IgG following the second immunization (P = 0.015 to 0.008), and Vi-specific IgG levels were comparable to those induced by the 165-kDa Vi-CRM197 (Fig. 1B). Pooled sera collected at 2 wk after the second injection from mice immunized with the different conjugates showed comparable bactericidal activity against a Citrobacter freundii strain expressing Vi (SI Appendix, Fig. S3).
Table 1.
Physical characteristics of Vi-CRM197 conjugates of different Vi size tested in mice
| Conjugate | Vi average number of repeating units* | Vi % OAc | Vi to CRM197 ratio (wt/wt) | % free Vi |
| 9.5-kDa fVi-CRM197 | 37 | 66 | 0.33 | 10.6 |
| 22.8-kDa fVi-CRM197 | 88 | 80.5 | 0.52 | <5† |
| 42.7-kDa fVi-CRM197 | 165 | 95 | 0.64 | 13.7 |
| 82.0-kDa fVi-CRM197 | 317 | 95 | 1.33 | 17.2 |
| 165-kDa Vi-CRM197 | 636 | 95 | 1.27 | <13† |
Calculation based on Vi monomer molecular weight of 259 corresponding to O-acetylated (OAc) and free acid forms.
% values refer to the ratio between the minimum quantifiable amount of free Vi detected by high-performance anion exchange chromatography coupled with pulsed amperometric detection to the total amount of PS in the conjugate.
Fig. 1.
Vi-specific IgG response in adult mice. Vi-specific IgG response expressed in enzyme-linked immunosorbent assay (ELISA) units induced in wild-type adult mice following immunization with unconjugated Vi of different sizes (A) and their corresponding CRM197 conjugates (B). Some of the constructs were tested in T cell-deficient mice (C). Subcutaneous injections containing 8 µg of Vi antigen were given at days 0 and 35 (A and B) or days 0 and 28 (C). Individual animals are represented by the dots; column heights represent geometric mean units. Sera at time 0 were below the limit of detection of the ELISA, corresponding to 3.3 EU/mL in the study of wild-type mice and to 1.3 EU/mL in the study of T cell-deficient mice.
To verify the T-dependent nature of the response to conjugate vaccines, we immunized T cell-deficient mice. Fig. 1C shows that conjugates prepared with Vi sizes of 9.5, 42.7, and 82.0 kDa did not elicit any antibody response, confirming that the response is T cell-dependent. The conjugate made with Vi size of 165 kDa induced an antibody response in T cell-deficient mice similar to unconjugated 165-kDa Vi, indicating a T-independent response. As expected for T-independent responses, no boost in antibody level was observed after the second immunization with either conjugated or unconjugated 165-kDa Vi. Of note, none of the conjugates tested elicited an anti-CRM197 IgG response in T cell-deficient mice.
Long-Chain Vi Conjugate Leads to Reduction of Vi-Specific IgG ASC in Spleen and Bone Marrow in Mice Immunized as Neonates.
To further dissect the mechanistic aspects of the T-dependent and T-independent responses, we selected the Vi fraction of 42.7 kDa as representative of short-chain conjugates and compared it with the 165-kDa long-chain Vi conjugate to test in neonatal mice (7 d old), which are more sensitive to PS-induced hyporesponsiveness than infant and adult mice (8). The neonatal mice were immunized twice, 3 wk apart, with 42.7-kDa Vi (indicated in the figures as fragmented Vi [fVi]) or 165-kDa Vi (indicated as Vi) conjugates together with alum, followed by a third immunization 2 wk later with the same conjugate, unconjugated fVi or Vi, or saline. Mice immunized with three doses of saline were used as negative controls.
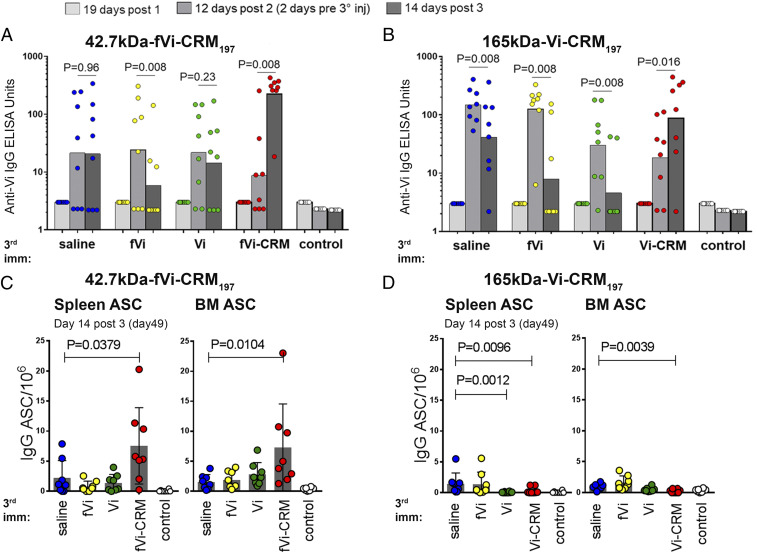
A third dose of short-chain conjugate (Fig. 2A) elicited a more significant increase in Vi-specific IgG than the increase elicited by a third dose of long-chain conjugate (Fig. 2B). The Vi-specific IgG levels at 14 d after three doses of 42.7-kDa or 165-kDa Vi conjugates did not differ (P = 0.4418; Fig. 2 A and B). However, the short-chain conjugate induced Vi-specific IgG with slightly higher avidity than the long-chain conjugate (SI Appendix, Fig. S4). Furthermore, mice primed with the 42.7-kDa Vi conjugate did not show any reduction in Vi-specific IgG levels after a third injection with saline or unconjugated 165-kDa Vi, although antibody levels were reduced after receipt of unconjugated 42.7-kDa Vi (Fig. 2A). In contrast, neonatal mice responded well to the priming with the long-chain Vi conjugate, but the response was short-lived and decreased dramatically after boosting with short- or long-chain unconjugated Vi (Fig. 2B).
Fig. 2.
The effects of Vi size in a Vi-CRM197 conjugate vaccine on the induction of Vi-specific IgG antibodies and ASCs in spleen and their homing to the BM. Neonatal mice were immunized with two doses of 42.7-kDa fVi-CRM197+alum (Left) or 165-kDa Vi-CRM197+alum (Right) at 3-wk intervals and then boosted 2 wk after the second dose with saline (blue circles), unconjugated 42.7-kDa fVi (yellow circles), unconjugated 165-kDa Vi (green circles), or comparable conjugates as in the first immunizations (red circles), all boosters without alum. (A and B) Vi-specific IgG response expressed in ELISA units in sera from mice primed with 42.7-kDa fVi-CRM197 (A) or 165-kDa Vi-CRM197 (B). Individual mice are represented by the dots; column heights represent geometric mean units. (C and D) Vi-specific IgG+ ASCs measured at 49 d after the first immunization with 42.7-kDa fVi-CRM197 (C) or 165-kDa Vi-CRM197 (D) in spleen (Left) and BM (Right). Results are expressed as number of spots/106 cells (mean ± SD) in spleen or BM; individual mice are represented by the dots. Statistical difference was calculated using the Wilcoxon matched-pairs signed rank test, where antibody levels were compared 2 d before and 14 d after booster (A and B), and the Mann–Whitney U test, where 42.7-kDa fVi, 165-kDa Vi, and 42.7-kDa fVi-CRM197/165-kDa Vi-CRM197 boosters were compared with saline booster (C and D).
We then asked whether immunization with conjugates containing Vi saccharides of different lengths affected the frequency of Vi-specific IgG+ ASCs in the spleen and bone marrow (BM). We found that the number of Vi-specific IgG+ ASC was higher at 14 d after the third dose of the short-chain Vi conjugate than after immunization with the long-chain Vi conjugate (Fig. 2 C and D) in both the spleen and the BM (P < 0.001). Furthermore, the third dose of 42.7-kDa Vi conjugate increased the number of Vi-specific IgG+ ASCs in spleen and BM compared with saline (Fig. 2C), while the number of Vi-specific IgG+ ASCs in mice primed with the long-chain conjugate remained low and even decreased after the booster dose (Fig. 2D).
Immunization with Long-Chain Vi Conjugate Induces Late Apoptosis of Vi-Specific B Cells in Spleen and Early Depletion of Vi-Specific B Cells in the BM.
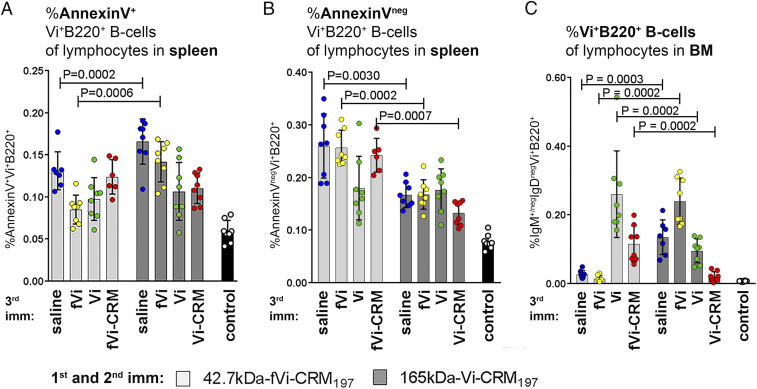
Next, apoptosis of Vi-specific B cells was assessed by direct labeling with fluorescent Vi. FACS analysis of Annexin V-positive Vi-specific B220+ B cells in spleen and BM was measured at 12 h after a third injection in mice immunized as neonates, as described above.
Mice primed with long-chain Vi conjugate showed higher frequencies of apoptotic Vi-specific B220+ B cells at 12 h after a third immunization with saline or unconjugated short-chain Vi compared with mice primed with short-chain conjugate. However, no difference was observed between mice that received a third dose of short- or long-chain Vi conjugate (Fig. 3A and SI Appendix, Fig. S5A). Strikingly, the frequencies of total Vi-specific B220+ B cells and live Annexin Vneg Vi-specific B220+ B cells were significantly higher in mice primed with 42.7-kDa Vi conjugate than in mice primed with long-chain Vi conjugate at 12 h after the third injection for all boosters except that with unconjugated long-chain Vi (Fig. 3B and SI Appendix, Fig. S5 B and C), indicating that the short-chain Vi conjugate induces less apoptosis than the long-chain Vi conjugate.
Fig. 3.
The 165-kDa Vi-CRM197 conjugate induced late apoptosis of Vi-specific B220+ cells in spleen but an early depletion in BM. Neonatal mice were immunized with two doses of 42.7-kDa fVi-CRM197 or 165-kDa Vi-CRM197 at a 3-wk interval, and 2 wk after the second dose, the mice received different boosters: saline (blue circles), unconjugated 42.7-kDa fVi (yellow circles), unconjugated 165 kD-Vi (green circles), or comparable conjugate as the in first two immunizations (red circles). Shown is the frequency of Annexin V+Vi+B220+ (A) and Annexin VnegVi+B220+ B cells (B) among lymphocytes in spleen and IgM+/negIgDnegVi+B220+ B cells among lymphocytes in BM (C) at 12 h after booster at day 35 in mice immunized with two doses of either 42.7-kDa fVi-CRM197 (light-gray bars) or 165-kDa Vi-CRM197 (dark-gray bars). Results are expressed as frequency (mean ± SD) in spleen of six to eight mice per group from one experiment; individual mice are represented by dots. Statistical difference was calculated using the Mann–Whitney U test where comparable boosters were compared in mice immunized with two doses of 42.7-kDa fVi-CRM197 or 165-kDa Vi-CRM197.
In BM, the frequency of mature IgM+/negIgDneg Vi-specific B220+ B cells was lower in mice primed with 165-kDa Vi conjugate than in those primed with 42.7-kDa Vi conjugate after a third immunization with unconjugated 165-kDa Vi or the same conjugate (Fig. 3C and SI Appendix, Fig. S5D). However, the frequency of mature IgM+/negIgDneg Vi-specific B220+ B cells was higher after a boost with saline or unconjugated 42.7-kDa Vi in mice primed with 165-kDa Vi conjugate than in mice primed with 42.7-kDa Vi conjugate (Fig. 3C and SI Appendix, Fig. S5D). Annexin V staining of the Vi-specific B220+ B cells in BM was negative at 12 h after the third immunization.
Short-Chain Vi Conjugate Induces More Prolonged Proliferation of Vi-Specific B Cells in Spleen than the Long-Chain Vi Conjugate.
We then tested whether the length of the Vi saccharide in the conjugates affected the status of proliferation of Vi-specific B cells at 5 d after the third dose. To this end, we stained for Ki67, a well-established marker of proliferation in lymphocytes (25, 26). No difference was observed between mice that received a third dose of short-chain or long-chain Vi conjugates, but in mice receiving saline at the third injection, the frequency of Ki67+ Vi-specific B220+ B cells in the spleen was higher in mice previously immunized with short-chain Vi conjugate than in those immunized with long-chain Vi conjugate (P = 0.0281; SI Appendix, Fig. S6 A and B). Similarly, in mice receiving saline or long-chain unconjugated Vi at the third dose, the frequency of Vi-specific B220+ B cells in the spleen was higher in mice immunized with the short-chain Vi conjugate than in those primed with the long-chain Vi conjugate (P = 0.0371 and 0.0155, respectively; SI Appendix, Fig. S6 A and B).
We next characterized Vi-specific B-cell subpopulations in spleen by CD138, which is expressed on plasmablasts/plasma cells (Vi+B220+CD138+) but not on memory or naive B cells (Vi+B220+CD138neg), and assessed their proliferative status. We found no difference in frequencies of Vi-specific plasmablasts or memory/naïve B cells, proliferating or nonproliferating, between mice that received a third dose of short-chain or long-chain Vi conjugates (SI Appendix, Fig. S7), with the exception of a slightly higher frequency of nonproliferating memory/naïve Vi-specific B220+ B cells (Ki67negVi+B220+CD138neg) in mice that received a third dose of the long-chain Vi conjugate. Strikingly, mice primed with long-chain Vi conjugate had lower frequencies of memory/naïve Vi-specific B220+ B cells, both proliferating and especially nonproliferating, at 5 d after a third immunization with saline (19 d after the second dose of long-chain Vi conjugate) compared with mice primed with short-chain conjugate (SI Appendix, Fig. S7 D–F).
Long-Chain Vi Conjugate Induces Hyporesponsiveness.
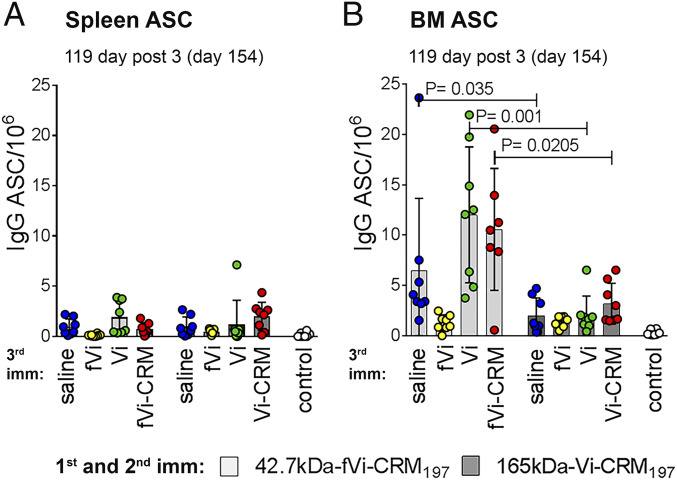
Finally, we evaluated the long-term effect of priming with the short or the long-chain Vi conjugates by measuring serum Vi-specific IgG up to 119 d after boost (154 d after first immunization of neonatal mice) and enumerating the Vi-specific IgG+ ASCs in spleen and BM.
There was a greater decline in Vi-specific IgG antibodies from 14 d to 119 d after the third dose of long-chain Vi conjugate (P = 0.008) than after the third dose of short-chain Vi conjugate (P = 0.05) (SI Appendix, Fig. S8). The number of Vi-specific IgG+ ASCs in spleen was low in all mice irrespective of the Vi conjugate received (Fig. 4A); however, the number of Vi-specific IgG+ ASCs that homed to and persisted in BM up to day 154 was significantly higher in mice primed with the short-chain Vi conjugate (Fig. 4B). Specifically, the number of Vi-specific IgG+ ASCs in BM was higher after third dose with saline (P = 0.0350), unconjugated long-chain Vi (P = 0.001), or short-chain conjugated Vi (P = 0.0205).
Fig. 4.
The 165-kDa Vi-CRM197 conjugate vaccine induces hyporesponsiveness, resulting in fewer Vi-specific ASCs that home to and persist in the BM. The mice were immunized with two doses of 42.7-kDa fVi-CRM197 or 165-kDa Vi-CRM197 at a 3-wk interval, and at day 35 the mice received a different booster: saline (blue circles), unconjugated 42.7-kDa fVi (yellow circles), unconjugated 165-kDa Vi (green circles), or comparable conjugate as in first two immunizations (red circles). Vi-specific IgG+ ASCs were enumerated at day 154 in spleen (A) and BM (B). Results are expressed as number of spots/106 cells (mean ± SD) in seven or eight mice per group in one experiment; individual mice are represented by dots. The Mann–Whitney U test was used to compare ASC numbers at day 154 in mice immunized with two doses of 42.7-kDa fVi-CRM197 or 165-kDa Vi-CRM197 and receiving comparable boosters.
A third injection with unconjugated 42.7-kDa Vi reduced the number of Vi-specific IgG+ ASCs in both spleen (Fig. 4A) and BM (Fig. 4B) compared with saline in mice primed with 42.7-kDa Vi conjugate (P = 0.03 in spleen and P = 0.0017 in BM).
We conclude that the long-chain Vi conjugate induces hyporesponsiveness, as reflected by a lack of long-term persistence of Vi-specific IgG+ ASCs in BM.
Discussion
Following the results of Vi conjugate vaccines in clinical trials (22, 23), we set out to identify the average size of Vi below which the unconjugated PS ceases to induce a significant Vi-specific IgG antibody response (i.e., no T-independent response) but would give a robust T-dependent response when conjugated to a carrier protein.
As observed in humans (22), in adult mice, the 165-kDa Vi conjugate induced a much larger Vi-specific IgG response than unconjugated PS, but a booster response was absent following a second immunization. In T cell-deficient mice, the long-chain Vi conjugate showed a similar antibody response as the unconjugated 165-kDa Vi, indicating that the conjugate is also an efficient T-independent antigen (Fig. 1). By producing Vi with different average sizes, we identified a critical size (∼82 kDa) below which both unconjugated and conjugated Vi PS can no longer act as T-independent antigens (Fig. 1). Unlike the 165-kDa Vi conjugate vaccine, the shorter Vi chain conjugates could boost Vi-specific IgG antibody levels following a second immunization in wild-type mice. The conjugates tested in this study, produced with Vi of different average sizes, were also different in terms of Vi:protein ratio (Table 1) and conjugate size. For both the 165-kDa and 42.7-kDa Vi, we had already verified the lack of impact of Vi conjugate loading and cross-linking on the immune response induced in mice (24).
Immune responses induced by the 42.7-kDa and 165-kDa Vi conjugates were then compared in a neonatal mouse model. In neonatal mice, the initial priming is likely to be extrafollicular, since the follicular dendritic cells (FDCs) barely exist so early; however, at the time of the third injection, the mice were 6 wk of age, with fully mature FDCs supporting an efficient memory B-cell response (7, 27). We found that immunization with long-chain Vi conjugates induced hyporesponsiveness, associated with depletion of Vi-specific IgG+ ASCs in spleen or BM observed 14 d after the third injection (Fig. 2D). Surprisingly, early apoptosis of Vi-specific B220+ B cells (Fig. 3A) or absence of proliferation at 5 d after boost (SI Appendix, Fig. S6B) was not observed, as was expected based on previous experience with meningococcal and pneumococcal conjugates (7, 9). Instead, the detrimental effect of the long-chain conjugate was delayed, as the highest frequency of apoptotic Vi-specific B220+ B cells was observed in mice primed with 165-kDa Vi conjugate and receiving saline as a third injection, i.e., 14 d and 12 h after the second conjugate immunization (Fig. 3A). Moreover, fewer proliferating Ki67+ Vi-specific B220+ B cells were observed at 5 d after receipt of saline as a third injection in mice immunized with long-chain Vi conjugate compared with those immunized with the short-chain Vi conjugate, i.e., 19 d after second conjugate immunization, reflecting reduced persistence of the response (SI Appendix, Fig. S6 A and B).
The delayed response may be explained by the observation that Salmonella Typhi induces a massive early extrafollicular response and delayed germinal center formation, with most of the isotype switching along with somatic hypermutations occurring at extrafollicular sites (28, 29). Indeed, after a boost with the long-chain Vi conjugate, we found that hardly any Vi-specific IgG+ ASCs persisted up to 14 d in the spleen (Fig. 2D) or homed to and persisted in the BM up to day 119 (Fig. 4B). In contrast, Vi-specific IgG+ ASC persisted in the BM for up to at least 119 d after boost with the short-chain Vi conjugate (Fig. 4B). However, the frequency of Vi-specific B220+ B cells in BM at 12 h after the boost with saline and unconjugated 42.7-kDa Vi was higher in mice immunized with 165-kDa Vi conjugate than 42.7-kDa Vi conjugate (Fig. 3C). This shows the ability of 165-kDa Vi conjugate to induce an efficient migration of ASCs to the BM, but these ASCs lack the capacity to persist up to 14 d after boost (Fig. 2D) and instead undergo apoptosis, which, however, was not detected at 12 h after boost. Furthermore, our results on B-cell subpopulations indicate that the lack of ASC persistence could possibly be due to delayed depletion of Vi-specific memory B cells by the long-chain Vi conjugate (SI Appendix, Fig. S7 D–F), which leads to the absence of regeneration of Vi-specific plasma cells from the memory pool in spleen (Fig. 2) and their homing to the BM and persistence and differentiation into long-lived memory plasma cells (Figs. 2 and 4B). This delay in hyporesponsiveness could be due to the combined T-dependent and T-independent properties of the conjugated long-chain Vi polysaccharide, where over an extended period, the detrimental effects of the T-independent override the T-dependent memory induction and seem to escalate with each dose of the long-chain Vi conjugate.
Polysaccharides are highly resistant to degradation and have been shown to persist in vivo for a long time (30, 31). Thus, a possible explanation for the persisting IgG titers in mice immunized with long-chain Vi conjugate could be the continuous induction of the extrafollicular response of short-lived plasma cells over extended periods due to retained T-independent properties of the conjugated long-chain Vi polysaccharide (28, 29). We observed that boosts with unconjugated shorter-chain Vi and longer-chain Vi primarily resulted in hyporesponsiveness in mice immunized with conjugates containing Vi of the same length. To our knowledge, this phenomenon has not been reported previously and warrants further exploration.
The results obtained are consistent with our hypothesis that when the property of the conjugated PS to act as a T-independent antigen is lost, the detrimental effects on memory and subsequent boosting are also negated. It would be of interest to compare the effects of preexposure to shorter-chain saccharides versus longer-chain saccharides in a prime-boost context, and the influence, if any, of age, but that is beyond the scope of this work.
In addition to the improved immunogenicity seen in mice, the use of shorter-chain Vi conjugates has some technical advantages (14). Although there is a loss of some Vi during fragmentation and sizing, it is possible to perform the conjugation reaction with a higher degree of control and better manufacturing consistency, due to the much lower viscosity of shorter PS. The yield of conjugate (expressed in terms of the Vi in the conjugation mixture) is higher and the product is easier to purify (particularly from unconjugated PS), characterize, and filter-sterilize. In this study, no minimal size was identified below which Vi could not induce a robust and persistent antibody response when conjugated to CRM197, but there likely is a minimum size, and it is known that other glycoconjugate vaccines with short oligosaccharides can fail to raise PS-specific antibodies if key epitopes are lost (32–36).
Our findings on Vi size contrast with current dogma for Vi conjugate vaccines (14, 37, 38). Previous studies have identified PS size as a critical parameter for the immunogenicity of Vi-based vaccines and have shown that smaller PS conjugates elicit lower levels of Vi-specific antibodies in both mice and rhesus monkeys after each of multiple doses (37). In this study, we have demonstrated the advantage of a shorter-chain Vi conjugate vaccine that elicits a T-dependent response only, does not cause hyporesponsiveness, but induces high, persistent Vi-specific IgG in serum and IgG+ ASCs in BM in neonatal mice. We suggest that the approach presented in this study can be expanded to other conjugated polysaccharides, where the polysaccharide still retains the correct epitopes but loses its T-independent induction ability and consequently creates a pure T-dependent antigen. We recently described this approach for the synthesis of a glycoconjugate vaccine against Shigella flexneri 6 (39). There are other examples in the literature suggesting that saccharides of shorter chain length, as opposed to higher molecular weight polysaccharides, may be better able to elicit T cell-dependent antibody responses (36, 40–42).
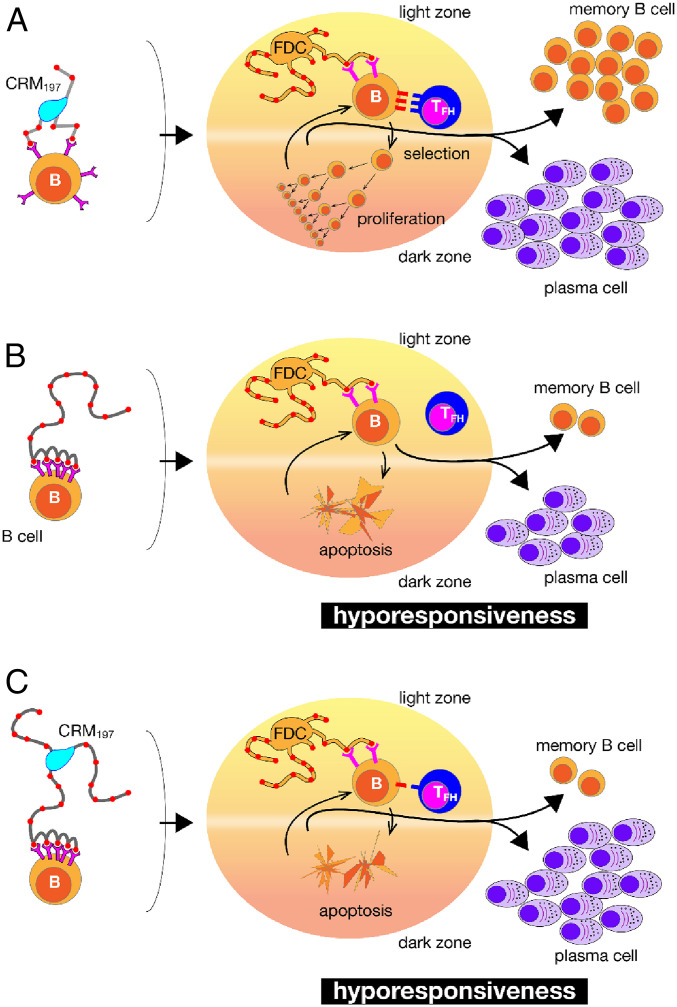
The implications of this work extend beyond a Vi conjugate vaccine and are summarized in the model shown in Fig. 5, which illustrates the interaction of memory B cells with different immunogens. Fig. 5A shows the behavior of a conjugate vaccine with a short PS. This can bind the B-cell receptors of B cells in the extrafollicular sites or be presented by the FDC to the B cells within the germinal center that process it and present the T-cell epitopes to the T follicular helper (Tfh) cells. This triggers the germinal center reaction that induces T-dependent affinity maturation, B-cell selection and proliferation, and finally plasma cells that produce the immediate antibody response and new memory B cells. Fig. 5B shows what happens with a long unconjugated PS. In this case, the repeated epitopes on the PS cross-link the B-cell receptors of the B cells in the extrafollicular space and induces short-lived proliferation, while in the germinal center they fail to engage follicular T helper cells, with a consequent apoptosis of memory B cells. This causes hyporesponsiveness and failure to boost during subsequent immunizations. Finally, Fig. 5C shows that the long-chain conjugate vaccine behaves mostly as the PSs shown in Fig. 5B because the PS length overrides the T-dependent germinal center reaction. In real life, we believe that most conjugate vaccines may have a mixed T-dependent and T-independent activity, which may explain many of the inconsistencies observed in clinical trials.
Fig. 5.
Schematic representation of the interactions of different PS immunogens with memory B cells in the extrafollicular space (Left) or within the germinal center (Right): conjugate vaccine with a short-chain PS (A), long unconjugated PS (B), or conjugate vaccine with long-chain PS (C).
Our data suggest a new paradigm for the rational design of glycoconjugate vaccines: first produce a PS that retains the correct antibody epitopes but is incapable of eliciting an antibody response in unconjugated format, then use that PS for conjugation. This paradigm should be readily testable following a similar procedure to that described here for Vi.
Materials and Methods
The overall objective of this study was to identify optimal Vi PS sizes at which unconjugated PS could not induce a T-independent antibody response but would induce a strong T-dependent antibody response when conjugated to a carrier protein. Detailed information on our experiments is provided in SI Appendix, Materials and Methods. In brief, our procedure was as follows: 165-kDa Vi PS from C. freundii sensu lato was fragmented, and populations of different sizes were separated and characterized. Corresponding CRM197 conjugates were synthesized and characterized. Unconjugated Vi of different sizes and corresponding conjugates were evaluated in wild-type and T cell-deficient mice for their ability to induce antibodies. Then 165-kDa Vi-CRM197 and 42.7-kDa fVi-CRM197 conjugates were tested in neonatal mice to compare the ability of conjugates of different Vi size to induce hyporesponsivness and apoptosis of Vi-specific B cells.
Long-chain Vi-CRM197 and fVi-CRM197 conjugates were synthesized and characterized as described previously (24) and as detailed in SI Appendix, Materials and Methods. Details of the immunization schemes and immunoanalyses are reported in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Eleonora Molesti for performing the enzyme-linked immunosorbent assays and Luisa Lanzilao for performing the serum bactericidal activity assays.
Footnotes
Competing interest statement: This work was undertaken at the request of and was sponsored by GlaxoSmithKline Biologicals SA. F.M., F.N., R.D.B., F.S., M.C., C.G., I.P., L.B.M., G.D.G., and R.R. are employees of the GSK group of companies. M.A. received a PhD studentship from GSK and University of Birmingham. C.A.M. was employee of Novartis Vaccines Institute for Global Health (acquired by the GSK group of companies in March 2015) at the time of the study. A.S. was an employee of GSK group of companies when the study was performed. F.M., L.B.M., C.A.M., and A.S. are listed as inventors on patents related to this work owned by the GSK group of companies. GVGH (GSK Vaccines Institute for Global Health), an Institute with a nonprofit mission, has partnered with Biological E Ltd., a vaccine manufacture located in Hyderabad, India, for the development of a Typhoid Conjugate Vaccine, which received Indian marketing authorization in March 2020.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005857117/-/DCSupplemental.
Data Availability.
All study data are included in the main text and SI Appendix.
References
- 1.Mond J. J., Lees A., Snapper C. M., T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Käyhty H., Karanko V., Peltola H., Mäkelä P. H., Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: No evidence of immunologic tolerance or memory. Pediatrics 74, 857–865 (1984). [PubMed] [Google Scholar]
- 3.Poolman J., Borrow R., Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev. Vaccines 10, 307–322 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Lesinski G. B., Westerink M. A., Vaccines against polysaccharide antigens. Curr. Drug Targets Infect. Disord. 1, 325–334 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli R., Glycoconjugate vaccines: Principles and mechanisms. Sci. Transl. Med. 10, eaat4615 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rappuoli R., De Gregorio E., Costantino P., On the mechanisms of conjugate vaccines. Proc. Natl. Acad. Sci. U.S.A. 116, 14–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brynjolfsson S. F. et al., Hyporesponsiveness following booster immunization with bacterial polysaccharides is caused by apoptosis of memory B cells. J. Infect. Dis. 205, 422–430 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Bjarnarson S. P. et al., The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur. J. Immunol. 35, 1037–1045 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Bjarnarson S. P., Benonisson H., Del Giudice G., Jonsdottir I., Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS One 8, e72588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anonymous; GBD 2017 Typhoid and Paratyphoid Collaborators , The global burden of typhoid and paratyphoid fevers: A systematic analysis for the global burden of disease study 2017. Lancet Infect. Dis. 19, 369–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebacq E., Comparative tolerability and immunogenicity of typherix or typhim Vi in healthy adults: 0, 12-month and 0, 24-month administration. BioDrugs 15 (suppl. 1), 5–12 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Overbosch D. et al., Combined typhoid fever and hepatitis A vaccine: Comparison of immunogenicity and safety to concomitant monovalent vaccine over 3 years. J. Travel Med. 12, 319–326 (2005). [DOI] [PubMed] [Google Scholar]
- 13.MacLennan C. A., Martin L. B., Micoli F., Vaccines against invasive Salmonella disease: Current status and future directions. Hum. Vaccin. Immunother. 10, 1478–1493 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szu S. C., Development of Vi conjugate - a new generation of typhoid vaccine. Expert Rev. Vaccines 12, 1273–1286 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Wain J., Hendriksen R. S., Mikoleit M. L., Keddy K. H., Ochiai R. L., Typhoid fever. Lancet 385, 1136–1145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin C. et al., Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: A randomised controlled, phase 2b trial. Lancet 390, 2472–2480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan V. K. et al., Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: A multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin. Infect. Dis. 61, 393–402 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Micoli F. et al., Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine 29, 712–720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Micoli F. et al., Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine 30, 853–861 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Rondini S. et al., Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM(197) glycoconjugate vaccine against Salmonella Typhi. J. Infect. Dev. Ctries. 6, 763–773 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Rondini S. et al., Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin. Vaccine Immunol. 18, 460–468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Damme P. et al., Safety, immunogenicity and dose ranging of a new Vi-CRM(1)(9)(7) conjugate vaccine against typhoid fever: Randomized clinical testing in healthy adults. PLoS One 6, e25398 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutta Z. A. et al., Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: Results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect. Dis. 14, 119–129 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Arcuri M. et al., The influence of conjugation variables on the design and immunogenicity of a glycoconjugate vaccine against Salmonella Typhi. PLoS One 12, e0189100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sercan Alp Ö. et al., Memory CD8(+) T cells colocalize with IL-7(+) stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur. J. Immunol. 45, 975–987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pracht K. et al., A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. Eur. J. Immunol. 47, 1389–1392 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Pihlgren M. et al., Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 170, 2824–2832 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Cunningham A. F. et al., Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J. Immunol. 178, 6200–6207 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Di Niro R. et al., Salmonella infection drives promiscuous B cell activation followed by extrafollicular affinity maturation. Immunity 43, 120–131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreo F. et al., Persistence of Streptococcus pneumoniae urinary antigen excretion after pneumococcal pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 28, 197–201 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Hamer D. H. et al., Assessment of the Binax NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage. Clin. Infect. Dis. 34, 1025–1028 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Laferriere C. A., Sood R. K., de Muys J. M., Michon F., Jennings H. J., Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: Length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 66, 2441–2446 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeters C. C. et al., A comparative study of the immunogenicity of pneumococcal type 4 polysaccharide and oligosaccharide tetanus toxoid conjugates in adult mice. J. Immunol. 146, 4308–4314 (1991). [PubMed] [Google Scholar]
- 34.Peeters C. C. et al., Preparation of polysaccharide-conjugate vaccines. Methods Mol. Med. 87, 153–174 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Wessels M. R. et al., Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect. Immun. 66, 2186–2192 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rana R. et al., Development and characterization of Haemophilus influenzae type b conjugate vaccine prepared using different polysaccharide chain lengths. Vaccine 33, 2646–2654 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Szu S. C. et al., Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect. Immun. 57, 3823–3827 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szu S. C., Bystricky S., Hinojosa-Ahumada M., Egan W., Robbins J. B., Synthesis and some immunologic properties of an O-acetyl pectin [poly(1→4)-alpha-D-GalpA]-protein conjugate as a vaccine for typhoid fever. Infect. Immun. 62, 5545–5549 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raso M. M. et al., GMMA and glycoconjugate approaches compared in mice for the development of a vaccine against Shigella flexneri serotype 6. Vaccines (Basel) 8, E160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poolman J. et al., Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 27, 3213–3222 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Benaissa-Trouw B. et al., Synthetic polysaccharide type 3-related di-, tri-, and tetrasaccharide-CRM(197) conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infect. Immun. 69, 4698–4701 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti L. C. et al., Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J. Clin. Invest. 89, 203–209 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and SI Appendix.