Abstract
Background and Aims
We conducted a systematic review and meta-analysis evaluating the relapse rate after therapeutic de-escalation in inflammatory bowel disease [IBD] patients who achieved deep remission [DR].
Methods
We searched MEDLINE, EMBASE, and major gastroenterology conferences up to July 2019 for studies reporting relapse in adult patients with DR who subsequently underwent therapeutic de-escalation. Eligible studies defined DR as at least a combination of clinical remission and mucosal healing/endoscopic remission. The primary outcome was cumulative 1-year and 2-year relapse rates after therapeutic de-escalation. Secondary outcomes were relapse rates in ulcerative colitis [UC] and Crohn’s disease [CD], relapse after anti-tumour necrosis factor-α [anti-TNFα] de-escalation, and the rate of disease response recapture following re-escalation.
Results
Thirteen studies encompassing 837 patients were identified. The cumulative relapse rate after therapeutic de-escalation was 28.7% within 1 year [12 studies], and 38.4% within 2 years [eight studies]. Relapse rates within 1 year and 2 years were comparable between UC [five studies; 25.4% and 37.4%] and CD [seven studies; 34.1% and 39.9%]. Ten studies reported de-escalation of anti-TNFα, of which 29.8% patients relapsed within 1 year and 41.4% within 2 years. Response recapture following re-escalation [eight studies] was 75.4%.
Conclusions
Despite achieving deep remission, therapeutic de-escalation in this patient population is associated with significant relapse risk within 1 year and 2 years. This risk is more pronounced in patients requiring anti-TNFα for management, likely because of more severe disease. Similar rates of relapse were reported among UC and CD within these time periods. These findings suggest that combined clinical and endoscopic remission should not be an impetus to consider therapeutic de-escalation.
Keywords: Endoscopy, quality of life, social-economic and psychological endpoints
1. Introduction
Since the early 2000s, gastroenterologists’ options of therapeutic agents to use in the treatment of inflammatory bowel disease [IBD] have expanded to include targeted therapies.1,2 The use of targeted therapies, specifically anti-tumour necrosis factor alpha [anti-TNFα] monoclonal antibodies, have become widespread due to their clinical efficacy and their ability to reduce inflammation, as evidenced by improvement in endoscopic, histological, and radiological parameters and serum biochemical markers. These changes subsequently prolong the length of clinical remission and improve quality of life while reducing the risk of serious complications, surgery, and hospitalisations.1,3–5
Despite the many benefits of anti-TNFα treatment, one question raised by both patients and gastroenterologists is if and when therapy de-escalation after achieving remission is possible.6 This concern stems not only from fear of side effects associated with long-term use of biologics, but also health care system-related or financial reasons.7,8 Anti-TNFα use has been shown to be one of the major drivers of cost in IBD care, accounting for 64% and 31% of the total cost in Crohn’s disease [CD] and ulcerative colitis [UC], respectively.9 Cost-effectiveness analysis has also demonstrated a fiscal advantage of short-term use over lifelong therapy.10 In addition, patients experience challenges with long-term adherence to biologics due to difficulty obtaining insurance approval, making infusion appointments, refilling injectables or gaining access to medications due to high costs, delayed reimbursement for medication, limited supply leading to long waiting lists, and limited access in certain geographical regions.11–14
Discontinuing either conventional or targeted therapies could increase the risk of disease relapse, and response recapture by therapy re-initiation is variable. Furthermore, particularly in the case of anti-TNFα therapy where anti-drug antibodies are common, therapeutic de-escalation also carries the risk of eliminating a specific drug from a patient’s therapeutic options in the future.1,6 Guidance on therapeutic de-escalation has been primarily based on expert opinion, with most recommending that the decision be made at the discretion of the gastroenterologist. The EPACT panel recommended that it is appropriate to stop biologic therapy, being given alone or in combination therapy, after 2 years for patients in clinical and endoscopic remission, despite acknowledging that studies had shown high relapse rates after discontinuation of biologic therapy.15,16
The term deep remission [DR] is a concept used in the literature to denote a state in which patients are stably free of clinical signs and symptoms of IBD, and additionally have a low risk of developing disease complications. The most common components that have been proposed to be surrogate markers for DR are clinical remission [CR] and endoscopic mucosal healing [MH].17 Several studies have demonstrated that achieving this combination is associated with more favourable outcomes. For example the EXTEND trial, using a definition of DR as Crohn’s Disease Activity Index [CDAI] ≤150 and complete endoscopic mucosal healing, showed that achieving this endpoint correlated with fewer hospitalisations, fewer surgeries, less activity impairment, better quality of life, and cost savings for patients.3,18 The CALM trial showed that timely escalation of therapy, based on clinical symptoms combined with biochemical markers, resulted in better clinical and endoscopic outcomes.19 However, several studies have also shown that persistent microscopic inflammation beyond clinical and endoscopic remission is associated with increased relapse rates, hospitalisation, colectomy, and colorectal neoplasia.20–23 Clearly, endpoints in disease management that consider all descriptors of disease severity and individual patients’ IBD history are needed to guide treatment and improve outcome.3,24
Adopting the most widely accepted definition of DR encompassing clinical remission and mucosal healing, our analysis aims to address the following questions. [1] What are the rates of disease relapse after achieving deep remission and subsequent therapeutic de-escalation? [2] What are the rates of disease relapse after achieving deep remission within stratified groups—Crohn’s disease and ulcerative colitis? [3] What are the rates at which therapeutic response can be recaptured after relapse?
In contrast to previous reviews on therapeutic de-escalation, we included only studies in which patients achieved at minimum clinical remission and mucosal healing/endoscopic remission, the most widely accepted definition of deep remission. We made an effort to include only studies with definitions of deep remission defined as at least clinical remission and mucosal healing/endoscopic remission, which is more stringent than previous meta-analyses on therapy cessation. We believe this information will help guide therapeutic management for patients and physicians who are considering de-escalation of therapy, specifically with regards to targeted anti-TNFα agents.
2. Methods
The current study was performed in accordance with the guidelines established by the PRISMA statement.25
2.1.Data sources and searches
We performed a systematic search of MEDLINE and EMBASE up to July 8, 2019, using the following search terms: [‘inflammatory bowel disease’ OR ‘IBD’ OR ‘crohn*’ OR ‘ulcerative colitis’ OR ‘CD’ OR ‘UC’ or ‘colitis’] AND [‘mucosal healing’ OR ‘deep remission’ OR ‘complete remission’ OR ‘full remission’ OR ‘endoscopic remission’]. This search was conducted without restrictions on year or language. The search strategy is detailed in the Appendix. We also manually searched through abstracts presented at major gastrointestinal conferences from 2012 through 2019 [Digestive Disease Week, United European Gastroenterology Week, European Crohn’s and Colitis Organisation, the American College of Gastroenterology Annual Scientific Meeting, Advances in Inflammatory Bowel Diseases, and the Crohn’s and Colitis Congress]. Citations of manuscripts included were also reviewed for possible additional studies to be considered for inclusion. Two authors [OA and AG] independently conducted this review. A third author [BZ] reviewed studies that were not agreed upon for inclusion.
2.2.Selection criteria
Randomised controlled trials [RCTs], including cluster RCTs, controlled [non-randomised] clinical trials, case control or nested case control studies, and cohort studies [retrospective and prospective], including conference abstracts, were evaluated for inclusion. We included studies examining the adult human population [18 years or older] with inflammatory bowel disease who have been treated and undergone therapeutic de-escalation following achievement of ‘deep remission’, defined as at least a combination of clinical remission and mucosal healing/endoscopic remission, which is the most commonly accepted definition. Attempts were made to contact the authors of primary studies for unpublished data. Eligible studies required therapeutic de-escalation and subsequent adequate follow-up of the patient population to evaluate relapse.
Case reports were excluded. Additionally, studies that did not define deep remission or did not identify components of deep remission to include at least clinical and endoscopic remission were excluded. Studies with a paediatric population were excluded.
The primary outcome of interest was the 1-year and 2-year relapse rates after therapeutic de-escalation in patients who achieved deep remission. Secondary outcomes of interest were relapse in ulcerative colitis and Crohn’s disease, relapse associated with de-escalation of classes of therapeutics, and achievement of disease response recapture with re-escalation following relapse.
2.3.Data extraction and risk of bias assessment
Two authors [AG and OA] independently extracted the following data onto a data collection form: first author’s name, last author’s name, publication year, country, single or multiple institutions, study design, type of IBD, type of medication used, medications withdrawn, concomitant or maintenance therapy used, definition of deep remission, definition of mucosal healing/endoscopic remission, definition of clinical remission, number of participants who relapsed at two pre-determined time points [1 year and 2 years], follow-up duration, rate of recapturing remission with re-retreatment, and any identified predictors of early vs late relapse. For studies which did not report the counts of those who relapsed or continued to be in remission at these intervals, the numbers were manually calculated using estimations from figures and total participants at enrolment or remaining participants at these time points.
All studies were deemed cohort studies based on the intervention of interest [therapeutic de-escalation]. Therefore, risk of bias was assessed independently by two authors [OA and BZ] using the Newcastle-Ottawa Scale.26 Discrepancies between the authors were resolved after discussion. Out of nine possible stars, studies were considered at high risk of bias if they received 0–3 stars, moderate risk if 4–6 stars, and low risk if 7–9 stars.
2.4.Data synthesis
Given the primary intention of this review, we anticipated that few randomised trials were conducted to evaluate the effect of therapeutic de-escalation in patients with deep remission. Therefore, in addition to generating a systematic review and meta-analysis on this topic, we decided to include cohort studies for our meta-analyses to obtain probabilities of relapse under predefined conditions. Given the inherent heterogeneity in the designs of the included studies [for example, retrospective versus prospective, definitions of deep remission, de-escalation protocols, patient follow-up, etc.], we calculated pooled event rates and corresponding 95% confidence intervals [95% CI] using the random-effects model as per DerSimonian and Laird and inverse variance method for dichotomous outcomes.27 Between-study heterogeneity was assessed with the chi square test with significance defined as p <0.1 and the I^2 test at >50%.28 Publication bias was evaluated with a funnel plot for each analysis and the Egger test. All analyses were performed using Comprehensive Meta-Analysis [version 3; Biostat, Englewood, NJ, USA, 2013]. One study removed sensitivity analyses heterogeneities were calculated for all meta-analyses [Supplementary Tables S3–S6, available as Supplementary data at ECCO-JCC online].
3. Results
3.1.Search results
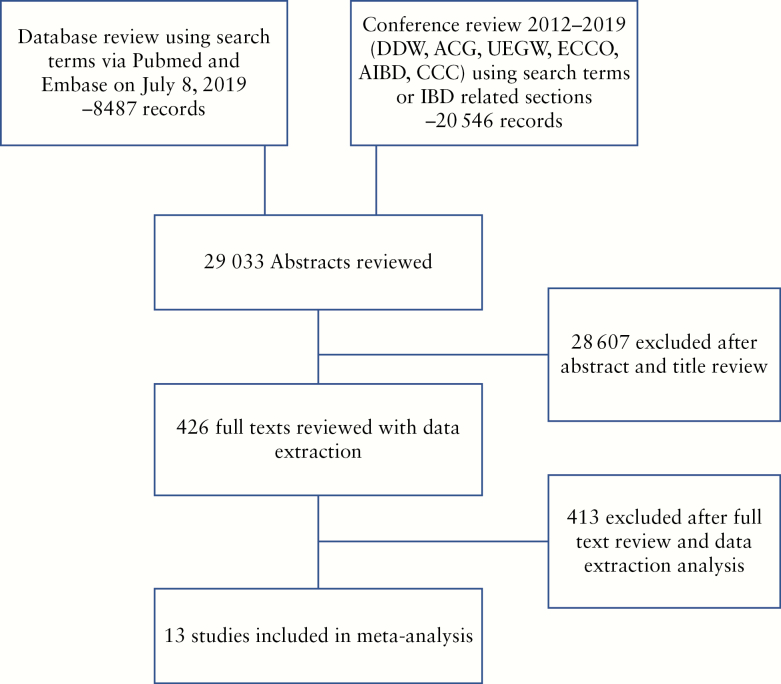
The implemented search strategy identified 29 033 publications in the initial search [Figure 1]. After screening of titles and abstracts and removing duplications, 426 articles underwent detailed review. After applying the exclusion criteria, 13 studies [nine manuscripts, four conference abstracts] encompassing 837 patients were selected for meta-analysis [Table 1].29–41 Among these patients, UC diagnosis was provided for 199 patients and CD diagnosis was provided for 239 patients. One year and two year relapse rates in Fiorino 2016 and Iborra 2019 were not directly reported;34,36 incidences of relapse were extrapolated from the manuscripts’ Kaplan-Meier survival analyses. Molander 2015 included five IBD-undifferentiated [IBD-U] patients in their UC group.37 Hisamatsu 2019 included patients aged 15–65; however, the majority of patients were likely over 18 years old based on the reported age average and standard deviation.39
Figure 1.
PRISMA diagram [Preferred Reporting Items for Systematic Reviews and Meta-Analyses].
Table 1.
Characteristics of studies included in meta-analysis.
| Study | Country | Study design | DR criteria [in addition to CR+ER] | Patients enrolled fitting minimal DR criteria | Baseline therapeutics | De-escalation strategy | Median follow-up time | Recapture data | |
|---|---|---|---|---|---|---|---|---|---|
| UC | CD | ||||||||
| Echarri 2013 | Spain | Pro | No activity on bowel MRI | - | 32 | IFX/ADA +/- AZA, MTX, 5-ASA | Anti-TNFα cessation, continue other therapeutics | 34 months | Yes |
| Rismo 2013 | Norway | Pro | No additional | - | 37 | IFX/ADA +/- AZA, MTX, 5-ASA | Anti-TNFα cessation, continue other therapeutics | Did not report | No |
| Bodini 2014 | Italy | Pro | Biomarker remission | 6 | 10 | Anti-TNFα monotherapy | Anti-TNFα cessation, then randomize to 5-ASA vs AZA | 48 weeks | No |
| Dart 2014 | UK | Retro | CRP <5 mg/dL | - | 6 | IFX/ADA +/- AZA/6MP | Anti-TNFα cessation, continue other therapeutics | Did not report | No |
| Bortlik 2015 | Czech Republic | Pro | No additional | 17 | 61 | IFX/ADA +/- AZA, MTX | Anti-TNFα cessation, continue other therapeutics | 30 months | Yes |
| Molander 2015 | Finland | Pro | FC <100 µg/g | 33* | 16 | IFX/ADA +/- AZA/6MP, MTX, 5-ASA | Anti-TNFα cessation, continue other therapeutics | 13 months | Yes |
| Hlavaty 2016 | Slovakia | Pro | No activity on abdominal imaging, CRP <5 mg/dL, FC < 50 µg/g | 3 | 8 | IFX/ADA +/- AZA, 5-ASA | Anti-TNFα cessation, continue other therapeutics | 497 days | Yes |
| Fiorino 2016 | Multiple | Retro | No additional | 111 | - | IFX +/- AZA, 5-ASA | Anti-TNFα cessation, continue other therapeutics | 24 months | Yes |
| Casanova 2017 | Spain | Retro | No additional | 161 | 258 | IFX/ADA +/- thiopurines, MTX, 5-ASA | Anti-TNFα cessation, continue other therapeutics | 29 months | Yes |
| Soufleris 2018 | Greece | Pro | CRP <5 mg/dL and/or FC <250 µg/g | - | 19 | Anti-TNFα monotherapy | Anti-TNFα cessation | 43 months | Yes |
| Buda 2019 | Italy | Retro | Biochemical remission | 24 | 33 | IFX 5 mg/kg every 8 weeks monotherapy | IFX 3 mg/kg every 8 weeks | 28.4 months [mean] | Yes |
| Iborra 2019 | Spain | Pro | Biochemical remission [normal values for CRP, haemoglobin, and FC] | 35 | 60 | AZA or 6MP monotherapy | AZA or 6MP cessation | 36.7 months | Yes |
| Hisamatsu 2019 | Japan | Pro | No additional | - | 19 | ADA + thiopurines +/- 5-ASA | Thiopurines cessation, continue other therapeutics | Did not report | No |
Pro, prospective; retro, retrospective; IFX, infliximab; ADA, adalimumab; AZA, azathioprine; MTX, methotrexate; CR, clinical remission; ER, endoscopic remission; DR, deep remission; 6MP 6-mercaptopurine; CRP, C-reactive protein; FC, faecal calprotectin; TNF, tumour necrosis factor; UC, ulcerative colitis; CD, Crohn’s disease; MRI, magnetic resonance imaging.
All included studies except Hisamatsu 2019 originated in Europe. Nine of the studies were prospective in design,29,30,33,35–40 and six were conducted at multiple institutions.32,34,36,37,39,40 One study was a randomised control trial [RCT] comparing mesalamine versus azathioprine in maintaining remission after anti-TNFα withdrawal.30 Eight studies required additional confirmation of deep remission beyond the strict inclusion criteria of steroid-free clinical and endoscopic remission.30–33,35–38 The median follow-up time ranged from 48 weeks to 43 months. Eleven studies reported de-escalation strategy involving anti-TNFα,29–35,37,38,40,41 of which three enrolled patients on anti-TNFα as monotherapy.30,31,33 None included other classes of biologics or small-molecule therapy. None were extensions of clinical trials intended for approval of novel therapeutics. Usage of biosimilars was not reported. The heterogeneity of all reported analyses are provided in Supplementary Table S2, available as Supplementary data at ECCO-JCC online.
3.2.Quality of studies and risk of bias
Using the points system of the Newcastle-Ottawa Scale [NOS], each study was evaluated for quality and risk of bias [Supplementary Table S1, available as Supplementary data at ECCO-JCC online]. Studies were granted a point for ‘adequacy of follow-up of cohorts’ if their reported outcomes accounted for dropout or it could be adequately controlled for based on available data. One study scored 4 out of 9, five studies scored 5 out of 9, five scored 6 out of 9, suggesting that most studies carried a moderate risk of bias. All studies except for Fiorino 2016 and Hisamatsu 2019 lacked a non-exposed cohort, which in this case would be patients with deep remission who did not undergo therapeutic de-escalation.34,39 Although Bodini 2014 was an RCT, the intervention of stopping anti-TNFα was not randomised and therefore it resembled a cohort study.30
3.3.Relapse from deep remission at 1 year and 2 years
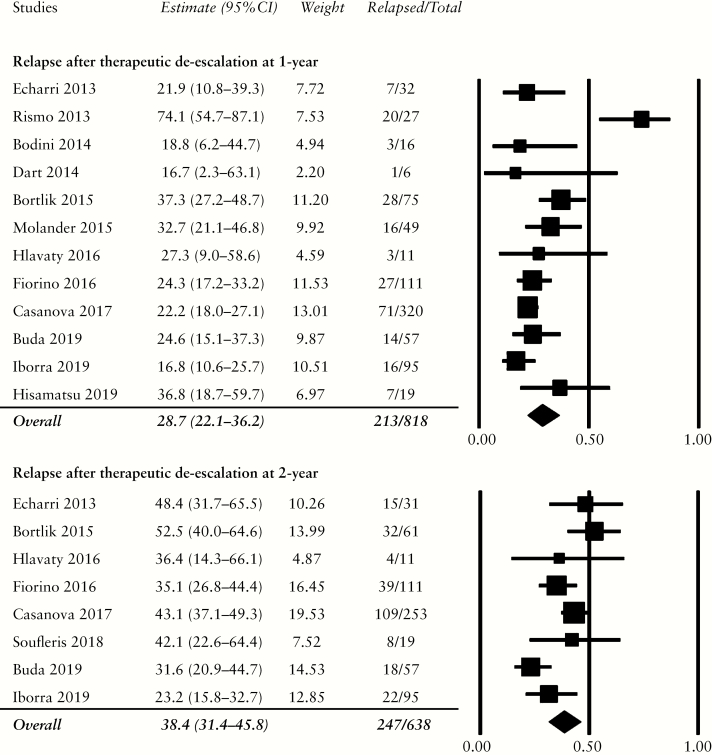
Twelve studies reported the rate of relapse within the first year of therapeutic de-escalation.29–32,34–41 Cumulatively, 213 of 818 patients, or 28.7% [95% CI: 22.1–36.2%], experienced disease relapse [Figure 2]. Relapse rose to 38.4% [95% CI: 31.4–45.8%] within 2 years, totalling 247 of 638 patients from the eight studies included.29,31,33–36,38,41 Funnel plots [Supplementary Figure S1, available as Supplementary data at ECCO-JCC online] and Egger’s test for relapse within 1 year and 2 years were performed to detect publication bias [1 year: Egger’s t-value = 0.92, p = 0.19; 2 years: Egger’s t-value = 0.32, p = 0.38].
Figure 2.
Cumulative rates of relapse in patients with deep remission following 1 year and 2 years of therapeutic de-escalation. Relapse rates were 28.7% within the 1st year and 38.4% within the first 2 years.
One study removed sensitivity analysis of relapse at 1 year demonstrated decreased heterogeneity after the exclusion of the Rismo 2013 study [Supplementary Table S3]; with this study removed from the analysis, the 1-year relapse rate decreased to 25.4% [95% CI: 21.4–29.8%]. For relapse at 2 years, the exclusion of Iborra 2019 decreased heterogeneity significantly; the 2-year relapse rate after removing this study rose to 41.4% [95% CI: 36.1–46.9%]. Of note, the Iborra 2019 study reported the withdrawal of azathioprine/mercaptopurine in patients not receiving biologic treatment, reflecting possibly lower disease severity.
3.4.Relapse for ulcerative colitis and Crohn’s disease
3.4.1. Ulcerative colitis
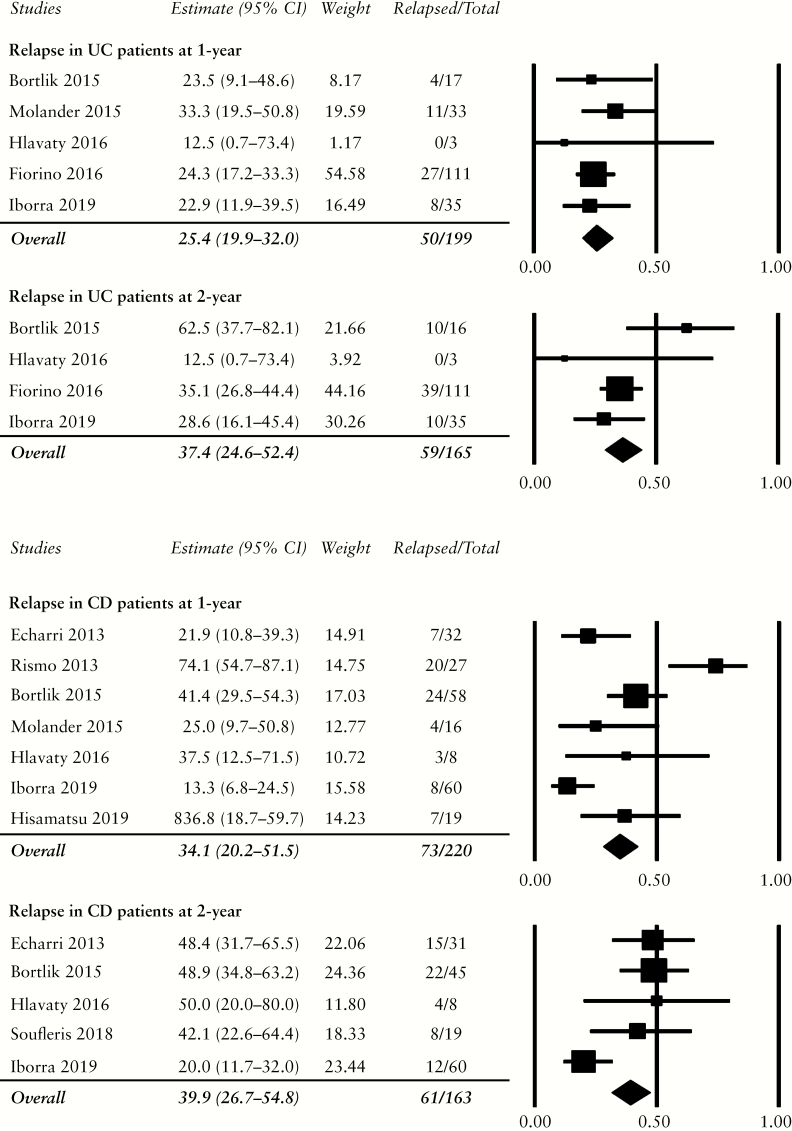
Five studies reported the rate of relapse within the first year of therapeutic de-escalation to be 25.4% [50 of 199, 95% CI: 19.9–32.0%].29,34–37 Four studies provided the 2-year data, with 37.4% relapsing [59 of 165, 95% CI: 24.6–52.4%] within this period [Figure 3].29,34–36 Funnel plot [not shown] and Egger’s test [1-year t-value = 0.47, p = 0.34; 2-year t-value = 0.04, p = 0.49] did not demonstrate publication bias.
Figure 3.
Relapse rates in patients with ulcerative colitis [UC] and Crohn’s disease [CD] at 1 year and 2 years. The pooled estimates of relapse were similar between the two diseases at both follow-up intervals.
One study removed sensitivity analysis demonstrated minimal heterogeneity among the studies which reported relapse at 1 year [Supplementary Table S4]. For relapse at 2 years, the exclusion of Bortlik 2015 significantly decreased the heterogeneity for this meta-analysis; with this study removed, the relapse rate decreased to 33.3% [95% CI: 26.1–41.2%].
3.4.2. Crohn’s disease
Seven studies reported the rate of relapse within the first year after therapeutic de-escalation to be 34.1% [73 of 220, 95% CI: 20.2–51.5],29,35–40 mainly driven by Rismo 2013 who reported 74% relapsers. The 2-year data were obtained from five studies, with 39.9% relapse [61 of 163, 95% CI: 26.7–54.8%] [Figure 3].29,33,35,36,38 There was no publication bias on funnel plot [not shown] nor Egger’s test [1-year t-value = 0.14, p = 0.45; 2-year t-value = 0.37, p = 0.37].
One study removed sensitivity analysis demonstrated substantial heterogeneity among all studies reporting 1-year relapse rate [Table S4]. For the 2-year relapse rate, the exclusion of Iborra 2019 significantly decreased the heterogeneity; with this study removed, the relapse rate increased to 47.6% [95% CI: 38.1–57.2%]. Following the exclusions of Bortlik 2015 from the 2-year relapse for UC and Iborra 2019 from the 2-year relapse for CD to minimise heterogeneity, the remaining studies suggested that patients with CD [49 relapsers of 103, four studies] might be more likely to relapse than patients with UC at 2 years [49 relapsers of 149, three studies].
3.5.Relapse after anti-TNFα de-escalation
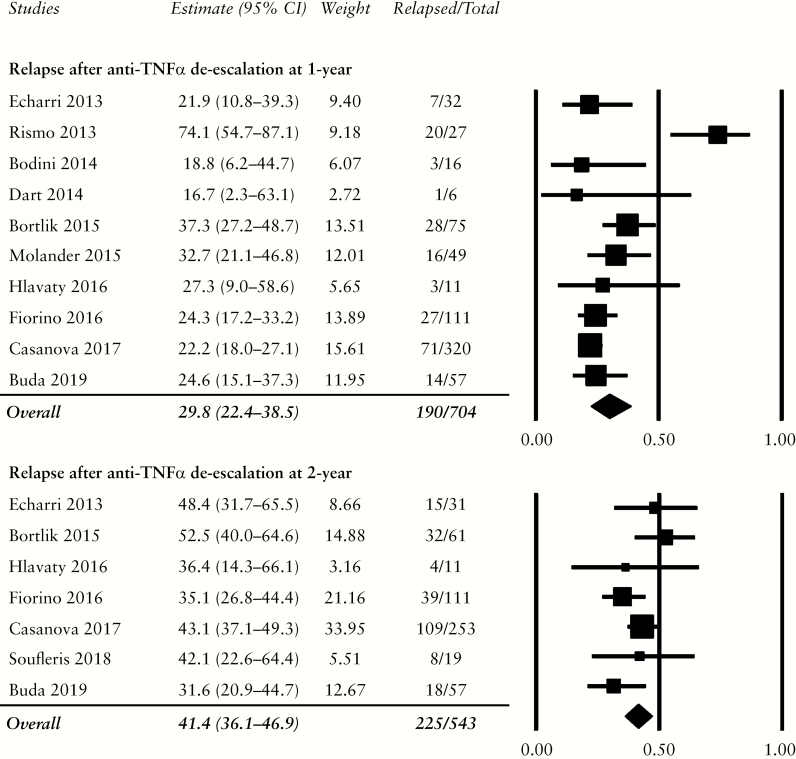
Ten studies reported the relapse rate within the 1st year,29–32,34,35,37–39,41 and seven studies within 2 years,29,31,33–35,38,41 after anti-TNFα de-escalation. Most studies continued the patients’ concurrent immunomodulators and/or aminosalicylates. Three studies enrolled patients on anti-TNFα as monotherapy.30,31,33 Bodini 2014 was a randomised controlled trial which assigned patients to either mesalamine or azathioprine after cessation of anti-TNFα to observe the medications’ efficacy of maintaining remission.30 Buda 2019 de-escalated infliximab from 5 mg/kg to 3 mg/kg every 8 weeks without the use of therapeutic drug monitoring to assess outcome.31 Finally, Fiorino 2016 enrolled 193 patients, of whom 111 discontinued infliximab and are included in this meta-analysis.34
Combined 1-year relapse after anti-TNFα de-escalation was 29.8% [190/704, 95% CI: 22.4–38.5%]. Relapse rate within 2 years was 41.4% [225/543, 95% CI: 36.1–46.9%] [Figure 4]. No publication bias was detected on both funnel plots [not shown] and Egger’s test [1-year t-value = 0.85, p = 0.21; 2-year t-value = 0.14, p = 0.45]. Of the three studies which included only patients on anti-TNFα monotherapy, the relapse rates were 23.4% [95% CI: 15.1–34.5%]30,31 and 34.3% [95% CI: 24.5–45.7%]31,33 for one year and two years, respectively. Instead of discontinuing anti-TNFα monotherapy altogether, Buda 2019 de-escalated the dose of infliximab and reported a relapse rate of 24.6% in Year 1 and 31.6% in Year 2, the latter of which was the lowest of all studies for this analysis.31
Figure 4.
Relapse rates after anti-TNFα de-escalation at 1 year and 2 years. Relapse was slightly higher when compared with cumulative rates of relapse, as patients who required treatment with anti-TNFα likely had more aggressive disease. TNF, tumour necrosis factor.
One study removed sensitivity analysis for relapse at 1 year demonstrated decreased heterogeneity after removal of Rismo 2013 [Supplementary Table S5]; following the exclusion of this study, the relapse rate decreased to 25.9% [95% CI: 22.1–30.1%]. For relapse at 2 years, low heterogeneity was detected among the included studies.
Of all studies included for this analysis, only Fiorino 2016 featured two arms comparing the outcome of patients who continued on anti-TNFα therapy versus those who discontinued it.34 The study reviewed the data of 193 eligible UC patients retrospectively. 111 patients discontinued infliximab and 82 patients continued. The median time to relapse was 3.6 years among those who discontinued compared with 7.6 versus those who did not. There was non-significantly increased risk of hospitalisation for the infliximab discontinuation group, but the risk of colectomy did not differ.
3.6.Relapse associated with azathioprine and aminosalicylates
Three studies investigated the roles of azathioprine and aminosalicylates at maintaining remission following anti-TNFα withdrawal. Bodini 2014 randomised patients in deep remission to de-escalate from anti-TNFα therapy to either mesalamine [2.4 g/day for UC, 3 g/day for CD] or azathioprine [2.5 mg/kg/day].30 Despite enrolling only 16 patients, three of 10 mesalamine-treated patients experienced relapse [median follow-up 14 weeks] whereas all six of azathioprine-treated patients maintained remission [median follow-up 55.5 weeks]. In the Fiorino 2016 study, 100 patients who discontinued anti-TNFα were followed to evaluate the efficacy of thiopurines, aminosalicylates, or the combination of the two in maintaining deep remission.34 The authors found that thiopurine monotherapy was superior to aminosalicylates and trended toward longer remission against combination therapy. Hisamatsu 2019 randomised patients to continue or discontinue thiopurines, which did not affect the remission rate nor the adalimumab trough levels at 52 weeks after discontinuation.39
The effect of withdrawing azathioprine/mercaptopurine on relapse in patients not treated with biologic therapy was assessed in the Iborra 2019 study.36 Most patients were started on high-dose salicylates. The relapse rates at 1 year and 2 years were 16.8% and 23.2%, respectively, with cumulative 5-year relapse around 46.5%, suggesting that therapeutic de-escalation should not be attempted unless necessary even among patients with moderate disease severity who do not require biologic therapy for disease control.
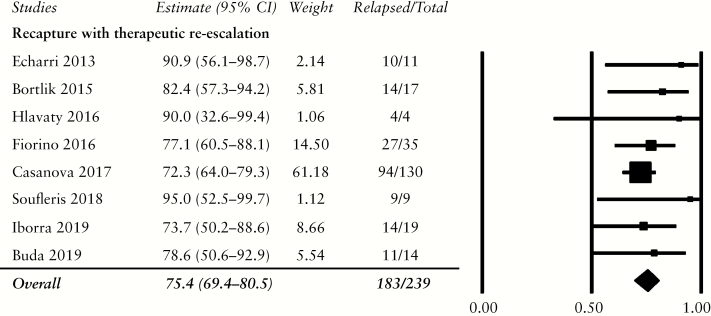
3.7.Recapture with therapeutic re-escalation
Eight studies provided data on the outcome after therapeutic re-initiation.29,31,33–36,38,41 Of 239 patients. 183 [75.4%, 95% CI: 69.4–80.5%] who relapsed after therapeutic de-escalation responded to the re-introduction of their previous therapies or another therapy [Figure 5]. One study removed sensitivity analysis detected no heterogeneity among the included studies [Supplementary Table S6].
Figure 5.
Disease response rate following therapeutic re-escalation.
4. Discussion
The ongoing development and adoption of novel targeted therapies for IBD has enhanced our ability to achieve disease remission in larger numbers of patients compared with conventional treatments.42 As more patients respond to these medical therapies, it has become possible to ask whether indefinite treatment with these new agents is required or if they may be stopped after reaching some theoretical endpoint. Clearly, knowing the safety and consequences of therapeutic de-escalation would be of critical importance for this decision. In reality, factors such as patient preferences, adverse side effects, health policy, or financial considerations often drive the choice to withdraw targeted treatments.43–45 This review identified common reasons for the decision to de-escalate therapy which include sustained remission, patient preference, side effects, pregnancy, and health care policy. Interestingly, research has demonstrated many motivations for de-escalation to be unjustified. For example, cessation of biologics therapy for pregnancy is unnecessary and dangerous for the patient and the fetus, and new recommendations from both the American Gastroenterological Association46 and the American College of Obstetricians and Gynecologists47 support continuing treatment through pregnancy and lactation. Similarly, the approval and adoption of biosimilar alternatives will likely improve the financial impact of continuing biologic therapy, potentially leading to less cost-driven discontinuation.48 Alternatively, the use of therapeutic drug monitoring assists in the optimisation of biologics therapy, enabling the consideration of monotherapy or immunomodulator withdrawal without significant clinical impact.49
The existing literature supports the continuation of therapy when a response to treatment is observed; however, this strategy was based primarily on anti-TNFα cessation studies of patient cohorts with heterogeneous treatment outcomes of clinical and/or endoscopic remission.1,16,50 Multiple studies have revealed that achieving clinical remission alone is insufficient to prevent bowel damage and complications of IBD. Indeed, based on more recent data, current treatment targets for Crohn’s disease and ulcerative colitis recommended by the International Organization for the Study of Inflammatory Bowel Disease incorporate endoscopic mucosal healing as a therapeutic objective in addition to clinical parameters.51–53 Thus, it is possible that the inability to maintain remission in patients who de-escalated therapy in previous studies was due to the failure to target the appropriate endpoint before medication withdrawal.
The combination of clinical remission and endoscopic mucosal healing has been termed ‘deep remission’ and has been associated with improved overall outcomes.18,54 We performed this study to investigate the consequences of therapeutic de-escalation in patients achieving deep remission without concomitant corticosteroid use. Applying the least stringent and most widely accepted definition of deep remission,17 we report the overall 1-year and 2-year relapse rates to be 28.7% and 38.4%, respectively, after therapeutic de-escalation. Similar rates are observed between ulcerative colitis and Crohn’s disease. Following one study removed sensitivity analysis to decrease heterogeneity for relapse among patients with Crohn’s disease at 2 years, our results are similar to the relapse rate of patients in deep remission (Crohn’s Disease Endoscopic Index of Severity [CDEIS] 0–3, no ulcers on endoscopy) from the STORI trial; the percentage of relapse for this sub-group was 37.1% [95% CI: 30.9–43.3%] at 1 year and 48.6% [95% CI: 42.0–55.2%] at 2 years [personal communication].16 Unsurprisingly, anti-TNFα cessation was the de-escalation strategy used in most studies. One group reported that although azathioprine maintains remission better than aminosalicylates after anti-TNFα withdrawal in ulcerative colitis, neither option alone nor the combined regimen was comparable to anti-TNFα continuation.34 Even in patients with mild-to-moderate disease severity not receiving biologic therapy, thiopurine continuation is warranted to prevent relapse in the setting of minimal contraindications.36 Nearly 80% of those who relapse after therapeutic de-escalation could be recaptured, but a subset of patients experienced adverse reactions and required alterations of their previous regimens. Consistent with previous findings,1,16,50 the overall significant rate of relapse within 1 and 2 years after therapeutic de-escalation strongly supports their continuation despite achieving deep remission, especially in patients who require anti-TNFα.
Whereas meta-analyses on therapeutic withdrawal have been previously completed, our study differs from earlier studies in several important aspects. First, we performed an exhaustive search including abstracts of all recent major gastroenterology conferences in addition to review of published manuscripts. We only included studies which strictly defined deep remission—at minimum, clinical remission with endoscopic evidence of mucosal healing. In studies which included subsets of patients with deep remission, we selectively extracted and included relapse information only for these patients. Whenever possible, we manually calculated the relapse rate at 1 and 2 years. Finally, we included recent studies published after the previous reviews.
There were several important limitations to our study. Despite a minimum requirement for deep remission, the exact definition differed among the studies. Similarly, relapse was defined differently. Both of these factors likely contributed to the heterogeneity in the outcomes of our analyses. Neither the differences in relapse rates between therapeutic continuation and withdrawal, nor between ulcerative colitis and Crohn’s disease, were reported in the studies, which precluded the calculation of pooled odds ratio. We also could not comment on the cessation of newer biologic agents or small molecules due to the paucity of data in literature. Finally, no reported studies on the cessation of non anti-TNFα biologics or small molecules existed during the period included in our literature review.
In conclusion, our findings of high rates of 1- and 2-year relapse, even in patients achieving deep remission as defined at minimum by clinical remission and endoscopic mucosal healing, suggest that therapeutic de-escalation should be approached with extreme caution. Although a majority of patients were able to recapture a response to anti-TNFα upon re-escalation, there were significant risks to this strategy. Clinical remission and endoscopic mucosal healing did not predict durable disease-free remission, and it is possible that some other combination of factors may be more successful. Future studies examining de-escalation after treatment with the newer targeted therapeutic options, as well as research on practical step-down strategies while maintaining remission, are needed.
Supplementary Material
Acknowledgments
The authors would like to thank Teresa Shao for her contributions to the literature search.
Appendix 1. Search strategy.
Pubmed/MEDLINE
The following search strategy implemented on July 8, 2019 retrieved 2605 references:
(‘inflammatory bowel disease’[All Fields] OR ‘IBD’[All Fields] OR ‘crohn*’[All Fields] OR ‘ulcerative colitis’[All Fields] OR ‘CD’[All Fields] OR ‘UC’[All Fields] OR ‘colitis’[All Fields]] AND [‘mucosal healing’[All Fields] OR ‘deep remission’[All Fields] OR ‘complete remission’[All Fields] OR ‘full remission’[All Fields] OR ‘endoscopic remission’[All Fields]).
EMBASE
The following search strategy implemented on July 8, 2019 retrieved 5882 references:
[‘inflammatory bowel disease’/exp OR ‘inflammatory bowel disease’ OR ‘ibd’ OR ‘crohn*’ OR ‘ulcerative colitis’/exp OR ‘ulcerative colitis’ OR ‘cd’/exp OR ‘cd’ OR ‘uc’ OR ‘colitis’/exp OR ‘colitis’] AND [‘mucosal healing’/exp OR ‘mucosal healing’ OR ‘deep remission’ OR ‘complete remission’/exp OR ‘complete remission’ OR ‘full remission’ OR ‘endoscopic remission’].
Conference presentation: part of this manuscript was presented at the American College of Gastroenterology, San Antonio, TX, 2019.
Funding
This work was supported by the National Institutes of Health [5T32DK007007-45 to BZ].
Conflict of Interest
The authors report no conflict of interests.
Author Contributions
BZ: study concept and design, data acquisition, data analysis, data interpretation, drafting and revising the article. AG: study concept and design, data acquisition, data analysis, data interpretation, drafting and revising the article. OA: study concept and design, data acquisition, data analysis, data interpretation, drafting and revising the article. LS: study concept and design, data analysis, data interpretation, drafting and revising the article, final approval of submission.
References
- 1. Gisbert JP, Marín AC, Chaparro M. The risk of relapse after Anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2016;111:632–47. [DOI] [PubMed] [Google Scholar]
- 2. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res 2018;11:215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–22.e415. [DOI] [PubMed] [Google Scholar]
- 4. D’Haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: a European multicenter trial. Gastroenterology 1999;116:1029–34. [DOI] [PubMed] [Google Scholar]
- 5. Geboes K, Rutgeerts P, Opdenakker G, et al. Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn’s disease. Curr Med Res Opin 2005;21:1741–54. [DOI] [PubMed] [Google Scholar]
- 6. Clarke K, Regueiro M. Stopping immunomodulators and biologics in inflammatory bowel disease patients in remission. Inflamm Bowel Dis 2012;18:174–9. [DOI] [PubMed] [Google Scholar]
- 7. Cleynen I, Van Moerkercke W, Billiet T, et al. Characteristics of skin lesions associated with anti-tumor necrosis factor therapy in patients with inflammatory bowel disease: a cohort study. Ann Intern Med 2016;164:10–22. [DOI] [PubMed] [Google Scholar]
- 8. Minozzi S, Bonovas S, Lytras T, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016;15:11–34. [DOI] [PubMed] [Google Scholar]
- 9. van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 10. Bodger K, Kikuchi T, Hughes D. Cost-effectiveness of biological therapy for Crohn’s disease: Markov cohort analyses incorporating United Kingdom patient-level cost data. Aliment Pharmacol Ther 2009;30:265–74. [DOI] [PubMed] [Google Scholar]
- 11. Shi HY, Ng SC. The state of the art on treatment of Crohn’s disease. J Gastroenterol 2018;53:989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Côté-Daigneault J, Bouin M, Lahaie R, Colombel JF, Poitras P. Biologics in inflammatory bowel disease: what are the data? United European Gastroenterol J 2015;3:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louis E. Stopping biologics in IBD— what is the evidence? Inflamm Bowel Dis 2018;24:725–31. [DOI] [PubMed] [Google Scholar]
- 14. Inotai A, Csanadi M, Petrova G, et al. Patient access, unmet medical need, expected benefits, and concerns related to the utilisation of biosimilars in Eastern European countries: a survey of experts. Biomed Res Int 2018;2018:9597362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pittet V, Froehlich F, Maillard MH, et al. ; EPACT-II Update Panellists When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohns Colitis 2013;7:820–6. [DOI] [PubMed] [Google Scholar]
- 16. Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70.e65; quiz e31. [DOI] [PubMed] [Google Scholar]
- 17. Gulati A, Alipour O, Shao L, Zhang B.. A systematic review and novel classification system to define deep remission. United European Gastroenterol J; 2019;7:586. [Google Scholar]
- 18. Zallot C, Peyrin-Biroulet L. Deep remission in inflammatory bowel disease: looking beyond symptoms. Curr Gastroenterol Rep 2013;15:315. [DOI] [PubMed] [Google Scholar]
- 19. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease [CALM]: a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 20. Korelitz BI. Mucosal healing as an index of colitis activity: back to histological healing for future indices. Inflamm Bowel Dis 2010;16:1628–30. [DOI] [PubMed] [Google Scholar]
- 21. Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg L, Nanda KS, Zenlea T, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol 2013;11:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis 2014;8:1582–97. [DOI] [PubMed] [Google Scholar]
- 24. af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol 2012;47:528–37. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 26. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale [NOS] for assessing the quality of nonrandomised studies in meta-analyses. http://http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed November 1, 2019. [Google Scholar]
- 27. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bortlik M, Duricova D, Machkova N, et al. Discontinuation of anti-tumor necrosis factor therapy in inflammatory bowel disease patients: a prospective observation. Scand J Gastroenterol 2016;51:196–202. [DOI] [PubMed] [Google Scholar]
- 30.Bodini G, Savarino V, Dulbecco P, Baldissarro I, Savarino E. —. IBD recurrence after stopping anti-TNF-alpha therapy: a prospective randomized controlled study comparing mesalamine and azathioprine.ad interim results. United European Gastroenterol J 2014;2:1. [Google Scholar]
- 31. Buda A, Facchin S, Lorenzon G, et al. Infliximab dose-reduction in inflammatory bowel disease [IBD] patients in prolonged deep remission: potential implications on de-escalation strategies in a real life clinical setting without a therapeutic drug monitoring [TDM] approach. Dig Liver Dis 2019;51:E101. [Google Scholar]
- 32. Dart RJ, Griffin N, Taylor K, et al. Reassessment of Crohn’s disease treated with at least 12 months of anti-TNF therapy: how likely is treatment withdrawal? Frontline Gastroenterol 2014;5:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soufleris K, Kafalis N, Fasoulas K, et al. Stopping anti-TNFa monotherapy in Crohn’s disease patients in deep remission and favourable disease characteristics is associated with a high rate of relapse. J Crohns Colitis 2018;12[Suppl 1]:S391.
- 34. Fiorino G, Cortes PN, Ellul P, et al. Discontinuation of infliximab in patients with ulcerative colitis is associated with increased risk of relapse: a multinational retrospective cohort study. Clin Gastroenterol Hepatol 2016;14:1426–32.e1. [DOI] [PubMed] [Google Scholar]
- 35. Hlavaty T, Krajcovicova A, Letkovsky J, et al. Relapse rates of inflammatory bowel disease patients in deep and clinical remission after discontinuing anti-tumor necrosis factor alpha therapy. Bratisl Lek Listy 2016;117:205–11. [DOI] [PubMed] [Google Scholar]
- 36. Iborra M, Herreras J, Boscá-Watts MM, et al. Withdrawal of Azathioprine in inflammatory bowel disease patients who sustain remission: new risk factors for relapse. Dig Dis Sci 2019;64:1612–21. [DOI] [PubMed] [Google Scholar]
- 37. Molander P, Färkkilä M, Ristimäki A, et al. Does faecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2015;9:33–40. [DOI] [PubMed] [Google Scholar]
- 38.Echarri A, Ollero V, Rodriguez J, Gallego J, Castro J.Predictors of relapse after discontinuing anti-TNF therapy in Crohn’s disease patients on deep remission. J Crohns Colitis 2013;7[Suppl 1]:71.
- 39. Hisamatsu T, Kato S, Kunisaki R, et al. ; DIAMOND2 Study Group Withdrawal of thiopurines in Crohn’s disease treated with scheduled adalimumab maintenance: a prospective randomised clinical trial [DIAMOND2]. J Gastroenterol 2019;54:860–70. [DOI] [PubMed] [Google Scholar]
- 40. Rismo R, Olsen T, Cui G, et al. Normalization of mucosal cytokine gene expression levels predicts long-term remission after discontinuation of anti-TNF therapy in Crohn’s disease. Scand J Gastroenterol 2013;48:311–9. [DOI] [PubMed] [Google Scholar]
- 41. Casanova MJ, Chaparro M, García-Sánchez V, et al. Evolution after Anti-TNF discontinuation in patients with inflammatory bowel disease: a multicenter long-term follow-up study. Am J Gastroenterol 2017;112:120–31. [DOI] [PubMed] [Google Scholar]
- 42. Hindryckx P, Vande Casteele N, Novak G, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug[s] for our patients? J Crohns Colitis 2018;12:105–19. [DOI] [PubMed] [Google Scholar]
- 43. Mahlich J, Matsuoka K, Sruamsiri R. Biologic treatment of Japanese patients with inflammatory bowel disease. BMC Gastroenterol 2018;18:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mak JW, Sung JJ. The use of biologics and biosimilar in Asian patients with IBD: are we ready? J Gastroenterol Hepatol 2019;34:1269–70. [DOI] [PubMed] [Google Scholar]
- 45. Waljee AK, Wallace BI, Cohen-Mekelburg S, et al. Development and validation of machine learning models in prediction of remission in patients with moderate to severe Crohn disease. JAMA Netw Open 2019;2:e193721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019;156:1508–24. [DOI] [PubMed] [Google Scholar]
- 47. ACOG Committee Opinion No. 776: Immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:e287–95. [DOI] [PubMed] [Google Scholar]
- 48. Rudrapatna VA, Velayos F. Biosimilars for the treatment of inflammatory bowel disease. Pract Gastroenterol 2019;43:84–91. [PMC free article] [PubMed] [Google Scholar]
- 49. Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019;17:1525–32.e1521. [DOI] [PubMed] [Google Scholar]
- 50. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015;149:1716–30. [DOI] [PubMed] [Google Scholar]
- 51. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE]: determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 52. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 53. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 54. Rogler G, Vavricka S, Schoepfer A, Lakatos PL. Mucosal healing and deep remission: what does it mean? World J Gastroenterol 2013;19:7552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.