Abstract
Background and Aims
Mesenchymal stem cells [MSCs] are used in preclinical and clinical studies for treatment of immune-mediated disorders, thanks to their immunomodulatory properties. Cell therapy with MSCs induces multiple effects in the immune system which ultimately lead to increase in the number of immune cells with regulatory phenotype. In this study, we investigated whether the beneficial effects of MSC therapy are maintained in the long term in a clinically relevant mouse model of colitis.
Methods
A single dose of adipose-derived MSCs [aMSCs] was infused into dextran sulphate sodium [DSS]-induced colitic mice during the induction phase of the disease. Following a latency period of 12 weeks, mice were re-challenged with a second 7-day cycle of DSS.
Results
DSS-induced colitic mice treated with aMSCs showed significant reduction in their colitic disease activity index during the second DSS challenge when compared with non-aMSC treated DSS-induced colitic mice. Strikingly, the long-term protection induced by aMSC therapy was also observed in Rag-1-/- mice where no adaptive immune memory cell responses take place. Increased percentages of Ly6G+CD11b+ myeloid cells were observed 12 weeks after the first inflammatory challenge in the peritoneal cavity, spleen, and bone marrow of DSS-induced colitic mice that were infused with aMSCs. Interestingly, upon re-challenge with DSS, these animals showed a concomitant increase in the regulatory/inflammatory macrophage ratio in the colon lamina propria.
Conclusions
Our findings demonstrate for the first time that MSC therapy can imprint an innate immune memory-like response in mice which confers sustained protection against acute inflammation in the long term.
Keywords: Inflammatory bowel disease, stem cell therapy, sustain protection
1. Introduction
Inflammatory bowel diseases [IBD], such as Crohn’s disease and ulcerative colitis, are chronic inflammation of intestinal mucosa affecting millions of people in developed countries. Although IBD aetiology is not yet fully understood, gut inflammation results from unregulated immune reactions mainly in genetically susceptible individuals.1 Current available treatments have aimed at reducing inflammation in an attempt to avoid disease recurrence or to prolong clinical remission periods. Despite recent advances, no treatment is fully effective for many refractory patients, so that surgery remains as the only alternative. New therapeutic approaches are then highly needed to complement the efficacy of current immunosuppressive and biologic agents.
Mesenchymal stem cells [MSCs] are used in a wide diversity of cell therapy protocols for treatment of inflammatory disorders, with very promising results.2–5 Although an array of immune responses have been described with respect to the immunomodulatory action of MSCs, such as induction of cells with a regulatory phenotype, inhibition of inflammatory cells, and secretion of anti-inflammatory molecules,6–12 among others, the precise mechanism of action of MSCs is not fully defined. The immune responses induced by MSCs can take place in a broad spectrum of in vivo conditions that include different sources of MSCs, cell doses, MHC context, and even the route of administration.13–16 In this regard, although the biodistribution of MSCs is strongly dependent on the route of administration, there is no clear correlation between the in vivo biodistribution of the MSCs and their capacity to modulate pathological immune responses.14,17,18 Although the general assumption is that the therapeutic action of MSCs is transient,10,19 recent studies have suggested that sustained beneficial effects also may be generated by MSC therapy.20–24 Moreover, it is well known that the immune system has the ability to mediate long-term responses influenced by previous insults.25 In light of this, in this study we examined whether MSC therapy could induce changes in the immune system able to sustain their beneficial effects during prolonged periods of time. To address this, we analysed the long-term effects of cell therapy with adipose-derived mesenchymal stem cells [aMSCs] in a mouse model of IBD induced by dextran sulphate sodium [DSS]. Following a latency period of 12 weeks, mice were re-challenged with DSS and their response to the inflammatory insult was evaluated. Our findings demonstrate that MSC therapy has a beneficial effect in immunocompetent as well as in Rag-1-/- colitic mice. Most importantly, these effects were maintained upon re-challenge with DSS, where an ameliorated colitis was observed in mice that had been treated with aMSCs during the first inflammation cycle. The observation of an increased regulatory/inflammatory macrophage ratio in the colon lamina propria in aMSC-treated colitic mice emphasises the key interplay between the infused aMSCs and the myeloid cells in the context of intestinal inflammation.
2. Materials and Methods
2.1. Mice
C57BL/6J, BALB/cJ and Rag-1-/- mice from the Jackson Laboratory were used. All experiments were performed in accordance with the corresponding regulations regarding experimental animal welfare [RD 223/1998 and Directive 2010/63/EU protocols]. The experimental protocol was reviewed and approved by the ethics committee for animal research at the CIEMAT and Comunidad de Madrid [based on the RD 53/2013].
2.2. Generation and characterisation of adipose-derived mesenchymal stem cells
C57BL/6J, BALB/cJ and human aMSCs were generated from abdominal adipose tissue as described in Supplementary Material, available as Supplementary data at ECCO-JCC online. Human adipose tissue samples were obtained after informed consent approved by the Spanish Ethics Committee [IIS-Fundación Jiménez Díaz, Madrid, Spain, PIC017-18_FJD]. aMSCs were defined according to the criteria of the International Society for Cellular Therapy [Supplementary Figure 1A and B, available as Supplementary data at ECCO-JCC online].26
2.3. Colitis induction and experimental design
Different concentrations of dextran sulphate sodium [DSS] were used with ranges from 1.5% to 3.5% in drinking water during 7 days ad libitum. A single dose of aMSCs [from 1 to 5 × 106 cells/mouse] was intraperitoneally infused on the indicated day. Following a resting period of 12 weeks with water, mice then received a second 7-day cycle of DSS [Figure 1A]. Disease activity index [DAI] was assigned according to the criteria described in Supplementary Material.27 Peripheral blood samples were evaluated by an automated blood cell-counter [Abacus, Diatron].
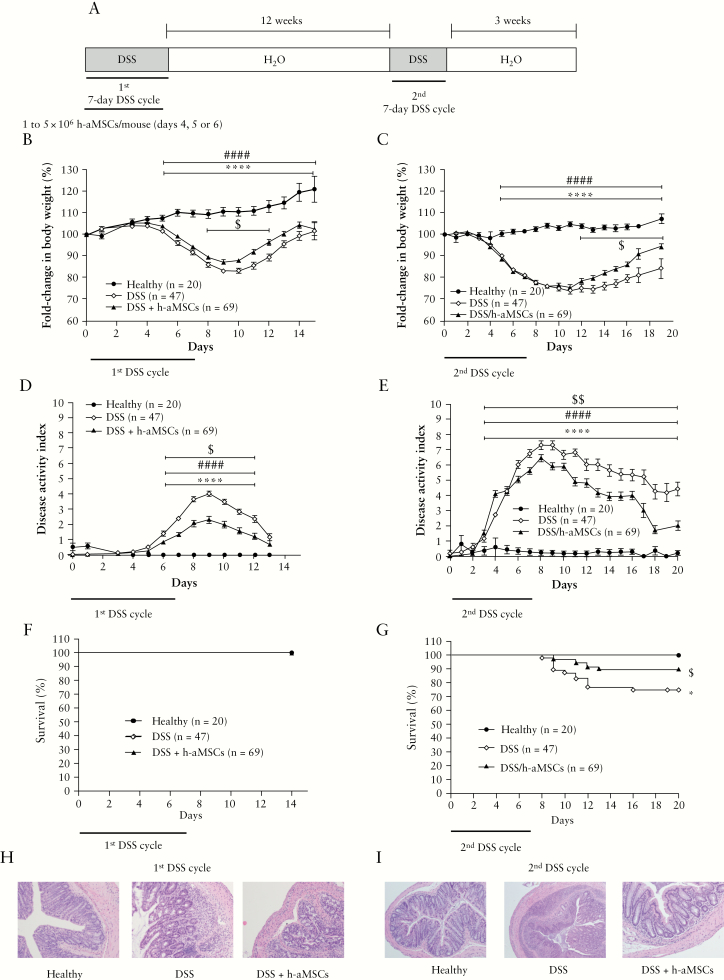
Figure 1.
Experimental design and DSS-induced colitis status of mice following intraperitoneal administration of human adipose-derived MSCs. Experimental design of DSS-induced colitis; [A] fold-change in body weight during the first [B] and the second [C] treatment with DSS; disease activity index during the first [D] and the second [E] treatment with DSS; survival during the first [F] and the second [G] treatment with DSS. Representative images of colon tissue [magnification 100X] at Day 9 following the first [H] and at Day 10 upon the second [I] treatment with DSS. Healthy, n = 20; DSS, n = 47, and DSS + h-aMSCs, n = 69. Data are presented by mean and standard error of the disease activity index and of the fold-change in body weights, with respect to Day 0 expressed as percentage of initial weight. Survival data are presented by percentage. Significance was analysed by the Mann-Whitney U test and log rank test and represented by *p <0.05 and ****p ≤0.0001 DSS vs heathy, ####p ≤0.0001 DSS + h-aMSCs vs healthy, and $p <0.05 and $$p <0.01 DSS + h-aMSCs vs DSS. Results correspond to 12 independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
2.4. Histological analysis
Colons were surgically removed and fixed with formalin overnight at 48 h. Tissue sections were embedded in paraffin and stained with haematoxylin/eosin.
2.5. Cell isolation
Mice were culled and mononuclear cells were isolated from the peritoneal cavity, spleen, bone marrow, and mesenteric lymph nodes [mLNs] using a cell strainer. Colon leukocyte isolation was carried out following the Lamina Propria Dissociation Kit manufacturer´s instructions.
2.6. Flow cytometry analysis
Isolated mononuclear cells were surface-stained with antibodies described in Supplementary Table 2, available as Supplementary data at ECCO-JCC online. For intracellular staining, the Foxp3/transcription factor staining buffer set was performed according to the manufacturer’s instructions. For intracellular analysis of cytokine expression, mononuclear cells were stimulated as described in Supplementary Material. Cells were collected on a BD LSR Fortessa flow cytometer. Data were analysed using FlowJo software.
2.7. Immunosuppression assays
Peripheral blood mononuclear cells [PBMCs] were isolated by density centrifugation gradient using Ficoll-Paque Plus from heparinised peripheral blood obtained from healthy donors after informed consent. Splenocytes were obtained from C57BL/6J mice, and the Ly6G+CD11b+ myeloid cells were sorted from total splenocytes by a BD Influx cell sorter using Ly6G and CD11b antibodies. CD4+ T cells were isolated from total splenocytes following Mojosort Mouse CD4 kit instructions.
Carboxyfluorescein diacetate N-succinimidyl ester [CFSE] labelling is described in Supplementary Material. Cell proliferation was determined by flow cytometry analysing the dilution of the mean fluorescence intensity in the CFSE-labelled CD4+CD3+DAPI- population [viable lymphocytes]. Data were analysed with FlowJo software.
2.8. Statistical analysis
Normal distribution was analysed by the Shapiro-Wilks test. The parametric test [Student t test] for normal distribution and non-parametric test [Mann-Whitney U test] were used for non-normal distribution. Analysis was performed using the GraphPad Prism 7.00.
All details regarding the material, methods, material companies, and the antibodies used are reported in Supplementary Material and Supplementary Tables 1 and 2, available as Supplementary data at ECCO-JCC online.
3. Results
3.1. Protective long-term effects of MSC therapy in DSS-induced colitis
Numerous immune responses have been implicated in the beneficial effect associated with cell therapy with MSCs.6–10 In this study, we examined whether these beneficial effects are also maintained in the long term in a clinically relevant experimental model of colitis.
At present, there is no consensus with respect to the most efficient MSC therapy protocol to be used for treatment of inflammatory disorders. Hence, we first carried out experiments in order to test the effects induced by different doses of human aMSCs [h-aMSCs, ranging from 1 to 5 × 106 h-aMSC/mouse], and also the influence of the day of h-aMSC infusion [Days 4 to 6] during the first cycle of DSS administration. As shown in Supplementary Figure 2, available as Supplementary data at ECCO-JCC online, a 7-day DSS cycle resulted in a marked body weight loss [left] and disease activity index [DAI, right] in non-aMSC treated DSS-induced colitic mice compared with healthy mice. Regardless of the h-aMSC dose used and day of infusion of the h-aMSCs, a similar degree of colitis attenuation was observed in all instances, which accords with previous results published in the literature.6,14,28
To study the long-term effects induced by MSC therapy, experiments were conducted according to the experimental design depicted in Figure 1A, consisting of two 7-day cycles of DSS administered 3 months apart. A single dose of h-aMSCs [range 1 to 5 × 106 h-aMSCs per mouse] was infused during the first DSS treatment [from Days 4 to 6]. As shown in Figure 1B, the infused aMSCs induced a significantly less pronounced decrease in body weight as well as a reduced DAI [Figure 1D] with respect to non-aMSC treated colitic mice. All mice corresponding to the different groups of treatment survived [Figure 1F]. Histologically, a better preserved colon morphology and an attenuated leukocyte infiltration were observed in h-aMSC treated colitic mice with respect to non-aMSC treated colitic mice [Figure 1H].
Following a latency period of 12 weeks, mice both treated and non-treated with h-aMSC reached normal levels of granulocytes, monocytes, lymphocytes, and platelets in peripheral blood [Supplementary Figure 3A, available as Supplementary data at ECCO-JCC online]. Additionally, the histology of the colons [Supplementary Figure 3B] and the body weights [Supplementary Figure 3C] were similar to control mice. Strikingly, when mice that were treated with h-aMSCs during the first DSS cycle [DSS/h-aMSC mice] were exposed to a second DSS cycle [1st DSS/h-aMSC + 2nd DSS mice], a significantly less pronounced decrease in body weight [Figure 1C] and reduced DAI [Figure 1E] were again observed compared with non-aMSC treated DSS-induced colitic mice [1st + 2nd DSS mice]. In addition to this, a significant increase in the survival of mice was observed in 1st DSS/h-aMSC + 2nd DSS mice with respect to [1st + 2nd] DSS mice [Figure 1G]. Histologically, the colon showed an attenuated leukocyte infiltration with respect to the [1st + 2nd] DSS mice together with a better-preserved colon morphology [Figure 1I].
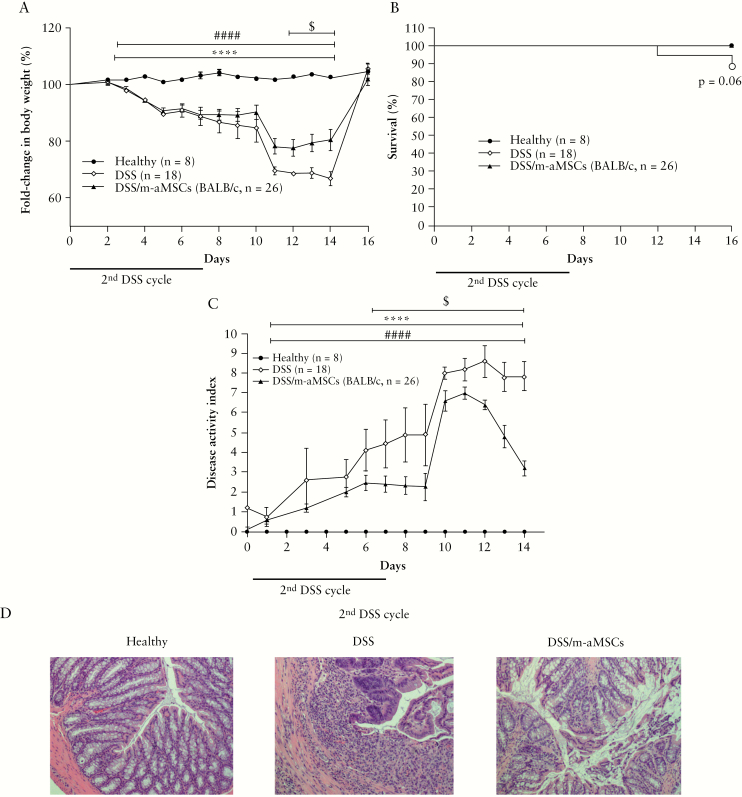
To investigate whether allogeneic mouse-derived aMSCs [m-aMSCs] mimicked the effects induced by h-aMSCs, which is a closer model to the one used in the clinic, we conducted similar experiments in which aMSCs from BALB/cJ mice were infused into C57BL/6J mice. As noted in the xenogeneic transplantation model, a less pronounced decrease in body weight and DAI was observed in DSS/m-aMSC with respect to colitic mice during the second DSS challenge [Figure 2]. In parallel, increased survival, better-preserved colon morphology, and attenuated leukocyte infiltration were observed in DSS/m-aMSC mice with respect to non m-aMSC infused colitic mice.
Figure 2.
Colitis status of mice determined by fold-change in body weight, survival, disease activity index, and histological analysis of colons of healthy, non-aMSC and allogeneic [from BALB/cJ mice] aMSC-treated colitic mice during the second challenge with DSS. Fold-change in body weight [A], survival [B], disease activity index [C], and representative images of colon tissue [magnification 100X] at Day 7 during the second challenge with DSS [D]. Healthy, n = 8; [1st + 2nd] DSS, n = 18; and 1st DSS/m-aMSCs + 2nd DSS, n = 26. Data are presented by mean and standard error of the mean of fold-change in body weight and disease activity index. Significance was analysed by the Mann-Whitney U test and represented by ****p ≤ 0.0001 [1st + 2nd] DSS vs healthy; ####p ≤0.0001 1st DSS/m-aMSCs + 2nd DSS vs healthy; and $p <0.05 1st DSS/m-aMSCs + 2nd DSS vs [1st + 2nd] DSS. Results correspond to three independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
All of these results indicate that a single intraperitoneal infusion of aMSCs can induce long-term protection against a recurrent inflammation.
3.2. The protective long-term effects of aMSCs are highly dependent on the early response to MSC therapy
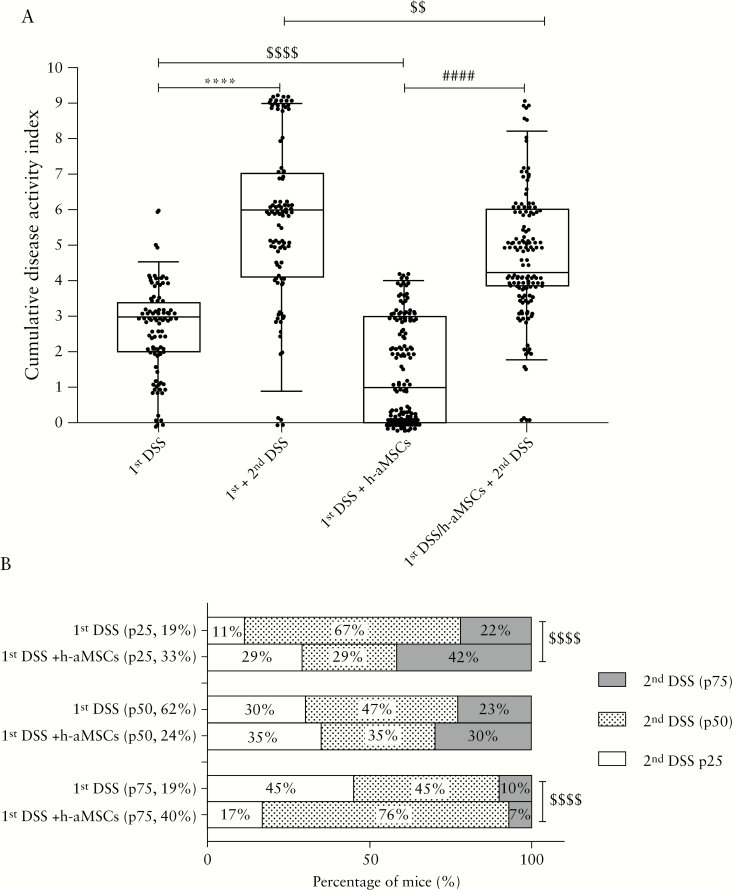
We next questioned whether highly respondent mice to the MSC therapy during the first DSS cycle were the same individuals that showed an attenuated colitis during the second DSS cycle. With this aim in mind, we calculated the cumulative disease activity index in non-aMSC and aMSC-treated colitic mice during the first DSS cycle, to stratify mouse responses according to their percentiles [p25, p50, and p75, Figure 3A]. As shown in Figure 3B, 29% of highly respondent mice to MSC therapy during the first DSS cycle [p25; 33%] were highly protected upon the second DSS cycle. This is in contrast to the 11% of non-aMSC treated DSS-induced colitic mice that had an attenuated response to the first DSS cycle [p25; 19%]. This suggests that the initial responses to MSC therapy govern the protection against a subsequent DSS challenge, thus pointing out that the long-term effects of MSC therapy are highly dependent on the first response. It is of interest that 17% of the DSS/h-aMSC colitic mice within p75 to the first DSS [p75, 40%] showed high protection upon the second DSS cycle [p25], suggesting that the MSC therapy induces an altered susceptibility to the inflammatory challenges that is maintained in the long term, although they were the h-aMSC treated colitic mice less protected during the first DSS cycle.
Figure 3.
Stratification and correlation of non-treated and treated with h-aMSCs in DSS-induced colitic mice based on percentile of cumulative disease activity index during the first and the second DSS cycles. Cumulative disease activity index of mice treated and non-treated with a single intraperitoneal dose of h-aMSCs during the first and second DSS cycles [A]. Contingency graph in percentage of colitic mice classified as treated and non-treated with h-aMSCs by the percentile [p25, p50, and p75] of their cumulative disease activity index during the first and second DSS cycles [B]. Data are represented in box plot graph by the median [p50 middle line] and interquartile ranges [p25 lower edge and p75 upper edge] of the cumulative disease activity index and the percentage of mice in the contingency graph in the first and second DSS cycles. Significance was analysed by the Mann-Whitney U test and Fisher´s test and represented by ****p ≤0.0001 DSS vs healthy; ####p ≤0.0001 DSS + h-aMSCs vs healthy; and $$p <0.01 and $$$$p ≤0.0001 DSS + h-aMSCs vs DSS. Results correspond to 10 independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
3.3. The adaptive immune response is dispensable for the protective long-term effects of MSC therapy in DSS-induced colitis
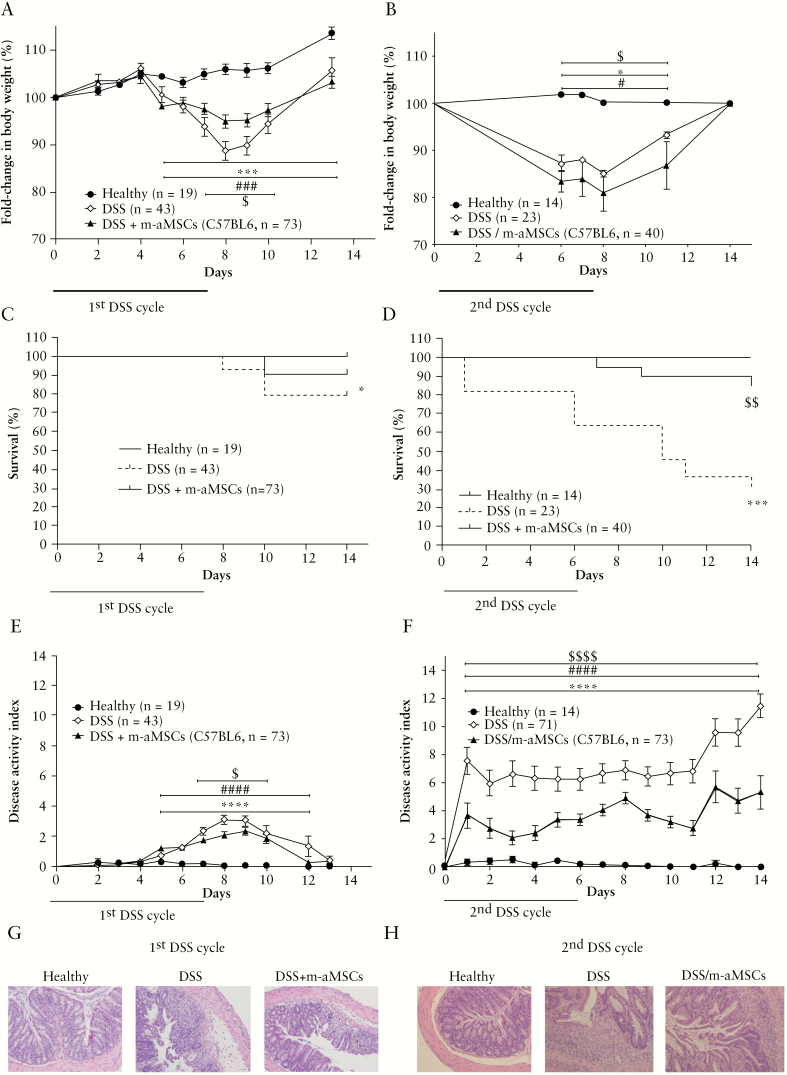
To investigate the immune responses responsible for the beneficial long-term effects induced by MSCs, similar experiments were conducted in Rag-1-/- mice in the absence of adaptive B and T cell responses.29 As observed in our previous experiments, cell therapy with m-aMSCs in Rag-1-/- colitic mice also showed significantly less pronounced decrease in body weight [Figure 4A], increased survival rate [Figure 4C], and reduced DAI [Figure 4E] with respect to DSS-induced colitic Rag-1-/- mice. This was accompanied by a better-preserved colon morphology and attenuated leukocyte infiltration in m-aMSC-infused colitic RAG-1-/- mice with respect to non-aMSC infused colitic Rag-1-/- mice [Figure 4G]. These results confirmed that innate immune responses are the key orchestrators of the immunomodulatory effects of aMSCs.
Figure 4.
Colitis status of Rag-1-/- immunodeficient mice in healthy and DSS-induced colitic mice following intraperitoneal administration of adipose-derived mouse MSCs. Fold-change in body weight during the first [A] and the second [B] treatment with DSS; survival during the first [C] and the second [D] treatment with DSS; disease activity index during the first [E] and during the second [F] treatment with DSS. Representative images of colon tissue [magnification 100X] at Day 8 following the first [G] and at Day 10 upon the second [H] treatment with DSS. Data are presented by mean and standard error of the disease activity index and the fold-change in body weight with respect to Day 0 expressed as percentage of initial weight. Survival data are presented by percentage over time. Healthy, n = 19; DSS, n = 43; and DSS + m-aMSCs, n = 73. Significance was analysed by the Mann-Whitney U test and long rank test and represented by *p <0.05, ***p <0.001 and ****p ≤ 0.0001 DSS vs healthy; #p < 0.05, ###p < 0.001, and ####p ≤ 0.0001 DSS + m-aMSCs vs healthy and $p <0.05, $$p <0.01, and $$$$p ≤0.0001 DSS + m-aMSCs vs DSS. Results correspond to 12 independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
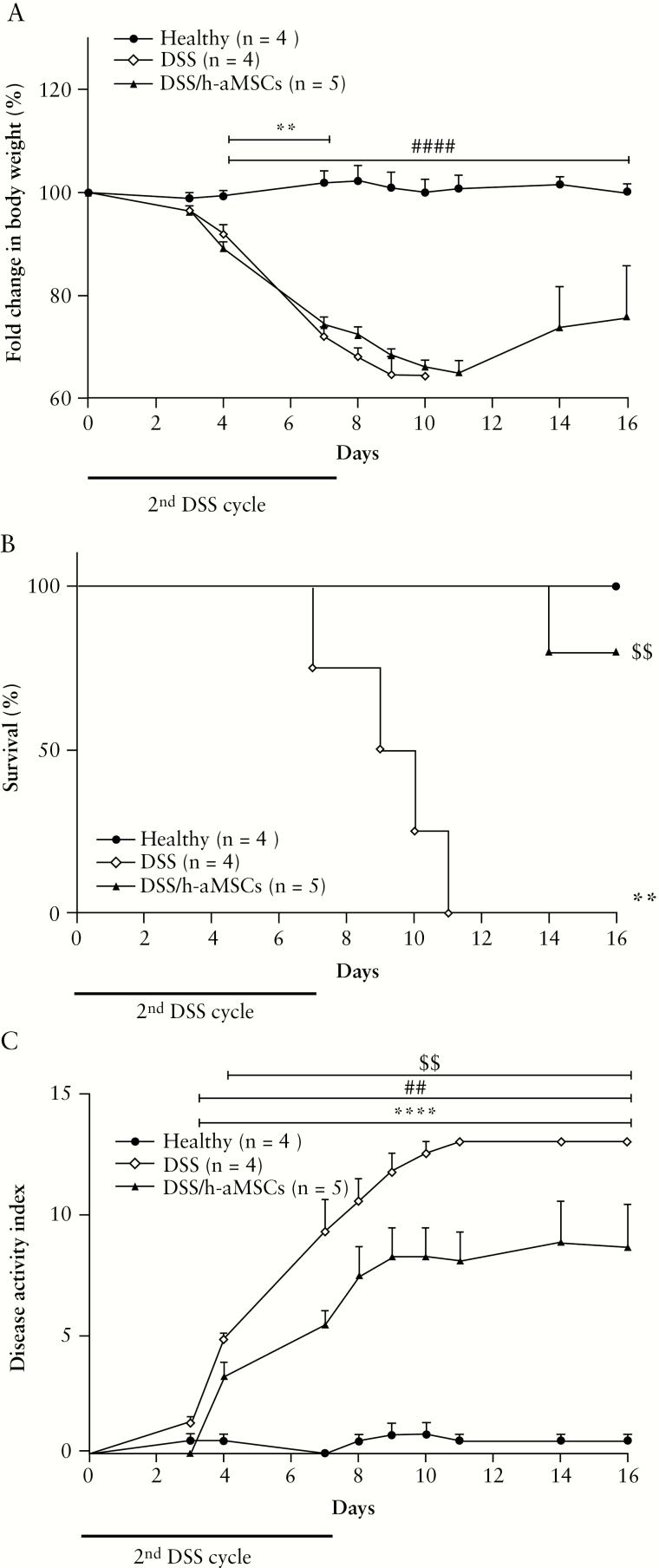
Remarkably, as observed in immunocompetent mice following the second DSS cycle, DSS/m-aMSC Rag-1-/- mice showed less pronounced decrease in body weight [Figure 4B] and a lower DAI than the non-aMSC treated DSS-induced colitic Rag-1-/- mice [Figure 4F]. Strikingly, 85% of DSS/m-aMSC colitic RAG-1-/- mice survived with respect to 27% survival of non m-aMSC treated colitic RAG-1-/- mice [Figure 4D]. Consistent with these observations, the colon morphology was better preserved and showed reduced leukocyte infiltration [Figure 4H] in the DSS-induced colitic mice infused with m-aMSCs after the second DSS cycle, with respect to non m-aMSC treated colitic mice. As was observed in immunocompetent mice, the beneficial effects induced by m-aMSCs were confirmed using xenogeneic aMSCs [from human adipose tissue, Figure 5].
Figure 5.
Colitis status of immunodeficient Rag-1-/- mice determined by fold-change in body weight, survival and disease activity index of healthy, non-aMSC and adipose-derived human aMSC-treated mice during the second challenge with DSS. Fold-change in body weight (A), survival (B) and disease activity index (C). Healthy, n = 4; (1st + 2nd) DSS, n = 4 and 1st DSS/h-aMSCs + 2nd DSS, n = 5. Data are presented by mean and standard error of the mean of the fold-change in body weight and disease activity index. Significance was analysed by the U Mann-Whitney test and represented by **p < 0.01 and ****p < 0.0001 (1st + 2nd) DSS vs heathy; ##p < 0.01 and ###p < 0.001 1st DSS/h-aMSCs + 2nd DSS vs healthy and $$p < 0.01 1st DSS/h-aMSCs + 2nd DSS vs (1st + 2nd) DSS. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
These results demonstrate the key role of innate immune memory responses in the sustained beneficial effects of cell therapy with MSCs for treatment of DSS-induced colitis.
3.4. Phenotypic analysis of myeloid populations induced by cell therapy with MSCs in DSS-induced colitis
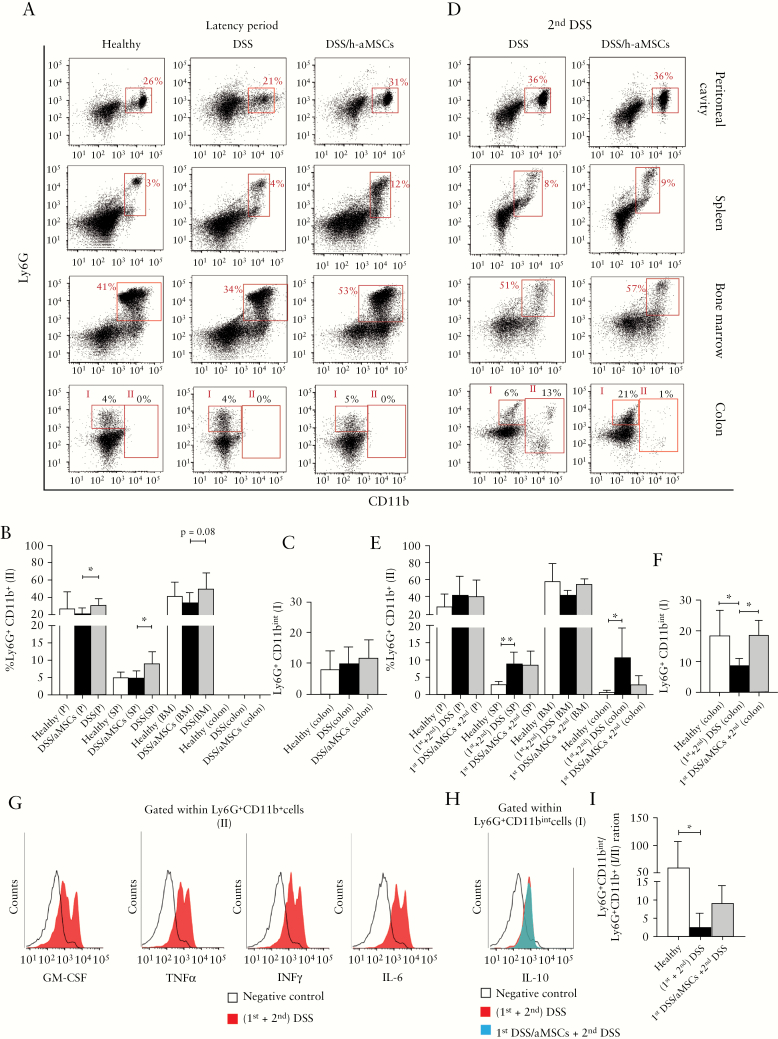
In order to dissect the mechanisms that are involved in the protective long-term effects induced by MSCs in the DSS-induced colitis, we analysed the immune cell populations that were present in different organs and tissues [peripheral blood, peritoneal cavity, spleen, bone marrow, and colon lamina propria] during the latency period of 12 weeks following the first DSS cycle and during the second challenge with DSS. As shown in Figure 6A and B, a significant increase in the percentage of Ly6G+CD11b+ myeloid cells was observed in the peritoneal cavity, spleen, and bone marrow, but not in the colon lamina propria, in DSS/aMSC mice, with respect to the DSS mice. As shown in Supplementary Figure 4A and 4B, available as Supplementary data at ECCO-JCC online, these Ly6G+CD11b+ myeloid populations co-expressed Ly6C, F4/80, MHC-II, CD11c, CD206, CD169, CX3CR1, iNOS, and Arg-1 markers with different levels of expression depending on the tissue studied. No clear differences among the groups of mice were observed.
Figure 6.
Frequencies of Ly6G+CD11b+ myeloid cells measured by flow cytometry in peritoneal cavity, spleen, bone marrow, and colon during the latency period and during the second DSS treatment in non-aMSC and aMSC-treated DSS-induced colitic mice. Representative dot-plots by flow cytometry [A] and frequency expressed as percentage of Ly6G+CD11b+ myeloid cells in peritoneal cavity [P], spleen [SP], bone marrow [BM], and colon [B] and frequency expressed as percentage of Ly6G+CD11bint myeloid cells in colons [C] in healthy [n = 5], non-aMSC [n = 6], and aMSC-treated mice [n = 8] 12 weeks after the first DSS challenge. Representative dot-plots by flow cytometry [D], frequency expressed as percentage of Ly6G+CD11b+ myeloid cells in peritoneal cavity, spleen, bone marrow, and colon [E], frequency expressed as percentage of Ly6G+CD11bint myeloid cells in the colons [F], and representative histograms of GM-CSF, TNFα, IFNγ and IL-6 expression gating on Ly6G+CD11b+ myeloid cells [gate II] [G] and IL-10 expression gating on Ly6G+CD11bint myeloid cells [gate I] [H] in non-aMSC [red] and aMSC-treated colitic mice [blue] during the second DSS challenge. Ly6G+CD11bint/Ly6G+CD11b+ ratio in the colon in healthy, non-aMSC, and aMSC-treated colitic mice during the second DSS challenge [I]. Data are presented by mean and standard error of the mean of the percentage of Ly6G+CD11b+ and Ly6G+CD11bint. Significance was analysed by the MannWhitney U test and represented by *p <0.05 and **p < 0.01. Results correspond to four independent experiments. DSS, dextran sulphate sodium; MSCs, mesenchymal stem cells.
Ly6G+CD11b+ myeloid cells from the peritoneal cavity co-expressed F4/80, MHC-II, CD206, and CX3CR1 molecules considered characteristic markers of alternative-activated macrophages derived from bone marrow monocytes [Supplementary Figure 4A].30,31 Based on the expression levels of Ly6C and Ly6G, the CD11b+ myeloid cells that increased in the spleen and in the bone marrow in h-aMSC treated mice during the 12-week latency period [Figure 6A, B and Supplementary Figure 4A] are defined as polymorphonuclear myeloid-derived suppressor cells [PMN-MDSCs Ly6CintLy6Ghigh] and immature myeloid cells [IMCs, Ly6CintLy6Gint].32 Moreover, we observed that the PMN-MDSCs from DSS/aMSC mice co-expressed iNOS and Arg-1 markers [Supplementary Figure 4B]. In addition to this, the MDSCs from the spleen also expressed IL-10, confirming their MDSC phenotype [Supplementary Figure 4B].32 Additionally, Ly6G+CD11b+ myeloid cells obtained from spleen in DSS/aMSC mice inhibited the proliferation of responder CD4+ T cells in an in vitro CFSE-proliferation assay using T cells as responder cells [Supplementary Figure 4C], confirming their identity as MDSCs. All these results suggest that MSC therapy induces in vivo reservoirs of different types of myeloid cells in different tissues, which are maintained in the long term.
To confirm whether these reservoirs of Ly6G+CD11b+ myeloid cells in the peritoneal cavity, spleen, and bone marrow also have a role in the long-term effect of MSC therapy, we monitored the dynamic of these populations during the second DSS cycle. There was a significant increase in the percentage of Ly6G+CD11b+ in the spleen and colon [gate II, Figure 6D and E] in mice re-challenged with DSS, with respect to the healthy mice. No differences were observed between DSS/aMSC-colitic mice and non-aMSC treated DSS induced-colitic mice. Ly6G+CD11b+ myeloid cells [gate II] increased in the colon of non-aMSC treated DSS-colitic mice co-expressed GM-CSF, TNFα, IFNγ, and IL-6, thus confirming their inflammatory profile [Figure 6G]. These myeloid populations also co-expressed Ly6C, F4/80, CX3CR1, iNOS, and MHC-II molecules that are indicative markers of inflammatory or M1 macrophages [Supplementary Figure 5, available as Supplementary data at ECCO-JCC online]. In contrast, DSS/aMSC-colitic mice had a decrease in the percentage of the inflammatory M1 macrophage population with respect to the non-aMSC treated DSS-induced colitic mice [Figure 6D and E], although these differences were not significant. In parallel, a significant increase in Ly6G+CD11bint [gate I] myeloid cells in the colon [Figure 6F] were observed. These cells co-expressed IL-10 [Figure 6H], Ly6C, F4/80, and Arg-1 molecules [Supplementary Figure 5], which is indicative of a regulatory M2 macrophage phenotype important in the proliferative phase of wound healing.33–35 As shown in Figure 6I, there is a tendency to increase the regulatory [Ly6G+CD11bint, gate I]/inflammatory [Ly6G+CD11b+, gate II] ratio in the colon of DSS/aMSC-colitic mice compared with non-aMSC colitic mice. These results showed a direct correlation between the regulatory/inflammatory balance and the respective inflammatory status of the non-aMSC and aMSC-treated colitic mice during the second DSS cycle. The differences in the myeloid cell components in the colon lamina propria were not observed during the latency period, suggesting that these cells may migrate to the inflamed colon in response to the secondary inflammatory challenge with DSS.
In summary, MSC treatment induces an accumulation of macrophages in the peritoneal cavity as well as increase of myeloid-derived suppressor cells and immature myeloid cells in the spleen and bone marrow, upon the first DSS cycle. These effects have also been observed up to 7 months following the first DSS cycle [data not shown]. Ultimately, these MSC-induced myeloid cells are the responsible for the increased regulatory/inflammatory macrophage ratio observed in the DSS/aMSC mice during the second inflammatory challenge.
4. Discussion
Mesenchymal stem cells are proposed as a novel cell therapy for treatment of inflammatory and immune-mediated disorders. Numerous promising results in preclinical studies and clinical trials support their use, even though their mechanisms of action are not fully defined.3,4,21,36,37 There has been a general consensus that the immunomodulatory effects of MSC therapy is transient.10,19 In this study, we investigated whether a protective long-term effect upon treatment with MSCs is maintained to a recurrent inflammatory challenge. To this end, we carried out two DSS treatments in mice 3 months apart with a single dose of adipose tissue-derived MSCs [aMSCs] administered only during the first DSS cycle. The second DSS cycle is comparable to a recurrent inflammation that occurs in IBD, in which the complete resolution of the inflammation is impaired.38 Strikingly, during the second DSS cycle we observed an attenuated degree of intestinal inflammation in DSS/aMSC-colitic mice, with respect to the non-aMSCtreated colitic mice, indicating the protective effects of MSC therapy in the long term. This would imply that the MSC therapy could confer an immune footprint that should be taken into consideration in the clinic. Of interest, previous studies pointed out the long-term effects of MSC therapy. In this sense, Lee et al. observed an attenuation of chronic DSS-induced colitis using multiple infusions of bone marrow-derived mesenchymal stem cell [BM-MSC] therapy during the acute induction with DSS.23 More recently, Alves et al. demonstrated that adipose-derived MSC administration alleviates trinitrobenzenesulphonic acid [TNBS]-induced murine colitis by correcting Th1/Th17/Treg imbalances that persisted up to 60 days.21 However, in this study, MSC-treated mice were not re-challenged chronically. In contrast to this study, durable and sustained long-term attenuated inflammatory responses in the gut in aMSC-treated Rag-1-/- mice, in the absence of adaptive immune responses. Results from clinical trials in transplant contexts and in complex perianal fistulas in Crohn’s disease also indicated the long-term effects of MSC therapy.20–24,39 However, no clear mechanisms were described in any of these studies.
In order to dissect the role played by the immune system in the long-term protection mediated by MSCs in colitic mice, experiments were conducted using Rag-1-/- mice where T and B cell responses are absent. Strikingly, DSS-induced colitic Rag-1-/- mice treated with aMSCs were also protected in the long term, as shown by the less pronounced decrease in body weight, increased survival, and reduced disease activity index in DSS/aMSC-colitic Rag-1-/- mice with respect to non-aMSC-treated colitic Rag-1-/- mice during the second challenge with DSS. According to our results in Rag-1-/- mice, Tregs were not required for the beneficial long-term effects induced by aMSCs in experimental colitis. These results clearly point out the key role played by an innate immune memory induced by MSC therapy. This striking observation is consistent with the view that myeloid cells are not simple housekeepers, but also key orchestrators of the immune response involved in homeostasis in the intestine and in the pathology of inflammatory bowel disease.33,34,40
To define the innate immune mechanisms that are involved in the beneficial long-term effects induced by the MSCs, we conducted an exhaustive and comparative analysis of myeloid populations in different organs and lymphoid tissues. Three months following the first DSS cycle, aMSC-treated mice showed a significant increase in the percentage of Ly6G+CD11b+ myeloid cells in the peritoneal cavity, spleen, and bone marrow, with respect to the non-aMSC-treated mice. No differences were observed in the colon lamina propria.
Ly6G+CD11b+ cells in the peritoneal cavity co-expressed high levels of F4/80 and CD206, markers of alternative activated macrophages [AAMs] and CX3CR1, which indicates their origin from bone marrow-derived monocytes.30,31 Based on the levels of expression of Ly6C and Ly6G markers,41 the CD11b+ myeloid population increased in the spleen and in the bone marrow in the DSS/aMSC mice, and were defined as granulocytic myeloid-derived suppressor cells [PMN-MDSCs, Ly6CintLy6Ghigh] and immature myeloid cells [IMCs, Ly6CintLy6Gint]. We also confirmed the MDSC and IMC phenotypes by the intracellular expression of iNOS, Arg-1, and IL-10 and by in vitro inhibition of T cell proliferation.
The observed increase in the proportion of Ly6G+CD11b+ cells in the peritoneal cavity, the spleen, and in the bone marrow during the resting 12-week period was no longer observed during the inflammatory re-challenge with DSS. Instead, a significant increase of Ly6G+CD11bint myeloid cells co-expressing F4/80, Arg-1, and IL-10 was observed in the colon lamina propria in the DSS/aMSC mice, with respect to DSS mice. A number of studies claimed that these populations are instrumental during the proliferative phase of wound healing.33,34,42,43 A decrease in Ly6G+CD11b+ cells co-expressing F4/80, CX3CR1, iNOS, MHC-II, GM-CSF, TNFα, IFN-γ, and IL-6 [indicative of their inflammatory profile] in the colon lamina propria in the DSS/aMSC mice, with respect to DSS mice, during the re-challenge with DSS was observed. This is in contrast to the rather similar phenotype of the Ly6G+CD11b+ myeloid cells found in the colon lamina propria both in DSS and DSS/aMSC mice during the latency period, thus suggesting the trafficking of these myeloid populations to the inflamed colon. These observations may suggest that the long-term inflammation protection conferred by MSCs is mainly due to an increase in the regulatory/inflammatory balance during the second inflammatory challenge in aMSC-treated colitic mice, with respect to non-aMSC-treated colitic mice. The regulatory myeloid cells found in the colon lamina propria most likely are the AAMs accumulated in the peritoneal cavity, or the MDSCs and IMCs found in the spleen and in the bone marrow.
In line with these results, a number of studies describe the link between the accumulation of AAMs, MDSCs, and IMCs and the subsequent migration to the inflamed tissues and their local conversion into regulatory/wound-healing macrophages. In this sense, these studies have demonstrated that non-classical CX3CR1+ monocytes, CCR2+ monocytes, or bone marrow monocyte precursors were the progenitors of regulatory macrophages that were responsible for wound healing after intestinal injury.33,34,44 Additionally, Quinn et al. described that the inflammation induces monocyte migration and accumulation of AAMs in the peritoneal cavity which then migrate and differentiate into tissue macrophages, inducing tolerance in the long term.45 They also suggested an immunological memory induced by the innate immune system.45–48Moreover, the induction and expansion of MDSCs and IMCs have been amply described in inflammation, infection, autoimmunity, and tumour development contexts as well as in cell therapy with MSCs.9,49–51According to Bronte et al.´s study, MDSCs and IMCs, accumulated in the spleen and in the bone marrow, can also migrate to the colon and develop towards macrophages with a regulatory profile.32 In the context of cell therapy, Vagnozzi et al. claimed that stem cell therapy improved heart function after ischaemia, through an induction of wound-healing F4/80+ macrophages that co-expressed CCR2+ and/or CX3CR1+.52 In addition, Biswas et al. indicated that exosomes released by MSCs are the key factor responsible for the conversion of MDSCs into M2 macrophages.53 Our results also agree with Du et al.´s findings, where the authors found that the insulin-like growth factor 2 [IGF-2] secreted by MSCs trained macrophages to become anti-inflammatory during their maturation, as shown by the elevated expression of programmed death-ligand 1 [PDL-1], which ultimately led to increased numbers of Tregs in the tissues.54 In our studies, the attenuated inflammation mediated by MSCs in the long term was also observed in Rag-1-/- mice, ruling out the key role played by Tregs.
We can conclude that the observed long-term modulation of inflammatory responses observed in DSS/aMSC colitic mice was mainly due to a sustained reservoir of alternative activated macrophages, myeloid-derived suppressor cells, and immature myeloid cells which remained in different lymphoid tissues and organs. All these events can attenuate subsequent inflammatory responses in the intestine, mainly due to an increase in the regulatory/inflammatory balance in the colon lamina propria. Thus, we have identified the induction of an anti-inflammatory innate immune memory by aMSCs which produces long-term beneficial effect in experimental colitis. In this sense, MSCs could be exploited as an alternative therapy for the induction of an anti-inflammatory sustained innate immunity.55 These results highlight the importance of cell therapy with MSCs as inductors of an innate immune memory, and point them out as attractive candidates for treatment of immune-mediated disorders in which the complete resolution of the inflammation is impaired.
Supplementary Material
Acknowledgements
Authors would like to thank Miguel Angel Martin for careful maintenance of the animals.
Part of this work was presented at the European Society of Gene and Cell Therapy Annual Congress [ESGCT / SETGYC] in Barcelona, Spain [2019] and at the International Conference on Innate Immune Memory [IIM] in Nijmegen, The Netherlands [2019].
Funding
This work was supported by Instituto de Salud Carlos III grants (PIE15/00048 and PI17/01161), and cofunded by the European Regional Development Fund (ERDF).
Conflict of Interest
The authors disclose no conflicts.
Author Contributions
Concept and design: ML-S and MIG; acquisition of data: ML-S, RH-S, MFG, and MIG; analysis and interpretation of data: ML-S and MIG; drafting and revising article: ML-S, JAB, and MIG; approval of the final version: ML-S, RH-S, MFG, JAB, and MIG.
References
- 1. Guan W. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res 2019, Dec 1. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lightner AL, Faubion WA. Mesenchymal stem cell injections for the treatment of perianal Crohn’s disease: what we have accomplished and what we still need to do. J Crohns Colitis 2017;11:1267–76. [DOI] [PubMed] [Google Scholar]
- 3. Álvaro-Gracia JM, Jover JA, García-Vicuña R, et al. . Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis [Cx611]: results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis 2017;76:196–202. [DOI] [PubMed] [Google Scholar]
- 4. Wang D, Zhang H, Liang J, et al. . Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant 2013;22:2267–77. [DOI] [PubMed] [Google Scholar]
- 5. Pérez-Simon JA, López-Villar O, Andreu EJ, et al. . Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 2011;96:1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou C, Wu XR, Liu HS, et al. . Immunomodulatory effect of urine-derived stem cells on inflammatory bowel diseases via downregulating Th1/Th17 immune responses in a PGE2-dependent manner. J Crohns Colitis 2020;14:654–68. [DOI] [PubMed] [Google Scholar]
- 7. Song WJ, Li Q, Ryu MO, et al. . TSG-6 secreted by human adipose tissue-derived mesenchymal stem cells ameliorates DSS-induced colitis by inducing M2 macrophage polarization in mice. Sci Rep 2017;7:5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim JY, Im KI, Lee ES, et al. . Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep 2016;6:26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Santalla M, Menta R, Mancheño-Corvo P, et al. . Adipose-derived mesenchymal stromal cells modulate experimental autoimmune arthritis by inducing an early regulatory innate cell signature. Immun Inflamm Dis 2016;4:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lopez-Santalla M, Mancheño-Corvo P, Menta R, et al. . Human adipose-derived mesenchymal stem cells modulate experimental autoimmune arthritis by modifying early adaptive T cell responses. Stem Cells 2015;33:3493–503. [DOI] [PubMed] [Google Scholar]
- 11. Abomaray FM, Al Jumah MA, Kalionis B, et al. . Human chorionic villous mesenchymal stem cells modify the functions of human dendritic cells, and induce an anti-inflammatory phenotype in CD1+ dendritic cells. Stem Cell Rev Rep 2015;11:423–41. [DOI] [PubMed] [Google Scholar]
- 12. Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol 2012;67:1–8. [DOI] [PubMed] [Google Scholar]
- 13. Mancheño-Corvo P, Lopez-Santalla M, Menta R, et al. . Intralymphatic administration of adipose mesenchymal stem cells reduces the severity of collagen-induced experimental arthritis. Front Immunol 2017;8:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang M, Liang C, Hu H, et al. . Intraperitoneal injection [IP], intravenous injection [IV] or anal injection [AI]? Best way for mesenchymal stem cells transplantation for colitis. Sci Rep 2016;6:30696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molendijk I, Barnhoorn MC, de Jonge-Muller ES, et al. . Intraluminal injection of mesenchymal stromal cells in spheroids attenuates experimental colitis. J Crohns Colitis 2016;10:953–64. [DOI] [PubMed] [Google Scholar]
- 16. Anderson P, Souza-Moreira L, Morell M, et al. . Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013;62:1131–41. [DOI] [PubMed] [Google Scholar]
- 17. Lopez-Santalla M, Mancheno-Corvo P, Escolano A, et al. . Comparative analysis between the in vivo biodistribution and therapeutic efficacy of adipose-derived mesenchymal stromal cells administered intraperitoneally in experimental colitis. Int J Mol Sci 2018, Jun 23. doi: 10.3390/ijms19071853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lopez-Santalla M, Mancheño-Corvo P, Escolano A, et al. . Biodistribution and efficacy of human adipose-derived mesenchymal stem cells following intranodal administration in experimental colitis. Front Immunol 2017;8:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol 2014;5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnhoorn MC, Wasser MNJM, Roelofs H, et al. . Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis 2020;14:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alves VBF, de Sousa BC, Fonseca MTC, et al. . A single administration of human adipose tissue-derived mesenchymal stromal cells [MSC] induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin Exp Immunol 2019;196:139–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panés J, García-Olmo D, Van Assche G, et al. ; ADMIRE CD Study Group Collaborators Long-term efficacy and safety of stem cell therapy [Cx601] for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 2018;154:1334–42.e4. [DOI] [PubMed] [Google Scholar]
- 23. Lee HJ, Oh SH, Jang HW, et al. . Long-term effects of bone marrow-derived mesenchymal stem cells in dextran sulfate sodium-induced murine chronic colitis. Gut Liver 2016;10:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho YB, Park KJ, Yoon SN, et al. . Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med 2015:4:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Natoli G, Ostuni R. Adaptation and memory in immune responses. Nat Immunol 2019:20:783–92. [DOI] [PubMed] [Google Scholar]
- 26. Dominici M, Le Blanc K, Mueller I, et al. . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006:8:315–7. [DOI] [PubMed] [Google Scholar]
- 27. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007;2:541–6. [DOI] [PubMed] [Google Scholar]
- 28. Gonçalves Fda C, Schneider N, Pinto FO, et al. . Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol 2014;20:18228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 1992;68:869–77. [DOI] [PubMed] [Google Scholar]
- 30. Gundra UM, Girgis NM, Ruckerl D, et al. . Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood 2014;123:e110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghosn EE, Cassado AA, Govoni GR, et al. . Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A 2010;107:2568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bronte V, Brandau S, Chen SH, et al. . Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schleier L, Wiendl M, Heidbreder K, et al. . Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut 2020;69:252–63. [DOI] [PubMed] [Google Scholar]
- 34. Farro G, Stakenborg M, Gomez-Pinilla PJ, et al. . CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut 2017;66:2098–109. [DOI] [PubMed] [Google Scholar]
- 35. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011;11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang J, Zhang H, Wang D, et al. . Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut 2012;61:468–9. [DOI] [PubMed] [Google Scholar]
- 37. Duijvestein M, van den Brink GR, Hommes DW. Stem cells as potential novel therapeutic strategy for inflammatory bowel disease. J Crohns Colitis 2008;2:99–106. [DOI] [PubMed] [Google Scholar]
- 38. Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun 2018;9:3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perico N, Casiraghi F, Todeschini M, et al. . Long-term clinical and immunological profile of kidney transplant patients given mesenchymal stromal cell immunotherapy. Front Immunol 2018;9:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murray PJ, Allen JE, Biswas SK, et al. . Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmitt H, Ulmschneider J, Billmeier U, et al. . The TLR9 agonist cobitolimod induces IL10 producing wound healing macrophages and regulatory T cells in ulcerative colitis. J Crohns Colitis 2020;14:508–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ihara S, Hirata Y, Hikiba Y, et al. . Adhesive interactions between mononuclear phagocytes and intestinal epithelium perturb normal epithelial differentiation and serve as a therapeutic target in inflammatory bowel disease. J Crohns Colitis 2018;12:1219–31. [DOI] [PubMed] [Google Scholar]
- 44. Hidalgo-Garcia L, Galvez J, Rodriguez-Cabezas ME, Anderson PO. Can a conversation between mesenchymal stromal cells and macrophages solve the crisis in the inflamed intestine? Front Pharmacol 2018;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quinn SM, Cunningham K, Raverdeau M, et al. . Anti-inflammatory trained immunity mediated by helminth products attenuates the induction of T cell-mediated autoimmune disease. Front Immunol 2019;10:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dominguez-Andres J, Netea MG. Long-term reprogramming of the innate immune system. J Leukoc Biol 2019;105:329–38. [DOI] [PubMed] [Google Scholar]
- 47. Gourbal B, Pinaud S, Beckers GJM, Van Der Meer JWM, Conrath U, Netea MG. Innate immune memory: An evolutionary perspective. Immunol Rev 2018;283:21–40. [DOI] [PubMed] [Google Scholar]
- 48. Saeed S, Quintin J, Kerstens HH, et al. . Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014;345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Su X, Yang L, Yin Y, et al. . Bone marrow mesenchymal stem cells tune the differentiation of myeloid-derived suppressor cells in bleomycin-induced lung injury. Stem Cell Res Ther 2018;9:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee HJ, Ko JH, Jeong HJ, et al. . Mesenchymal stem/stromal cells protect against autoimmunity via CCL2-dependent recruitment of myeloid-derived suppressor cells. J Immunol 2015;194:3634–45. [DOI] [PubMed] [Google Scholar]
- 51. Parekkadan B, Upadhyay R, Dunham J, et al. . Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology 2011;140:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vagnozzi RJ, Maillet M, Sargent MA, et al. . An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020;577:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biswas S, Mandal G, Roy Chowdhury S, et al. . Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer. J Immunol 2019;203:3447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Du L, Lin L, Li Q, et al. . IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab 2019;29:1363–75.e8. [DOI] [PubMed] [Google Scholar]
- 55. Mourits VP, Wijkmans JC, Joosten LA, Netea MG. Trained immunity as a novel therapeutic strategy. Curr Opin Pharmacol 2018;41:52–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.