Abstract
Immune checkpoint inhibitors (ICIs) have radically changed the clinical outcome of several cancers with durable responses. CTLA-4 (cytotoxic T lymphocyte antigen-4), PD-1 (programmed cell death protein 1) or PDL-1 (programmed cell death ligand protein 1) represent ICIs that can be used as monotherapy or in combination with other agents. The toxicity p\rofiles of ICIs differ from the side effects of cytotoxic agents and come with new toxicities like immune-related adverse events. Typically, these toxicities occur in all organs. However, the main organs affected are the skin, digestive, hepatic, lungs, rheumatologic, and endocrine. Most of the immune toxicity that occurs is low grade but some more severe toxicities can occur that require a rapid diagnosis and appropriate treatment. The recognition of symptoms by physicians and patient is necessary to resolve them rapidly and adapt treatment to allow the toxicity to resolve.
Keywords: immune check point inhibitor, toxicity, corticosteroids, immunosuppressive treatments
Introduction
Immune Checkpoint Inhibitors (ICI) have radically changed the treatment of several cancers. As a result, overall survival (OS) has increased in several types of cancer including melanoma, non-small cell lung cancer, renal cell carcinoma, and head and neck cancer. Additionally, new indications for ICIs continue to emerge.1
Of note, in addition to an increased anti-tumour immunity, the mechanism of action of ICIs reveals a new toxicity profile called immune-related adverse events (irAEs). All organs can be affected by these new toxicities, although the more frequently affected organs include the skin, digestive, and endocrine organs. The majority of toxicities caused by ICIs are low in severity, but some are more serious and require multidisciplinary management of side effects.2 To reduce the risk of experiencing severe toxicities, gathering information on different immune toxicities has been necessary and treatment practices have needed to adapt quickly. This review is an overview of the different toxicity profiles of ICIs and a discussion of their management strategies.
Pathophysiology
Immune Checkpoint Physiology
Cancer pathophysiology is characterised by the accumulation of several variable genetic alterations and the expression of neoantigens, which can lead to the presentation of peptides bound by major histocompatibility class I (MHCI) molecules on the surface of cancer cells.3 These cancer-specific peptide MHCI complexes can be recognised by CD8 T-cells.4
According to the cancer-immunity cycle, activated effector T cells traffic to and infiltrate the tumour bed. Thereafter, immune cells specifically recognise cancer cells via the interaction between the T-cell receptor (TCR) and cognate antigens bound to MHCI. The interaction between the TCR and MHCI ultimately target the cancer cell for destruction by immune cells.5 The dysregulation of this system is one of the causes of cancer development.6
Several signals are required for T-cell activation against tumour cells. These include the recognition of neoantigens bound to MHCI, many co-stimulation factors, and cytokine stimulation that is finely tuned to T-cell activation. T-cells also express inhibitory immune checkpoints that negatively regulate the T-cell pro-inflammatory response. This negative regulation is responsible for self-tolerance and limits the damage linked to the activation of the immune system.7 Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) and Programmed Cell Death Protein 1 (PD-1) are examples of immune checkpoints in the lymphoid organs and in the microenvironment, respectively.2 The ligands of CTLA-4 are CD80/86, which are present on the surface of antigen presenting cells (APCs) (dendritic cells, macrophages, B cells). Programmed Cell Death Ligand Protein 1 and 2 (PDL-1 and PDL-2), which are present on the surface of both APCs and tumour cells, are the ligands of PD-1.2
The identification of T-cell inhibitory signals led to the development of the new class of immunotherapy using immune checkpoint inhibitors (ICI). ICIs specifically hinder immune effector inhibition and potentially expand pre-existing anticancer immune response.5
Immune Checkpoint Inhibitors (ICI) Physiology
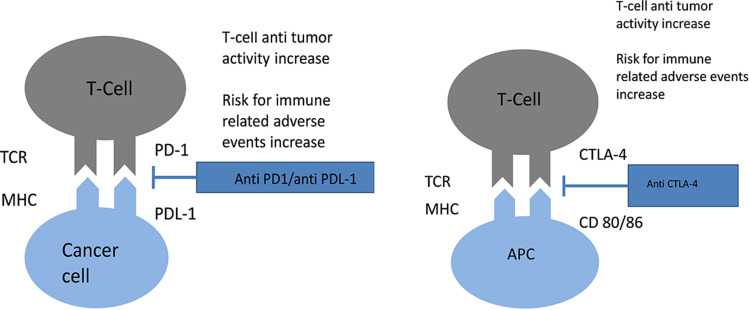
In mouse models, the suppression of the CTLA-4 gene causes death of mice due to lymphoproliferation. This observation revealed the critical negative regulatory role of CTLA-4.8,9 The suppression of the PD-1 gene in mice has more limited and variable autoimmunity, which includes arthritis and cardiomyopathy.10 Tumour cells upregulate CTLA-4 and PD-1, which decreases T-cell activity and promotes tumour growth. Blocking PD-1/PDL-1 and CTLA-4/CD28 co-signaling with ICI treatment blocks intrinsic negative regulation of the immune system and increases the cytotoxic T-cell and cytokine response against tumours (Figure 1).1,7
Figure 1.
Mechanism of action of both anti CTLA-4 and anti PD-1/PD-L1 agents.
Pathophysiology of Toxicities
irAEs can occur due to diverse, complex, and poorly understood mechanisms, which are probably interconnected. These irAEs appear to be a consequence of an excessive immune response secondary to the loss of immunological self-tolerance.1,7 Once the toxicity occurs, it could evolve independently.
One of the mechanisms of ICI toxicity has been underlined by the presence of a T-cell infiltrate found in the myocardium of patients with myocarditis. Interestingly, no B-cells or antibodies were found from the only two cases reported.11 Another possible mechanism of ICI toxicity was highlighted after the demonstration of the presence of similar T-cell clones between the tumour and myocardium thinking the presence of same antigens between the tumour and normal tissue.11 The mechanism of antigenic similarity is also described in the appearance of vitiligo in patients with advanced melanoma treated with ICIs. In this situation, melanocytes are destroyed by T-cell activity in response to similar antigens against melanoma and normal tissue.12
Previous reports have shown that patients with pre-existing anti-Thyroglobuline antibodies, and who are treated with ICIs, would be more likely to develop more thyroid disorders.13 This observation describes the possibility that ICI treatment could activate pre-existing humoral autoimmunity in a patient.
Other studies have shown a correlation between CTLA-4 gene polymorphisms and the development of irAEs with anti-CTLA-4 treatments.14 The role of cytokines in the occurrence of irAEs following ICI treatment is also discussed. For example, a high level of Il-17 was found in patients who developed colitis with anti-CTLA-4 treatment.14 An increase in inflammation due to complement activation was also highlighted in these patients.
Another mechanism of irAEs was the high expression of CTLA-4 antigen in normal tissue, such as the pituitary gland responsible for hypophysitis in patients treated with anti-CTLA-4 treatment. In an autopsy case series, high pituitary CTLA-4 expression was associated with clinical and severe hypophysitis pathology.15
The role of other genetic factors and the microbiota in the development of irAEs are also currently being investigated.2 Mechanisms of irAEs are still poorly understood and are likely to occur as a result of the conjunction of several non-exclusive phenomena.
Pre-Therapeutic Assessment
There are no predictive factors for identifying which patients will develop immune toxicity secondary to ICI treatment. Nonetheless, in certain situations close monitoring can be considered.
Medical History
A complete medical history that includes the collection of information about personal and family history with autoimmune and inflammatory conditions is essential. This is important as patients with a family history of autoimmune diseases are at higher risk of developing irAEs.16,17 For example, in patients with personal or family history of HLA B27 or HLA DRB1 genotypes, ICI treatment can unmask a predisposition to rheumatological complications such as rheumatoid arthritis or ankylosing spondylitis.18–20 It is important to note that a familial medical history of autoimmune diseases is not a contra-indication to using ICI therapies, but requires close monitoring.
Medication
As of yet, no medications have increased the toxicity of ICI therapies. However, some medications could blind toxicity and decrease the efficacy of ICIs. A review of all the medications a patient is taking should be completed and reported.
Since corticosteroid therapy is frequently used in oncology patients, many patients will be on ICI therapy and corticosteroids concurrently. Corticosteroids have a strong immunosuppressive action on adaptive immunity since they decrease antigen presentation, expression of co-stimulation markers like CD-80 or CD-86 and, ultimately, result in an ineffective T-cell response.21,22 Therefore, corticosteroid use has been associated with a decrease in the effectiveness of ICI treatment.23
In a cohort study of 640 patients with non-small cell lung cancer being treated with anti-PDL-1, 90 (14%) patients received corticosteroid doses ≥ 10 mg at the instauration of treatment. This therapeutic dose was significantly associated with a decrease in progression-free survival (hazard ratio: 1.3; P = 0.03) and a decrease in OS (hazard ratio: 1.7; P < 0.001).24 If feasible, patients on corticosteroid therapy should stop treatment or, alternatively, decrease to the lowest effective dose with a cut off of 10 mg/day. If this is not possible, a higher dose of corticosteroid could be continued along with ICI therapy, keeping in mind that ICI therapy would be likely to have a lower efficacy.
Other important medications to monitor include antibiotics. In an unplanned analysis from two trials of previously treated non-small-cell lung cancer randomly assigned to receive atezolizumab (n = 757) or docetaxel (n = 755), patients receiving antibiotics in atezolizumab arms had shorter OS (8.5 versus 14.1 months, HR 1.32, 95% CI 1.06–1.63, P = 0.01). This observation was not found for the docetaxel arms.25 Similar data were also observed for other cancer types like renal cell carcinoma.26 This phenomenon could be explained by the role of the microbiota. Preclinical models suggest that the microbiota influences a patient’s response to ICI therapy. Routy et al showed that the use of antibiotics 2 months prior to ICI therapy, and up to 1 month post ICI initiation, modified the microbiota and was responsible for a decrease in the tumour response.27
In a small cohort of melanoma patients treated with ICIs (n=43), the analysis of fecal microbiome samples suggested that different microbiota compositions resulted in different responses to ICIs. These data were also observed in patients with lung cancer.28 Like corticosteroids, the use of antibiotics is not a contra-indication for starting ICI therapy, but the initiation of antibiotics at the start of ICI treatment must be carefully considered and used with caution.
The influence of proton pump inhibitors (PPI) should also be considered. Derosa et al reported a negative effect of PPI therapy for patients treated with anti PDL-1. The negative effects were attributed to the effect that PPIs have on the composition of the gut microbiome.26 Even though there are few data concerning the relationship between PPIs and ICIs and, given that PPIs have a less important role compared to corticosteroids or antibiotics, the use of PPIs could be considered only if necessary before initiation of ICI therapy.
Imaging
As for other anti-tumour agents, it is necessary to obtain up to date computed tomography (CT) scans (TAP/CTAP) or magnetic resonance imaging (MRI) data to have a good estimate of tumour volume prior to starting treatment with ICIs. Close clinical monitoring in the first weeks of ICI therapy is recommended due to the possible hyper-progression phenomena in certain patients (6 to 29%). An early scan evaluation should also be done if there is any doubt of hyperprogression.29 Other organs like the brain should also be monitored as new lesions could emerge with ICI treatment.
Biology
Because ICIs could cause toxicities in any organ, a biological assessment is also necessary to screen patients at risk or certain pre-existing anomalies.30 Hematological investigation (NFS-platelets, TP-TCA), renal function (ionogram, magnesium, calcium, urea, creatinine, proteinuria), liver function (AST, ALT, bilirubin, GGT, APL), diabetes (glucose), pancreatic abnormalities (lipase), cardiac markers (BNP, troponin), endocrinal function (TSH, T4, cortisol at 8 a.m., testosterone, LH, FSH), and muscle integrity (CPK) should be analyzed.
Given that ICI treatment could cause potential reactivation of viral infections, viral serology (HBV, HCV, HIV) and PCR detection for CMV and EBV are also recommended. An evaluation of inflammation levels are also routinely screened (VS, CRP, LDH).
Due to the possibility of ICI treatment causing cardiac toxicity, a baseline electrocardiogram and an evaluation of ventricular function (if possible) is recommended.
Toxicity Management
The management of toxicity while on ICI therapy requires a rapid communication of information to physicians. It is also necessary to inform patients of the potential risks of ICI treatment and they should be informed to communicate any reactions quickly to their physician.
Almost all published trials on ICI treatment toxicity are retrospective, which makes it difficult to establish a universal management strategy.2 Different scientific societies have developed guidelines, major institutions have developed specific multidisciplinary meetings on ICI toxicities, and most of the prospective trials created their own guidelines. As such, several heterogeneous algorithms have been created and summarize in Supplementary data. Despite these useful tools, more specific approaches with additional emerging information have been established, especially by community oncologists.
It is important to note that even if the presentation of ICI toxicities mimics autoimmune diseases, their treatments are not similar. For example, the efficacy of corticosteroids in autoimmune hepatitis is low compared to the high efficacy of corticosteroids for ICI related hepatitis.31 This phenomenon might be explained by different types of immune infiltration. In ICI-related hepatitis, there are more diffuse, cytotoxic T-cell predominant lobular infiltrate patterns with fewer CD4 T cells and plasma cells in the liver parenchyma compared to patients with primary autoimmune hepatitis.32
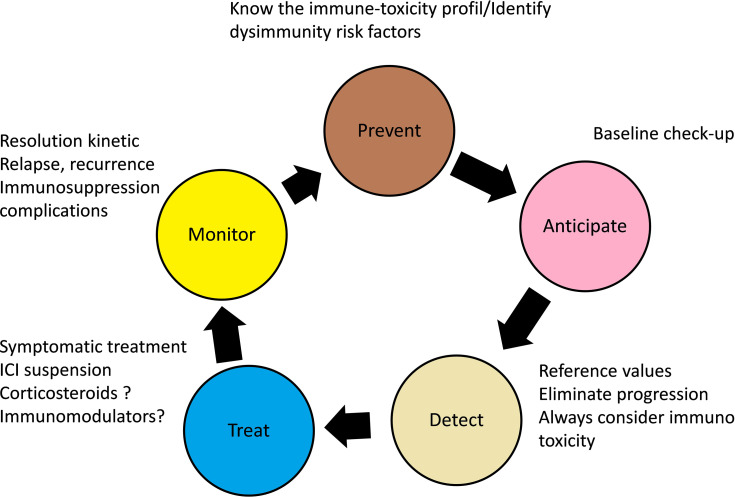
Since the underlying mechanism of ICI immunotoxicity is related to the inflammation of healthy tissues due to a loss of self-tolerance loss, suspending ICI treatment may not be sufficient to treat ICI reactions. Thus, corticosteroid therapy is usually the first-line treatment. Other immunomodulators (mycophenolate mofetil, tacrolimus, cyclosporine, or anti-thymocyte antibodies) may also sometimes be necessary to treat ICI toxicity if a resistance to corticosteroids occurs.1,2 The clinical principles of managing immunotoxicity related to ICI treatment are summarised in Figure 2; a strict collaboration between physicians and patients is necessary.33
Figure 2.
General principles of immunotoxicity management inspired from Menzies et al.33
ICI Treatment Suspension
Unlike chemotherapy, a dose reduction is currently not recommended if ICI toxicity occurs. Postponing and stopping ICI treatment is currently the first step in managing ICI toxicity.33 Whether to suspend ICI treatment temporarily or permanently depends on the type, grade, and the duration of toxicity as well as whether corticosteroid therapy and immunomodulatory treatment were used in the management. Typically, a potentially life- threatening Grade 4 toxicity for the patient should warrant treatment discontinuation.
For endocrine toxicities, hormonal supplementation often permits the continuation of ICI treatment without the use of corticosteroids. In general, suspending ICI treatment is not necessary for rheumatic toxicity. However, low dose corticosteroids and management by a specialist is often required.
Optimal Use of Corticosteroid Therapy
Once moderate toxicity appears secondary to ICI treatment, suspending treatment and supportive care are generally no longer sufficient for management and corticosteroid therapy should be quickly initiated. A multidisciplinary team should be involved in the decision to administer corticosteroids but should not delay their initiation.
There are multiple methods of corticosteroid administration (oral prednisone, IV methylprednisolone, local budesonide administration, topicals, eye drops). In a retrospective study investigating advanced melanoma, up to 35% of patients required corticosteroid therapy for managing ICI toxicity.34 Contraindications to the use of corticosteroid therapy still exist, especially in cases of severe active infection. Corticosteroid use must be carefully considered in severe unbalanced psychiatric conditions. However, in cases of severe ICI toxicity, the balance is generally in favour of use of corticosteroids.
Patients receiving long-term corticosteroid treatment should regularly be screened for side effects (diabetes, osteoporosis, muscular atrophy, Cushing’s syndrome, glaucoma, sleep disorders and psychiatric disorders). Immunosuppression induced by corticosteroid therapy can lead to a greater susceptibility to bacterial or even fungal infections (aspergillosis). Prophylaxis of pneumocystosis with trimethoprim-sulfamethoxazole, atovaquone, or pentamidine may be indicated once a prednisone or equivalent dose of > 25 mg/day is maintained for more than 4 weeks.1,2
The tapering of steroids is also an important step in the management of immune toxicity caused by ICI treatment. This period could result in rebound symptoms and close monitoring is required during the taper period.
Use of Immunomodulatory Therapies
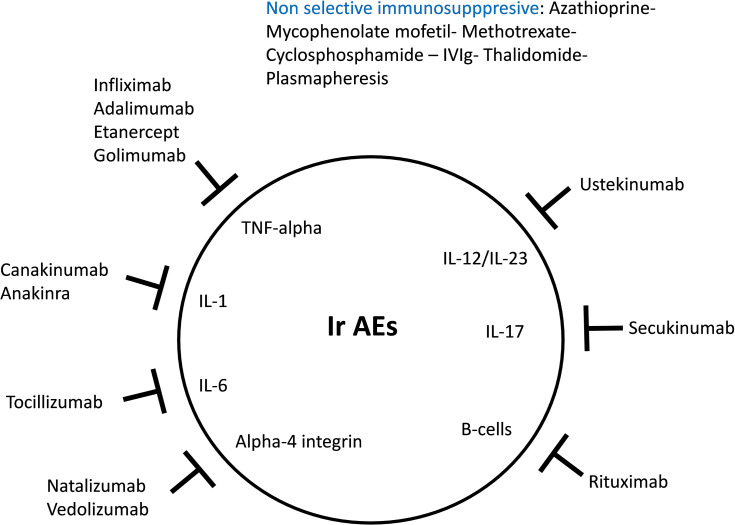
In cases where cortico-refractory toxicity occurs, or according to the nature and the severity of ICI toxicity, the use of immunomodulators as first-line therapy must be discussed (Figure 3).35 Myocarditis requires a rapid therapeutic response and patients treated with high doses of steroids have lower cardiac major adverse events than patients treated with lower doses.36
Figure 3.
Mechanism of action of immune modulating medications inspired from Martins et al.35
Other immunosuppressive therapies are also being investigated. In a cohort of 781 patients treated with ICIs, 92 patients presented with diarrhea. In this study, the correlation between the grade of diarrhea and the severity of endoscopic or histological analysis was poor. However, patients with higher endoscopic severity scores as a result of ulcers or pancolitis require infliximab more often. Consequently, endoscopic evaluation could help physicians identify whether to use infliximab over steroids.37
Immunomodulators generally result in remission of the toxicity more quickly than corticosteroid therapy. However, since they can cause cardiac, vascular, and renal toxicity, clinical and biological monitoring by a specialised team is required. Additionally, prophylaxis therapy for pneumocystosis is essential during treatment with several drugs particularly with infliximab.2
Other therapies are also being evaluated for treating several ICI toxicities. For example, vedolizumab (anti-integrin α4β7 antibody used in inflammatory bowel diseases) is currently being investigated as an agent tor treating cases of immuno-induced colitis.38 Abatacept (fusion protein inhibiting T cell activation via the recruiter CTLA-4), which is an effective treatment for rheumatoid arthritis, should also be explored in cases where ICI treatment causes rheumatological toxicity.39
Resuming ICI Treatment
Apart from certain skin (86% reversibility) and endocrine (46% reversibility) toxicities, the majority of irAEs (including grade 3–4) are reversible within a few weeks. The possibility of restarting ICI treatment should therefore always be discussed between the clinician and the patient.1 A medical history of grade 3–4 toxicity from ICI therapy often constitutes an exclusion criterion, even though there are no prospective trials on this subject. The exception is for endocrinopathy, rheumatologic, and several skin toxicities. Any life-threatening toxicity is a definitive contraindication for restarting ICI treatment.2
Whether to resume ICI treatment depends on the severity of the initial toxicity, but the number of subsequent therapeutic possibilities are often limited. Therefore, an objective tumour response will be a strong argument for considering the re-initiation of ICI treatment.2
Another ICI class may possibly be offered to the patient. Indeed, according to several retrospective studies, it seems that the toxicity of anti PD-1/PDL-1 does not predict a toxicity to anti-CTLA-4.33
Since there is a lack of data on whether to recommend anti-PD-1 use if toxicity from anti-PDL-1 occurs, or vice versa, there are no recommendations in this regard. Furthermore, the toxicity was not similar in cases where anti PD-1 or anti PDL-1 were introduced after immune toxicity occurred with one of these agents.
In a trial among melanoma patients who had to stop anti CTLA-4 therapy, only 3% experienced a recurrence in toxicity with anti PD-1 and 34% developed a new irAE.33 The delay between the last dose of the first ICI and the first dose of the second ICI seems to be an important factor in the recurrence of toxicity.30
Among 38 patients (24 grade 1/2, 14 grade 3/4 and 29 treated with corticosteroid) with bronchial carcinoma who were retreated with the same anti-PD-1 agent after treatment suspension post toxicity, 50% had no further immune-related adverse events and only 24% had a recurrence of toxicity. A new toxicity was developed by 26% of patients who resumed treatment. Among the 19 patients who developed a recurrent irAE, nearly all were manageable and 16/19 (84%) improved to grade 0–1 events with treatment. Two treatment-related deaths occurred despite treatment with high dose corticosteroids, anti-TNF agents, and other immunosuppressants. The mortality rate due to irAEs in the retreated cohort was 5%.40
Although recurrent immune adverse events are usually less severe than the initial events (probably because of heightened surveillance), a decision to restart ICI treatment is likely to depend on the severity of the prior event, the availability of alternative treatment options, and the overall status of the cancer.40 For toxicities requiring the use of corticosteroid therapy, it is recommended to suspend ICI treatment as long as the prednisone equivalent dosage remains above 10 mg/day.
According to some authors, resuming ICI treatment can be associated with a low dose corticosteroid therapy (< 10mg/day) to prevent an irAE relapse.
Recently Dolladille et al41 descrip a pharmacovigilance cohort of of 24,079 irAEs associated with at least one ICI and 452 of 6123 irAEs associated with ICI rechallenges (7.4%) were analysis. This cohort study find 28.8% of recurrence rate of the same irAE. Colitis, hepatitis and pneumonitis were associated with a higher recurrence rate, whereas adrenal events were associated with a lower recurrence rate compared with other irAEs.
Different Toxicity Profiles
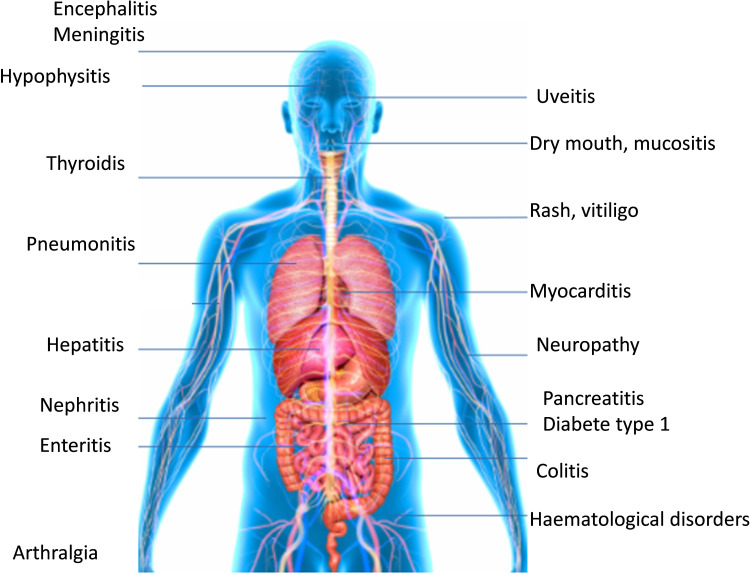
The most frequent irAEs include cutaneous, digestive, hepatic, and endocrine toxicities, although almost all organs can be affected (Figure 4).2 Each study investigating irAEs defines the severity of toxicity differently, thus it is essential to grade the toxicities according to the Common Terminology Criteria for Adverse Events (CTCAE) to limit evaluation bias.1 A toxicity labeled as Grade 3 or higher is considered as a severe toxicity. These irAEs occur mainly in the first 6 months of treatment, but can occur up to 1 year after stopping ICI treatment.30 Even though few data have described the safety profile of long-term ICI exposure, a prolonged ICI treatment greater than 2 years does not appear to increase the incidence of irAEs.1,2 However, the frequency of irAEs is still relatively low compared to other etiologies such as disease progression or consequent infections and these must be ruled out first.42
Figure 4.
Organs affected by immune-related adverse events.
The diagnosis of irAEs can be difficult for clinicians and is often based on several patient factors (clinical factors, CT scans, serological or hormonal assays, pathology).1 Some biomarkers have been described as etiologic factors of irAEs (hypereosinophilia, high level of IL 17), but are not yet used in practice.7 Although some toxicities can appear early, knowing the time of onset to determine whether the irAE is acute or chronic helps diagnosis by the clinician.1
Acute Toxicity (≤8 Months)
Acute toxicities are defined as toxicities that occur less than 8 months post treatment initiation.
Skin Toxicity
Skin toxicities are usually the first to occur after the initiation of ICI treatment and often occur within the first 2 weeks.2 Typically, four types of skin reactions are described: inflammatory, immuno-bullous, keratinocyte alteration (acantholytic dyskeratosis), or melanocytic (regression of nevus, prurigo, vitiligo).30
Rash and pruritus are the most frequent irAEs. Rash and pruritus comprise 50% of skin toxicities with anti-CTLA-4, 40% of cases with anti PD-1, and 60% of biotherapy cases. Severe skin toxicity, defined as grade 3–4 remains below 10% of cases.34 Cutaneous toxicities are more frequent in patients with melanoma than other localisations. Approximately 24.3% patients treated with Ipilimumab developed a rash43 and 35% developed pruritus since materia.44 Typically, the rash is maculopapular in nature, is slightly erythematous, and appears on the chest and limbs. This type of rash has been reported in 40%, 32%, and 26% of patients with metastatic melanoma treated with anti-CTLA-4 and anti PD-1treatment, anti-CTLA-4 treatment alone, and anti-PD-1 monotherapy, respectively.44 However the occurrence of grade 3–4 events was low (<2% for patient treated with anti-CTLA-4).45
The appearance of vitiligo can occur up to 3 weeks post initiation of treatment with ICIs (10% with pembrolizumab vs 2% with ipilimumab) and almost exclusively occurs in the context of advanced melanoma. Vitiligo is a frequent toxicity for melanoma patients. Vitiligo predominately occurs in the upper extremities and has been associated with a better response to anti-tumour therapy in many studies.34
Cases of alopecia, mucositis, cutaneous xerosis, photosensitivity, lichenoid, or psoriasis from reactions have been described less frequently. Exceptional cases of DRESS syndrome, Steven-Johnsons Syndrome, Lyell (toxic epidermal necrolysis), bullous pemphigoid, and Sweet syndrome have been observed.7,30 A dermatological consultation as well as a skin biopsy must be considered as soon as a grade 2 toxicity appears to exclude contact dermatitis, viral rash, vasculitis, atopic dermatitis, folliculitis, and toxiderma.30 Typically skin biopsies reveal the presence of a perivascular lymphocytic infiltrate.46
Skin toxicities are treated with local treatments, oral, or IV anti-inflammatories depending on the grade of toxicity. In cases of severe or persistent toxicity, high dose steroids or immunosuppressive treatments are required. Antihistamines can be used for treating pruritus as well as cold compresses or oatmeal baths. Topical corticosteroids or tricyclic antidepressants can also be used in the most disabling cases. Even though they are rare, cases of mucositis and dry syndrome can be treated with good oral hygiene, lidocaine, and cortisone mouthwashes.7
Gastrointestinal Toxicity
Digestive irAE including gastrointestinal and hepatic toxicity typically occur within 6 to 7 weeks.30 With anti-CTLA-4 therapy, gastrointestinal toxicity is one of the most common. The symptoms that develop include diarrhea in 33% of patients and colitis in 8 to 22% patients. Diarrhea also occurs in 6.8 to 19% of patients on anti PD-1/PDL-1therapy.44,47 The onset of diarrhea occurs relatively quickly, usually before six weeks. Notably, experiencing digestive toxicity with anti-CTLA-4 treatment is not predictive of digestive toxicity while on anti PD-1 or anti PDL-1 therapy. Combination therapy results in a higher frequency (44%) of digestive toxicity that occurs earlier on, but such cases are not more severe than toxicities associated with anti-CTLA-4 therapy alone.44
The most common symptoms of ICI therapy-associated enterocolitis include diarrhea, abdominal pain, rectal bleeding, weight loss, fever, vomiting, and oral or anal ulcerations. Dysphagia or epigastric pain can be signs of damage to the upper digestive tract. Biologically, anemia, inflammatory syndrome, and hypoalbuminemia may also be present.
Since there are no specific biomarkers for this toxicity,7 it is important to inform the patient of the risks of changes in bowel transit times to avoid the development towards severe grade 3–4 diarrhea and the increased risk of digestive perforation and peritonitis. If diarrhea is present, a stool culture ± parasitological and virological examination is needed to eliminate the classical enteropathogens like Clostridium Difficile. Diverticulitis and chronic inflammatory bowel diseases are other differential diagnoses that also need to be ruled out.
Recto-sigmoidoscopy is recommended for grade 3–4 and persistent grade 2 gastrointestinal toxicity and is sufficient to confirm the diagnosis; it also eliminates the need for a differential diagnosis but should not delay the treatment. The colorectal mucosa is typically erythematous with a loss of vascularisation, ulcers, and mucosal erosions. If biopsies are performed, colitis with neutrophilic and eosinophilic infiltrate as well as the presence of focal or diffuse cryptic abscesses can be observed. Microscopic colitis and intestinal pseudo-obstruction have also been reported with anti PD-1. The presence of chronic inflammation with a granulomatous reaction can make differential diagnosis more difficult. In upper digestive disorders, a fibroscopy could highlight esophageal ulcers as well as images of gastritis or duodenitis.30
The treatment of gastrointestinal irAEs depends on the intensity of symptoms. Depending on the treatment centre, infliximab may be used as the first line.48 In severe grade 3–4 colitis other immunosuppressants can be used. Mycophenolate mofetil 500 to 1000mg twice a day and tacrolimus have been effective. Vedolizumab and anti Il-17 therapy are currently under investigation for this indication.38 Several studies have also evaluated the efficacy of budesonide for prophylaxis but have not shown a decreased incidence of diarrhea with ICI treatment.34
Other less frequently occurring digestive disorders have also been described. The most common is an elevated level of pancreatic enzymes like amylase and lipase. A diagnosis of acute pancreatitis cannot be made if there is no pain present or there are no available CT images and corticosteroid therapy seems effective. Esophagitis, celiac disease, and enteric neuropathy have also been described.7 More studies are necessary to assess the risk of developing chronic inflammatory bowel disease after ICI treatment.
Liver Toxicity
Hepatic toxicity resulting from ICI treatment generally occurs within 6 to 14 weeks after initiation of ICI treatment.7 Often asymptomatic (isolated cytolysis, cholestatic icterus), it occurs in 5 to 10% of patients on anti-PDL-1 therapy, and in up to 25–30% of patients on anti-CTLA-4 therapy.49 Grade 3 toxicity is also more frequent with combination therapy (14%) than with monotherapy (2%). However, the incidence of Grade 3 toxicity is generally identical with anti-CTLA-4 (1 to 7%) and anti-PD-1 (1 to 6%) therapies.1
To avoid liver toxicity, liver function must be checked prior to each infusion. At the incidence of any disturbance to hepatic function it is necessary to eliminate acute systematically alcoholic hepatitis, use of hepatotoxic drugs, thrombosis, or a viral infection (HAV, HBV, HCV, HEV). An abdominal ultrasound is systematically performed to eliminate the possibility or progression of liver metastasis. In the case of immuno-mediated hepatitis, peri-portal edema and hepatomegaly can be observed.50 A liver biopsy is also recommended for any grade 3–4 toxicity. Histologically, sinusoid histiocytosis with endothelitis is observed with ICI liver toxicity and sometimes portal inflammation with cholangitis makes a differential diagnosis with NASH difficult.51
The management of hepatic toxicity depends on the grade of toxicity. In cases of refractory corticosteroid hepatitis, immunomodulatory treatment with mycophenolate mofetil 500 to 1000 mg twice a day can be discussed. The administration of tacrolimus or anti-thymocyte antibodies for two days must be discussed in cases of fulminant hepatitis. Since infliximab can result in hepatic toxicity, it is contraindicated in these situations.
Chronic Toxicity
Endocrine Toxicity
ICI-induced thyroiditis accounts for a significant portion of the irAEs. Thyroid dysfunction is low with anti-CTLA-4 therapy (1 to 6.8%)45,52 but occurs in 5 to 10% of patients during treatment with anti-PDL-1 monotherapy53–55 and in up to 20% of patients with anti-PD-1 and anti- CTLA-4 combination therapy.47 Interestingly, hypothyroidism occurs more frequently (up to 9%) with high doses of anti-CTLA-4 at 10 mg/kg.56 Although severe grade 3–4 thyroid toxicity is rare, regular thyroid assessments measuring TSH (thyroid stimulating hormone) and T4 levels are recommended.
In most cases, thyroid dysfunction is discovered incidentally from blood test analysis. However, symptoms of hypothyroidism like weight gain, depression, constipation, chills, and fatigue can be an alert for physicians. If peripheral hypothyroidism occurs, which is defined as having high TSH with low T4 levels, treatment with L-thyroxine at a dose of 0.5–1 µg/kg should be initiated. ICI treatment can be continued but thyroid function should be monitored before each infusion.30 Hyperthyroidism often precedes hypothyroidism and must be addressed, especially if the patient presented with no symptoms previously. Strict monitoring is necessary in cases of low TSH since there is an increased risk of developing hypothyroidism a second time.
Cases of hyperthyroidism often present with thyrotoxicosis syndrome, which is more clinically alarming and include symptoms like weight loss, sweating, palpitation, and anxiety.7,30 This occurs in 6% of patients on pembrolizumab versus 2% of patients on ipilimumab. Every immune-mediated case of hyperthyroidism should be treated with anti-TSH receptor antibody (TRAK), anti-TG, or anti-TPO antibodies. A specialist should be consulted and a thyroid scintigraphy is recommended. Symptomatic treatment of hyperthyroidism includes the use of beta-blockers. Treatment with carbimazole, methimazole, or propylthiouracil can be used for patients who are positive for TRAK or are symptomatic. Corticosteroid therapy is not recommended for immune-mediated hyperthyroidism except in rare cases of painful thyroiditis, in which oral prednisolone 0.5 mg/kg can be used. For patients who have severe symptoms, ICI treatment should be discontinued.
It is important to note that a low TSH level and a low T4 level should prompt screening for hypophysitis. Hypophysitis due to ICI treatment appears in 4–17% of cases of anti-CTLA-4 treatment. This is a dose-dependent toxicity. Hypophysitis also occurs in up to 16% of cases of combination therapy. Cases of hypophysitis with anti-PD-1 or anti-PDL-1 are exceptionally rare, with an incidence of 0.5%.57,58 It is important to note that the prevalence of hypophysitis is likely to be underestimated since its clinical presentation is non-specific and it is often diagnosed late.
The time to onset of hypophysitis is approximately 8–9 weeks after treatment initiation.7 Although hypophysitis has a variable clinical presentation, symptoms can include headache, visual disturbances, mood disorders, symptoms of hypothyroidism, adrenal insufficiency (hypotension, hypoglycemia, hyponatremia, hyperkalemia), or hypogonadism (amenorrhea, impotence). Rare cases of diabetes insipidus have also been described.1 The biological assessment for hypophysitis includes TSH, T4 levels, adrenocorticotropic hormone (ACTH), follicular stimulating hormone (FSH), luteinizing hormone (LH), testosterone, insulin-like growth factor 2 (IGF-1), prolactin, and cortisol levels at 8:00 AM. If the hypophysitis is global, all hormonal levels are low and the cortisol level at 8:00 AM collapses. A brain MRI should be performed and typically pituitary edema with supra-sellar convexity and heterogeneous contrast enhancement indicates hypophysitis. It can also eliminate the possibility of other differential diagnoses like carcinomatous meningitis or metastasis of the base of the skull.30 The diagnosis of hypophysitis can be complex and is based on a combination of clinical signs, hormonal tests, and is accompanied by a suggestive brain MRI.
A specialist consultation is recommended. According to CTCAE 4.0, there is no precise grading classification of hypophysitis.1
Hypophysitis management depends on the symptomatology and includes hormone replacement in cases of asymptomatic or mild symptoms. Additionally, introduction of corticosteroids can be used in cases of moderate to severe symptoms and ICI treatment should be discontinued. Long-term supplementation for > 2 years is necessary for the majority of patients and a card for adrenal insufficiency must be given to the patient.
ICI-induced type I diabetes is another rare irAE (<1%) that occurs with ICI treatment. It is more frequent with anti-PD-1 and anti-PDL-1 than with anti-CTLA-4 therapy.59 ICI-induced type I diabetes could develop quickly and result in hyperosmolar coma. Regular blood sugar tests should be completed to screen for ICI-induced type I diabetes. In some cases, it can present as acute ketoacidosis. The anti-GAD and anti-islet antibody assays are still necessary for the differential diagnosis of type 1 versus type 2 diabetes.
According to the CTCAE 4.0, there is no grade classification of ICI-induced Type 1 diabetes. In all cases, the treatment is insulin therapy. The role of corticosteroid remains unclear, but its use would make the control of diabetes more difficult. ICI treatment can be resumed as soon as glycemic decompensation is controlled.30
Primary adrenal insufficiency and hypoparathyroidism are other endocrine disorders rarely described in the literature.7 According to some authors, in cases where patients are experiencing fatigue without endocrine damage, corticosteroid therapy with a low-dose of prednisolone <20 mg/day is tolerated.1
Lung Toxicity
Pulmonary toxicity is one of the later occurring toxicities and can occur within 7–24 months after the initiation of treatment, but could occur more early. Although it is rare (5% incidence with anti-CTLA-4 and 3% with anti-PD-1 or anti-PDL-1), pulmonary toxicity has the highest rate of mortality.55,60,61 Pulmonary toxicity can affect up to 10% of patients on combination therapy and up to 2% of these cases are grade 3–4.62 The incidence of pneumonitis was rather similar across melanoma, NSCLC, and renal cell-carcinoma tumours. However, it seems that more treatment-related deaths due to pneumonitis occur in patients with NSCLC.63
Given the severity of pulmonary toxicity, a chest CT scan should be performed as soon as any new respiratory symptoms like dyspnea, cough, hypoxia, chest pain, and crackles appear. It is important to note that pulmonary adverse events are more often related to disease progression, notably for lung cancer or lung metastasis. Electrocardiogram, arterial blood gas analyses, and a dosage of cardiac enzymes are also necessary to exclude other diagnoses like acute pulmonary edema and pulmonary embolism.
In immune mediated pulmonary toxicity, bilateral pulmonary infiltrate, ground-glass opacities, and an interlobular septal thickening are typically described on the CT scan. A differential diagnosis of these non-specific images should be performed to rule out carcinomatous lymphangitis, organised cryptogenic pneumonia, non-specific interstitial pneumonia, hypersensitivity pneumonitis, or acute respiratory distress syndrome.64,65 Recently, an infection with COVID-19 presented that was similar to ICI treatment-induced pneumonitis.66 Fibroscopy of bronchoalveolar lavage could be performed and typically shows a lymphocytic inflammatory reaction. This test could be interesting for the differential diagnosis of pneumonitis versus pulmonary infection. A pulmonary biopsy is generally not required and is only warranted if there is still doubt about the etiology.
Rheumatological Toxicity
Rheumatological symptoms like myalgia and arthralgia can occur in 2–12% of patients treated with anti-PDL-1, and in 1–8% of patients on anti-CTLA-4 treatment.67 Given the inflammatory nature of arthralgia, it should signal the possibility of an immuno-mediated toxicity. Rheumatological toxicity is rarely serious (1% grade 3–4). Clinically, one or more of arthritis with synovitis and tenosynovitis can be observed. Cases of vasculitis, myositis, polymyositis, and temporal arteritis are also possible.68
Symptomatic treatments with paracetamol and/or NSAID could be used. A low dose of corticosteroids is often necessary for moderate symptoms In case of refractory presentation anti- TNF-α should be considered.68
Rare Toxicities
Kidney Toxicity
Immune mediated tubulointerstitial nephritis is rare. It occurs in 1% of patients on anti-PD-1/PDL-1 or anti-CTLA-4 therapy, and in 5% of patients on combination therapy.69 Renal failure typically occurs within 13 weeks of initiating ICI treatment and it is not always reversible. The ICI dose administered to the patient does not depend on prior renal function. The possibility of renal toxicity justifies a renal assessment prior to each infusion that includes ionogram, urea, and creatinine.30 Other causes of increased creatinine levels like renal obstruction or infection must also be ruled out. A renal biopsy could be performed to clarify the diagnostic.
The management of tubulointerstitial nephritis depends on the grade of the toxicity. However, symptomatic treatment should avoid using nephrotoxic drugs and correcting hypovolemia.
Lupus nephritis has been described with anti-CTLA-4 therapy.70 In a small case series of 13 patients treated with ICIs who developed renal dysfunction, the prevalent pathologic lesion was acute tubulointerstitial nephritis in 12 patients with lymphocytic infiltration and one patient presented with thrombotic microangiopathy.71
Neurological Toxicity
Neurological toxicity caused by ICI treatment is extremely variable and rare. Less than 3% of patients experience neurological toxicity from ICIs. However, recent studies have described up to 6% of cases of neurological toxicity with anti-PD-1 therapy, and up to 12% with combination therapy.30 The median time to neurotoxicity onset is 13 weeks after initiating treatment with anti-CTLA-4 therapy.72
The neurotoxicity resulting from ICI treatment can be central toxicity or peripheral toxicity. Central neurotoxicity includes encephalitis, reversible posterior leukoencephalitis, aseptic meningitis, and transverse myelitis while peripheral toxicity includes Guillain Barré syndrome, myasthenia, radiculoneuritis, demyelination, and polyneuropathy. Cranial nerve damage can also sometimes be observed.1 If central neurological damage is suspected, a brain MRI or an electroencephalogram can eliminate other causes like brain metastases, carcinomatous meningitis, hydrocephalus, and epilepsy.30 In neuromeningeal disease, a lumbar puncture shows pleocytosis of < 500 cells/mm3, which are predominantly lymphocytic, and proteinorachia < 1.5 g/L.
Although the diversity of neurological disorders does not allow classification by grade, ICI treatment must be suspended as soon as neurologic toxicity symptoms appear and until the etiology of the symptoms is defined. If moderate or severe neurologic symptoms occur, ICI treatment must be permanently discontinued.1
The treatment of immune-mediated neurotoxicity uses oral or IV corticosteroid therapy at a dose of 0.5–1 mg/kg and up to 2 mg/kg for severe symptoms. Corticosteroid tapering should be achieved in 4 to 8 weeks.30 If there is any suspicion that herpetic meningitis is occurring, corticosteroid therapy should be postponed and empirical treatment with IV acyclovir should be initiated and continued until results from Herpes simplex virus (HSV) PCR are available. In cases of myasthenia gravis or Guillain Barré syndrome, treatment with plasmapheresis or IV immunoglobulins should be discussed.30
Ocular Toxicity
Immune mediated ophthalmic injuries are rare and occur in <1% patients.73 All ophthalmic structures can be affected in immune mediated ophthalmic injuries. Toxicities include conjunctivitis, keratitis, episcleritis, uveitis, orbital inflammation, retinal disease, and choroidal diseases. Any ophthalmological symptoms like blurred vision, irritation, or redness should result in a consultation with a specialist as soon as possible. If any visual loss occurs, oral or IV corticosteroid therapy at a dose of 1–2 mg/kg should be initiated. In cases of episcleritis or uveitis that is not responding to local corticosteroids, ICI treatment should be suspended.1
Cardiac Toxicity
Rare cases of arrhythmia, myocarditis, pericarditis, and Takotsubo have been described with ICI treatment.74 Like many other toxicities, cardiac injury seems to occur more frequently with combination therapy. Monitoring cardiac enzymes like BNP and troponin are recommended during pre-therapeutic assessment but their monitoring is not routinely recommended during follow-up. In cases where recent dyspnea has occurred, a cardiological consultation should be requested as soon as the diagnosis is suspected.30 A high dose of oral or IV corticosteroid therapy (1–2 mg/kg) should be initiated rapidly in the intensive care unit as well as symptomatic treatment including a vasopressor. Treatment with infliximab or mycophenolate mofetil should be discussed if there is no rapid improvement.30
Hematological Toxicity
Isolated cases of disseminated intravascular coagulation, acquired hemophilia, idiopathic thrombocytopenic purpura, and autoimmune hemolytic anemia have been reported following treatment with ICIs. These occurred more frequently in patients treated for Hodgkin’s lymphoma. Effects of ICIs on bone marrow can also be observed like myelosuppression, isolated neutropenia, and myelodysplasia. In most cases, a bone marrow biopsy shows lymphocytic infiltrates without another cause.
Treatment of hematological toxicities from ICIs involves oral or IV corticosteroid therapy as well as the administration of granulocyte colony stimulating factor (GCSF) in cases of neutropenia. In refractory cases, IV immunoglobulins or cyclosporine therapy may be offered. A complete blood count (CBC) remains essential to measure prior to each infusion, although hematological anomalies are rarely identified following these analyses.1
Toxicity Profile of Anti-PD-1/PDL-1
Clinical studies investigating anti-PD-1 (nivolumab, pembrolizumab) or anti-PDL-1 agents (atezoliumab, avelumab, durvalumab) found that 39–70% of patients develop toxicity of all grades, combined.75–78 A large systematic review and meta-analysis of clinical trials investigating patients treated with anti-PD-1 or anti-PDL-1 therapy revealed that fatigue, pruritus, and diarrhea were the most common adverse events (AEs) described. The most frequent serious AE’s defined as grade 3 or higher were fatigue, anemia, and elevated aspartate aminotransferase. Anti-PD-1 agents were associated with a greater number of grade 3 AEs and an overall higher number of AEs compared to anti-PDL-1 agents (OR: 1.58; 95% CI: 1.00–2.54) but had a similar toxicity for all AEs compared to anti-PDL-1 agents (OR: 1.00; 95% CI: 0.78–1.32).65 The major causes of treatment-related deaths (n = 82) were respiratory (n = 39, 48%), cardiovascular (n = 9, 9.8%), infectious (n = 7, 8.5%), hematologic (n = 5, 6.1%), and hepatic (n = 3, 3.7%) causes.
In contrast to anti-CTLA-4 therapy, the toxicity of anti-PD-1 or PDL-1 therapy was not dose related. A dose escalation of nivolumab from 0.1–10 mg/kg of body weight every 2 weeks demonstrated no increase in toxicity.79 Similar results were shown for pembrolizumab with a dose escalation from 2–10 mg/kg.80
Toxicity Profile of Anti-CTLA-4 Agents
In clinical studies investigating treatment with the anti-CTLA-4 agent ipilimumab, 60–85% of patients developed a toxicity of any grade. The median time to onset of anti-CTLA-4 toxicities was 6 weeks. The timeline of anti-CTLA-4 toxicity occurs earlier than with anti-PD-1 or anti-PDL-1 agents.7 Pruritus, digestive damage, and pituitary damage are more frequently described with anti-CTLA-4 treatment than with anti-PD-1 or anti-PDL-1 therapy.2 Additionally, toxicities from anti-CTLA-4 therapy are more severe than with other ICIs (20–30% grade 3–4 vs, 10–15%).2 A dose related toxicity of anti-CTLA-4 therapy was identified by Ascierto et al after the evaluation of different ipilimumab doses (3mg/kg vs 10mg/kg) in melanoma.81 The most relevant toxicities according to each treatment are summarised in Table 1.
Table 1.
Immune-Related Adverse Events Associated with Anti CTLA-4 or Anti PD-1 or Anti PDL-1 or Association of Anti PD-1 and Anti CTLA-4
| Anti CTLA-4 (Any Grade (Grade ≥3))43,44,51,53,55,71 | Anti PD-1/PDL-1(Any Grade (Grade ≥3))43,46,51-54,75,77,78 | Anti CTLA-4 + Anti PD-1 (Any Grade (Grade ≥3))43 | |
| Rash | 14.553 −3971 (0.844 −3.151) | 477 −25.943 (047 −1.151) | 40.3 (4.8) |
| Pruritis | 24.444 −4371 (044 −1.151) | 7.246 −23.251 (043 −0.554) | 33.2 (1.9) |
| Diarrhea | 22.755 −4973 (3.153 −1071) | 6.846 −24.351 (046 −2.553) | 44.1 (9.3) |
| Hepatitis * | 1.253 −2171 (044 −5.751) | 1.153 −6.251 (143 −1.151) | 17.6 (8.3) |
| Hypothyroidis | 1.544 −971 (044 −0.451) | 878 −11.678 (044 −0.455) | 15 (0.3) |
| Arthralgia | 5.153 −10.851(043 −0.451) | 577 −12.651 (043 −0.453) | 10.5 (0.3) |
Note: The table showed the major immune related adverse events in large phase 3 clinical trial in metastatic or adjuvant setting including the lower and upper rate of toxicities.
Abbreviations: CTLA-4, cytotoxic T lymphocyte associated protein 4; PD-1, program cell death 1; PDL-1, program cell death ligand 1.
Toxicity Profile of Combination Therapy
Despite better tumour response rates, numerous studies show that the risk of developing toxicity with combination therapy increases by 50% compared to monotherapy (relative risk = 1.5).7 Patients receiving a combination of ICIs, which is most likely the combination of anti-PD-1 and anti-CTLA-4 therapy, are also likely to present with earlier toxicity.31 A meta-analysis evaluating the use of combination therapy also highlights more severe toxicities. In the meta-analysis, 55% of events were of grade 3–4 severity with combination therapy versus 16% grade 3–4 events with nivolumab alone, and 27% grade 3–4 events with ipilimumab alone.34
Immunotherapy in High Risk Populations
Patients with Auto Immune Diseases
Patients with autoimmune or inflammatory diseases have been constantly excluded from ICI clinical trials, so little prospective data exist in this high-risk population. Despite the lack of data, some retrospective studies have evaluated the efficacy and toxicity of ICIs in patients at risk of autoimmune flare-ups.
Among 397 patients treated with anti-PD-1 therapy who were included in a prospective study and who experienced toxicity, 45 had a history of autoimmune diseases like vitiligo, psoriasis, thyroiditis, Gougerot syndrome, rheumatoid arthritis, vasculitis, lupus, sarcoidosis, idiopathic thrombopenic purpura, and myasthenia gravis. Additionally, more than 50% were still symptomatic at the beginning of ICI treatment. The majority of patients (n = 25, 55.6%) were symptomatic, but few of them (n = 7, 15.6%) were still receiving immunosuppressive therapy with corticosteroids, methotrexate, hydroxychloroquine, or others at the time of ICI treatment initiation. The results also showed that in the autoimmune group, 44% of patients presented with an irAE versus 23.8% in the control group. Additionally, 50% of these irAEs were identified as a “flare-up” of the underlying autoimmune disease. The suspension of ICI treatment was only necessary for four patients with underlying autoimmune conditions including acute colitis, microscopic colitis, acute tubulointerstitial nephritis associated with Sjögren’s syndrome, and a flare-up of myasthenia gravis. There was no significant difference in response rate and overall survival between the two groups.82
In another trial, the occurrence of an autoimmune flare-up was more frequent in patients with uncontrolled disease before the initiation of ICI treatment (60% of all flare-ups) and in those still requiring immunosuppressive therapy at initiation (50% of all flare-ups).33 Moreover, the median onset of irAEs appeared much earlier in these patients (5.4 months vs 13 months). The response rates were also identical between patients with and without flare-ups.33
Although patients with autoimmune diseases appear to have an increased risk of experiencing irAEs, anti-PD-1 therapy is beneficial with relatively frequent but moderate grade toxicity. The suspension of ICI treatment in these patients is rarely required. A medical history of auto-immune diseases is not a contraindication to ICI treatment but a discussion with the patient is necessary to inform them of the risk of flare-ups, the possibility of new immune disorders, the progression of the cancer, and alternative treatments.33,82
Regarding anti-CTLA-4 therapy, similar treatment irAEs were identified in another retrospective study evaluating the use of ipilimumab for patients with advanced melanoma. Among 30 patients with a history of autoimmune disease including rheumatoid arthritis, psoriasis, and controlled chronic inflammatory bowel disease, 43% of patients were still treated with immunomodulators at the initiation of anti-CTLA-4 therapy. An auto-immune “flare-up” occurred in 27% of patients with a median time of onset of 1 month after the start of treatment. All were treated with corticosteroid therapy. A high grade 3–5 toxicity occurred in 33% of patients, which was unrelated to the underlying autoimmune disease. In this study, 50% of the patients developed neither a flare-up nor an irAE. Regarding the efficacy of the treatment, an objective response rate of 20% was observed, including a complete response in a patient who presented with a flare-up of rheumatoid arthritis. The median overall survival found in this study was 12.5 months.83
These data indicate that the use of anti-CTLA-4 treatment in the context of pre-existing autoimmune or inflammatory disease is possible. ICI treatment for patients with auto immune diseases is responsible for autoimmune decompensation in approximately 20 to 30% of cases but this is easily manageable.
The use of immunomodulators for autoimmune or inflammatory disorders seem to reflect an advanced illness and could decrease the efficacy of ICI treatment. Accordingly, it is preferable to use corticoids treatment at doses < 10 mg/day for patients with autoimmune diseases. After an interdisciplinary team discussion, a benefit-risk balance assessment, and close monitoring, patients with a history of autoimmune or inflammatory disease should still be able to benefit from ICI treatment.33,82,83
Older Patients
Older patients are currently underestimated in studies, but represent an important cohort of patients treated for cancer. Chemotherapy toxicity occurs more frequently in older patients than in younger patients.40 Older adults’ comorbidities and their age-related immune system impairment might affect the effectiveness and tolerance of ICI treatment. Moreover, older patients are known to have a higher prevalence of auto-antibodies and one can also expect that ICI treatment may reveal subclinical autoimmune diseases.41 A meta-analysis of randomised trials was conducted to investigate the efficacy of ICI treatment in older patients compared to younger adults. The efficacy of anti-CTLA-4 or anti-PD-1/PDL-1 therapy for different indications was analysed in 1244 older patients and compared to 2078 younger patients. The results revealed that there was no statistically significant difference in overall survival between younger and older patients (p = 0.93).
The frequency, grade, and characteristics of irAEs among patients who were older than 65 years and younger than 65 years were also analysed. This study showed no statistically significant difference in the incidence of irAEs, and the irAE profile was similar in both groups.41
Another study evaluating safety and efficacy of ICI treatment in real life conditions showed no difference in OS, PFS, or irAEs between patients older than 70 years old or younger patients.84 However, Baldini et al analysed patients treated with anti-PDL-1 therapy and showed an increase in the incidence of grade 2 or higher AEs in patients older than 70 years (33% versus 25%, p = 0.03). There was also a greater incidence of multiple toxicity compared to younger patients but the efficacy was similar between the groups with no difference in median PFS or median OS.85
According to these meta-analyses, the effectiveness of ICI treatment is independent of the patient’s age. It is important to note that the definition of older patients is not the same in every study, therefore it is difficult to have agreeable results. To our knowledge, there are no studies on how to treat irAEs specifically in older patients, but dose adjustment is not recommended, similar to any other situation with anti PDL-1 therapy.34,86 Since older patients who have cancer are often taking additional medications for other comorbidities, it is important to state that ICI treatment is not metabolised by cytochrome P450 enzymes, therefore enzymatic competition is not expected. Patients treated with anticoagulant or antiplatelet therapy must be carefully monitored in cases where colitis symptoms are identified, given the risk of gastrointestinal hemorrhage.41 Moreover, the use of some symptomatic treatments such as antihistamine for pruritus or corticosteroids may expose older patients to iatrogenic events such as worsening diabetes, disturbed mental status, hypertension, and delirium.41 A geriatric assessment could help identify older patients who will benefit from ICI treatment.86
Since half of all malignancies are diagnosed in patients older that 65 years old, dedicated studies are necessary for the proper and safe use of ICI treatment in the older patient population.8,41 Since there are no differences in treatment efficacy compared to younger patients, and toxicity levels are acceptable, there are no restrictions to using ICI treatment in older patients, but close monitoring is important.
Hepatic Insufficiency and Renal Failure
ICIs belong to a large family of monoclonal antibodies. They are eliminated via the reticuloendothelial system and are not excreted hepatically or renally. Therefore, no dose adjustment is necessary for patients with hepatic insufficiency or renal failure. Currently, the dose of ICI no longer depends on weight, but corresponds to a fixed dose. According to retrospective studies, no increases in toxicity were observed in cirrhotic or renal failure patients.34
The high molecular weight of anti-PD-1 and anti-CTLA-4 antibodies suggest that these agents are not removed by dialysis and no dose adjustment is required. Two single-centre trials highlight their efficacy in patients on dialysis but there is a possible increased risk of irAEs.87,88
Another trial with a small sample of patients also showed that the activity of anti-PD-1 therapy was similar compared to patients with normal renal function, and no unexpected irAEs occurred.89
Transplanted and Allografted Patients
Transplant and allograft patients are always excluded from prospective trials. As a result, no real prospective data exist. A recent literature review published in 2019 evaluated the safety of ICI treatment for 39 patients who had had a previous solid organ transplantation. Allograft rejection occurred in 41% of patients at similar rates for anti-CTLA-4 and anti-PD-1 therapy. The median time to rejection was 21 days. Among patients with graft rejection, graft loss occurred in 81% and death was reported in 46% of patients.90 Solid organ transplantation is not an absolute contraindication to using ICI treatment, but there is a high rate of rejection and mortality for these patients. A benefit-risk analysis must be done and is dependent on the type of graft.
Unlike pulmonary or cardiac grafts, a lost kidney transplant could be managed by dialysis. A multidisciplinary consultation is always necessary prior to initiation. According to other studies, anti-CTLA-4 would be less likely to cause graft rejection compared to anti-PD-1/PDL-1 therapy.2,30 For patients with bone marrow transplants who are not on long-term immunosuppressive therapies, the efficacy of ICIs may be better than in solid organ transplant patients. That said, the risk of graft versus host disease (GVHD) seems to increase with ICI treatments.2
HIV
Patients with viral infections such as HIV or active viral hepatitis were previously excluded from ICI treatment studies. However, these patients are more often exposed to tumour pathologies. Certain retrospective studies appear to favour the use of ICIs since the efficacy and toxicity profile appears comparable to other agents.91–93 Additionally, no viral reactivation was observed in these studies.94
Similarly, pembrolizumab has been used in HIV patients with advanced cancer. The patients in this study had to be stable with a CD4 count greater than or equal to 100 cells/μL, they had to be on antiretroviral therapy (ART) for 4 or more weeks, and had to have an HIV viral load of less than 200 copies/mL.95 A multidisciplinary discussion between an oncologist and infectious disease specialist is recommended before initiating treatment. Apart from any tumour pathology, a Canadian trial is currently testing the efficacy of ICI treatment in HIV-positive patients with increasing viral load while on antiviral triple therapy.96 ICI treatment could therefore provide these patients with a dual effect with regard to controlling viral replication and tumour growth.
Variability in ICI Responses
Immunosuppression – Efficacy Relationship
The use of corticosteroid therapy or immunomodulatory therapies for immunotoxicity could interfere with the effectiveness of ICI treatment. To investigate whether the management of immunotoxicity would interfere with the effectiveness of subsequent ICI treatment, a retrospective study evaluating the resumption of treatment with anti-PD-1 after the onset of toxicity with anti-CTLA-4 therapy in patients with advanced melanoma was conducted. Among 67 patients who required corticosteroid therapy or immunomodulatory treatment for toxicity with anti-CTLA-4 therapy (included 86% grade 3–4 events), the tumour response rate with anti-PDL-1 therapy was 40%. However, in the five patients still receiving immunosuppressive therapy at the initiation of anti-PD-1, the response rate did not exceed 15%. This was in contrast to the 44% response rate in non-treated patients at the anti-PD-1 initiation.33
For immune induced colitis, the use of corticosteroid therapy or infliximab does not seem to modify the tumour response rate.30 According to several other retrospective studies, the response rate would not be modified in these patients.63,97 More data from prospective studies are necessary to evaluate the interaction between immunosuppressants and ICI treatments.
Toxicity – Efficacy Relationship
Immune related adverse events are caused by immune activation, and the relationship between ICI treatment toxicity and efficacy is regularly questioned. The relationship between toxicity and efficacy is complex because patients with toxicity represent a very heterogeneous group. This is due to different toxicity grades, whether a decision to use an immunosuppressive treatment was made, and whether ICI treatment was suspended during and after the event.
In melanoma, the onset of vitiligo or arthritis appears to be correlated with a better tumour response.98
In a retrospective study that included 271 patients treated with anti-PD-1 therapy for advanced bronchial carcinoma, 116 patients (42.8%) presented with an immunotoxicity, of whom 56 required a temporary or permanent suspension of ICI treatment. Corticosteroid therapy was initiated in approximately 20% of cases. The results of this study are in favour of decreased overall survival in patients who had to stop ICI treatment (median OS of 8.3 months vs 14.5 months, p = 0.008). Overall survival also appears lower in patients with Performance Status > 1, liver metastases, and grade 3–4 toxicity (HR OS = 2.29 95% CI (1.05–4.98); p = 0.036). If all grades were combined, the only toxicity associated with a decreased overall survival would be the appearance of colitis with nivolumab (p = 0.01). No toxicity was associated with an improvement in overall survival in this study.98 These results should be interpreted with caution because patients and the type of care management was very heterogeneous among different centres.
In cases of metastatic renal cell carcinoma, a real-world efficacy and safety study showed better overall survival in patients who developed irAEs of any grade.99 Similarly, 1747 patients from seven clinical trials investigating metastatic or locally advanced urothelial cancer treated with anti-PDL-1, reported immune toxicity in 64% of responding patients and in 34% of patients who did not respond to treatment.100 Similar data were documented for nivolumab treatment of non-small cell lung carcinoma.101 Similar Eggermont et al analysis the effect of irAEs for patient treated with pembrolizumab in adjuvant setting for melanoma. The occurrence of an irAEs was associated with a longer recurrence free survival in the pembrolizumab arm.102
Rheumatic disorders are interesting predictors of response. Kostine et al showed a better tumour response rate for patients with rheumatic AEs compared with patients without rheumatic AEs (85.7% vs 35.3%; P<0.0001).103
At minimum, the general consensus is that irAEs are not required to obtain a benefit from ICI treatment. The lack of standard guidelines for managing immunotoxicity and the conflicting results from these studies should influence the development of new prospective trials investigating this subject.12
Conclusion
Immune checkpoint inhibitors currently represent one of the most effective treatments against cancer and are increasingly being used as first-line agents for many indications. With the growing use of ICIs, a new toxicity profile has appeared. This included immune related adverse events. Although immune related adverse events are often reversible and are rarely lethal, they must be rapidly recognised by clinicians to optimise their management.
The management of immunotoxicity usually involves corticosteroid therapy or the use of immunomodulators. For those patients who are not eligible to receive other therapeutic options, early and adequate management of toxicity eventually allows ICI treatment to resume. More studies are required to understand more fully the mechanism of immune related adverse events and to develop more targeted therapies.
The tolerance profile of ICIs is, overall, better than that of chemotherapy. This allows their use to be extended over a wider population. However, the prospective data are still scarce in patients with underlying autoimmunity and immunosuppression. Given that ICI treatment increases overall patient survival and the indications for immunotherapy will continue to expand, new toxicities may soon emerge.
Funding Statement
No funding for this review.
Disclosure
Felix Lefort reports personal fees from Ipsen, outside the submitted work. A Ravaud has received honoraria for consultancy from Pfizer, Astra Zeneca, MSD, BMS, Roche, and Ipsen; is a member of the GU for Pfizer, Astra Zeneca, MSD, BMS, Roche and Ipsen; and has received transportation and housing for meetings from Pfizer, Astra Zeneca, BMS and Ipsen; and reports grants, personal fees, and non-financial support from Pfizer and Merck GA, and personal fees, non-financial support from BMS, Ipsen, MSD, Astra Zeneca, and Novartis, outside the submitted work. M Gross-Goupil is a member of GU board for Pfizer, Astra Zeneca, MSD, BMS, Roche and Ipsen; has received transportation and housing for meetings from Pfizer, BMS, Janssen, Ipsen, Roche, MSD, Amgen, Astellas; has received honoraria for symposiums from MSD, BMS, Pfizer.; and reports personal fees, non-financial support from BMS, MSD, Astra Zeneca, Roche, and Merck, during the conduct of the study. A Daste has received honoraria for consultancy for Astra-Zeneca, BS, MSD, Roche, and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 3.Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol. 2011;3(1):17–30. doi: 10.1039/C0IB00046A [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Cerottini J-C, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12(1):337–365. doi: 10.1146/annurev.iy.12.040194.002005 [DOI] [PubMed] [Google Scholar]
- 5.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12(7):489–492. doi: 10.1038/nrd4066 [DOI] [PubMed] [Google Scholar]
- 7.Hryniewicki AT, Wang C, Shatsky RA, Coyne CJ. Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J Emerg Med. 2018;55(4):489–502. doi: 10.1016/j.jemermed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- 9.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985 [DOI] [PubMed] [Google Scholar]
- 10.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- 11.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer. 2017;123(S11):S11:2143–53. doi: 10.1002/cncr.30444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimbara S, Fujiwara Y, Iwama S, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018;109(11):3583–3590. doi: 10.1111/cas.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romo-Tena J, Gómez-Martín D, Alcocer-Varela J. CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun Rev. 2013;12(12):1171–1176. doi: 10.1016/j.autrev.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 15.Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. doi: 10.1016/j.ajpath.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockadedysimmunetoxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi: 10.1093/annonc/mdv623 [DOI] [PubMed] [Google Scholar]
- 17.Eun Y, Kim IY, Sun J-M, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep. 2019;9(1):14039. doi: 10.1038/s41598-019-50574-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steven NM, Fisher BA. Management of rheumatic complications of immune checkpoint inhibitor therapy – an oncological perspective. Rheumatology. 2019;58(Supplement_7):vii29–vii39. doi: 10.1093/rheumatology/kez536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology. 2019;58(3):476–480. doi: 10.1093/rheumatology/key358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leipe J, Christ LA, Arnoldi AP, et al. Characteristics and treatment of new-onset arthritis after checkpoint inhibitor therapy. RMD Open. 2018;4(2):e000714. doi: 10.1136/rmdopen-2018-000714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Y, Bender IK, Konstantinidis AK, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013;121(9):1553–1562. doi: 10.1182/blood-2012-05-432336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garant A, Guilbault C, Ekmekjian T, Greenwald Z, Murgoi P, Vuong T. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: a systematic review. Crit Rev Oncol Hematol. 2017;120:86–92. doi: 10.1016/j.critrevonc.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 24.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. doi: 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 25.Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 26.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 28.Jin Y, Dong H, Xia L, et al. The diversity of gut microbiome is associated with favorable responses to anti–programmed death 1 immunotherapy in chinese patients with NSCLC. J Thorac Oncol. 2019;14(8):1378–1389. doi: 10.1016/j.jtho.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 29.Frelaut M, Le Tourneau C, Borcoman E. Hyperprogression under Immunotherapy. IJMS. 2019;20(11):2674. doi: 10.3390/ijms20112674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 31.McGuire HM, Shklovskaya E, Edwards J, et al. Anti-PD-1-induced high-grade hepatitis associated with corticosteroid-resistant T cells: a case report. Cancer Immunol Immunother. 2018;67(4):563–573. doi: 10.1007/s00262-017-2107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31(6):965–973. doi: 10.1038/s41379-018-0013-y [DOI] [PubMed] [Google Scholar]
- 33.Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376. doi: 10.1093/annonc/mdw443 [DOI] [PubMed] [Google Scholar]
- 34.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346. doi: 10.1001/jamaoncol.2016.1051 [DOI] [PubMed] [Google Scholar]
- 35.Martins F, Sykiotis GP, Maillard M, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20-e54-e64. [DOI] [PubMed] [Google Scholar]
- 36.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278. doi: 10.1136/esmoopen-2017-000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66(5):581–592. doi: 10.1007/s00262-017-1962-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234. doi: 10.1001/jamaoncol.2015.4368 [DOI] [PubMed] [Google Scholar]
- 40.Santini FC, Rizvi H, Wilkins O, et al. Safety of retreatment with immunotherapy after immune-related toxicity in patients with lung cancers treated with anti-PD(L)-1 therapy. J Clin Oncol. 2017;35(15_suppl):9012. doi: 10.1200/JCO.2017.35.15_suppl.9012 [DOI] [Google Scholar]
- 41.Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):1–7. doi: 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helissey C, Vicier C, Champiat S. The development of immunotherapy in older adults: new treatments, new toxicities? J Geriatr Oncol. 2016;7(5):325–333. doi: 10.1016/j.jgo.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 43.Minkis K, Garden BC, Wu S, Pulitzer MP, Lacouture ME. The risk of rash associated with ipilimumab in patients with cancer: a systematic review of the literature and meta-analysis. J Am Acad Dermatol. 2013;69(3):e121–8. doi: 10.1016/j.jaad.2012.12.963 [DOI] [PubMed] [Google Scholar]
- 44.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merrill SP, Reynolds P, Kalra A, Biehl J, Vandivier RW, Mueller SW. Early administration of infliximab for severe ipilimumab-related diarrhea in a critically ill patient. Ann Pharmacother. 2014;48(6):806–810. doi: 10.1177/1060028014528152 [DOI] [PubMed] [Google Scholar]
- 49.Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38(6):976–987. doi: 10.1111/liv.13746 [DOI] [PubMed] [Google Scholar]
- 50.Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol. 2015;39(8):1075–1084. doi: 10.1097/PAS.0000000000000453 [DOI] [PubMed] [Google Scholar]
- 51.Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31(4):1071–1077. doi: 10.1007/s10637-013-9939-6 [DOI] [PubMed] [Google Scholar]
- 52.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 53.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 55.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 56.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol. 2016;28(4):278–287. doi: 10.1097/CCO.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 58.Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. 2019;181(3):211–219. doi: 10.1530/EJE-19-0238 [DOI] [PubMed] [Google Scholar]
- 59.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38(9):137–138. doi: 10.2337/dc15-0889 [DOI] [PubMed] [Google Scholar]
- 60.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi: 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 62.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 64.Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–290. doi: 10.1056/NEJMc1505197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–717. doi: 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calabrò L, Peters S, Soria JC, et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir Med. 2020;S2213-2600(20)30170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benfaremo D, Manfredi L, Luchetti MM, Gabrielli A. Musculoskeletal and rheumatic diseases induced by immune checkpoint inhibitors: a review of the literature. Curr Drug Saf. 2018;13(3):150–164. doi: 10.2174/1574886313666180508122332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 69.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 70.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–212. doi: 10.1056/NEJMc0904283 [DOI] [PubMed] [Google Scholar]
- 71.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638–647. doi: 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- 73.Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288–294. doi: 10.1097/CCO.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 74.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 76.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 77.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-l1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 81.Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. doi: 10.1016/S1470-2045(17)30231-0 [DOI] [PubMed] [Google Scholar]
- 82.Danlos F-X, Voisin A-L, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21–29. doi: 10.1016/j.ejca.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 83.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corbaux P, Maillet D, Boespflug A, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur J Cancer. 2019;121:192–201. doi: 10.1016/j.ejca.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 85.Baldini C, Martin Romano P, Voisin AL, et al. Impact of aging on immune-related adverse events generated by anti-programmed death (ligand)PD-(L)1 therapies. Eur J Cancer. 2020;129:71–79. doi: 10.1016/j.ejca.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 86.Van Holstein Y, Kapiteijn E, Bastiaannet E, van den Bos F, Portielje J, de Glas NA. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer. Drugs Aging. 2019;36(10):927–938. doi: 10.1007/s40266-019-00697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cavalcante L, Lutzky J, Amin A. Ipilimumab was safe and effective in two patients with metastatic melanoma and end-stage renal disease. Cancer Manag Res. 2015;47–50. doi: 10.2147/CMAR.S73389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park S, Daniels GA. Anti-PD-1 therapy in patients with end-stage renal disease on dialysis: a single-center case series. J Clin Oncol. 2017;35(15_suppl):14553. doi: 10.1200/JCO.2017.35.15_suppl.e14553 [DOI] [Google Scholar]
- 89.Vitale MG, Baldessari C, Milella M, et al. Immunotherapy in dialysis-dependent cancer patients: our experience in patients with metastatic renal cell carcinoma and a review of the literature. Clin Genitourin Cancer. 2019;17(5):903–908. doi: 10.1016/j.clgc.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 90.Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7(1):106. doi: 10.1186/s40425-019-0585-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pu D, Yin L, Zhou Y, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine. 2020;99(5):e19013. doi: 10.1097/MD.0000000000019013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ostios-Garcia L, Faig J, Leonardi GC, et al. Safety and efficacy of PD-1 inhibitors among HIV-positive patients with non–small cell lung cancer. J Thorac Oncol. 2018;13(7):1037–1042. doi: 10.1016/j.jtho.2018.03.031 [DOI] [PubMed] [Google Scholar]
- 93.Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5(7):1049. doi: 10.1001/jamaoncol.2018.6737 [DOI] [PubMed] [Google Scholar]
- 94.Shah NJ, Al-Shbool G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7(1):353. doi: 10.1186/s40425-019-0771-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uldrick TS, Gonçalves PH, Abdul-Hay M, et al. Assessment of the safety of pembrolizumab in patients with hiv and advanced cancer-a Phase 1 study. JAMA Oncol. 2019;5(9):1332. doi: 10.1001/jamaoncol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat Commun. 2019;10(1):814. doi: 10.1038/s41467-019-08798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ksienski D, Wai ES, Croteau N, et al. Efficacy of nivolumab and pembrolizumab in patients with advanced non–small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clin Lung Cancer. 2019;20(1):97–106. [DOI] [PubMed] [Google Scholar]
- 99.on behalf of the Italian Nivolumab Renal Cell Cancer Early Access Program group, Verzoni E, Cartenì G, Cortesi E, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program. J Immunother Cancer. 2019;7(1):99. doi: 10.1186/s40425-019-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maher VE, Fernandes LL, Weinstock C, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730–2737. doi: 10.1200/JCO.19.00318 [DOI] [PubMed] [Google Scholar]
- 101.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse eventswith nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eggermont A, Kicinski M, Blank C, et al. Associationb etween immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6(4):519–527. doi: 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77(3):393–398. doi: 10.1136/annrheumdis-2017-212257 [DOI] [PubMed] [Google Scholar]