Abstract
During human diamine oxidase (DAO) ELISA development we noticed that in serum DAO concentrations appear to be higher when compared to plasma. Neutrophils contain DAO in the specific granules and we hypothesized that DAO is released from neutrophils during serum coagulation. If activation of neutrophils can release DAO, its concentrations might be elevated in vivo after lipopolysaccharide (LPS) administration and in bacteremic patients. Using blood from healthy volunteers DAO concentrations were measured ex vivo in serum, citrate, EDTA and heparin plasma over several hours and after activation of neutrophils. Lipopolysaccharide and granulocyte-colony stimulating factor (G-CSF) were administered to 15 and 8 healthy volunteers, respectively and DAO concentrations were measured at different timepoints. DAO antigen levels were also determined in three different subcohorts of patients with culture-proven bacteremia and high C-reactive protein (CRP) levels. DAO concentrations were elevated in a time-dependent manner in serum but not in EDTA or citrate plasma (P < 0.01). Neutrophil activation using phorbol myristate acetate (PMA) and zymosan dose-dependently caused DAO concentrations to be elevated more than 10-fold at both 22°C and 37°C (both P-values <0.001). Administration of LPS to healthy volunteers released DAO from neutrophils (P < 0.001). Of the 55 different bacteremic patients selected from three independent cohorts only 3 (5.4%) showed highly elevated DAO concentrations. Serum DAO concentrations do not accurately reflect circulating enzyme levels but coagulation-induced neutrophil activation and consequently DAO release. Only a few bacteremic patients show high DAO concentrations able to degrade histamine rapidly.
Keywords: bacteremia, diamine oxidase, lipopolysaccharide, neutrophil activation, specific granules
Introduction
Diamine oxidase (DAO) was first described almost nine decades ago due to its histamine degradation activity.1 In humans high DAO mRNA levels and activity were found in the gastrointestinal tract, kidney, and placenta.2 Plasma DAO concentrations increase at least 100-fold during pregnancy,3,4 but the physiological function of this rise is not clear. Expression in the placenta is restricted to fetus-derived extravillous trophoblast cells (EVTs) and therefore DAO is not of maternal origin (uterine decidua cells) as was believed to be the case for decades.5 A few publications described the presence of DAO in the specific granules of both neutrophils and eosinophils and its release after activation.6 Herman et al. and Melamed et al. showed that DAO is released from human granulocytes in a complement-dependent manner.7,8 What could the function of DAO release from activated neutrophils be?
One possibility would be to degrade histamine. The synthesis of new histamine can be induced by LPS and certain strains of Pseudomonas aeruginosa in neutrophils.9,10 Diamine oxidase could locally counteract the pro-inflammatory effects of histamine. Neutrophils are not only key for effective antimicrobial defense, but are also clearly responsibly for causing tissue damage.11 DAO could dampen an excessive immune response by inactivating histamine. Although some animal studies, including a primate model, support the hypothesis that histamine plays an important role in the development of endotoxic shock,12–14 the role of histamine during septic conditions in humans is not clear. Neugebauer et al. published that increased histamine concentrations are a predictor of mortality in sepsis patients.15 Unfortunately, these data were never confirmed by further studies. Jacobs et al. measured normal histamine concentrations in septic shock patients.16 One possible explanation for normal histamine levels in septic shock patients could be that DAO is released and is able to degrade histamine in vivo as well ex vivo. We recently published that DAO antigen concentrations are strongly increased during mast cell activation in mastocytosis patients and that DAO is possibly degrading histamine not only in vivo but also ex vivo in blood collection tubes or during downstream assays.17 The increase in DAO concentrations is possibly caused by the release of the heparin proteoglycan serglycin from activated mast cells, which displaces DAO from the heparin-sensitive heparan sulfate glycosaminoglycan binding sites in the gastrointestinal tract.
Quantification of DAO antigen or activity in serum seems to be still considered useful in the diagnosis of histamine intolerance,18,19 although the value of the results in relation to histamine intolerance is highly questionable.20,21 Some data are published showing that DAO is not present in serum21 but others demonstrated DAO activity in serum with a highly sensitive and relatively specific radioactive putrescine assay.22 We recently published the development of a human DAO ELISA, which allows us to accurately quantify hDAO antigen concentrations in serum, plasma and other body fluids. The mean DAO plasma concentration is approximately 1 ng/mL, which is physiologically likely not really relevant, because for example 8 ng/mL DAO in plasma of pregnant women degrade 100 ng/mL histamine with a half-life of 54 min.4,17 Nevertheless, 63 ng/mL DAO would only need 6.7 min to reduce the histamine concentration by 50%. At high local DAO tissue concentrations histamine might be rapidly inactivated. What is the source of DAO measured in serum or plasma? Is it leaking from the gastroinestinal tract or the kidneys or neutrophils?
We present data that DAO measured in serum is mainly derived from activated neutrophils and can be elevated manifold compared to citrate or EDTA plasma. In vivo neutrophil activation with a single LPS injection also caused DAO release, but most patients with high C-reactive protein (CRP) and culture-proven bacteremia have low circulating DAO levels unable to degrade histamine efficiently.
Material and methods
Standard Greiner Vacuette® citrate, heparin and EDTA blood collection tubes were used to prepare serum or plasma (Greiner Bio-One, Kremsmuenster, Austria). For serum preparation in Figure 1(b) we used BD Vacutainer® Rapid Serum Tubes (RST) tubes (Becton Dickinson, Vienna, Austria). Tubes were not moved during the different incubation times. After obtaining serum and plasma all samples, if not otherwise indicated, were frozen for at least 24 h at −30°C before DAO ELISA measurements. Phorbol myristate acetate (P1585, Sigma-Aldrich, Vienna, Austria) was dissolved in DMSO and stored in small aliquots at −30°C. Zymosan (Z4250, Sigma-Aldrich, Vienna, Austria) was activated by incubating 10 mg in 1 mL PBS for 1 h at 99°C. After centrifugation for 30 min at 3000 rpm the pellet was dissolved in 1 mL PBS, stored in the fridge and used within 1 week. Both PMA and zymosan were added as a 20× solution with appropriate matrix solutions as controls. Blood dilution was therefore 5%. Heparin blood collection tubes were used for PMA and zymosan experiments. DAO concentrations measured in citrate plasma were multiplied by 1.17, because 1 part citrate solution is mixed with 9 parts whole blood, but citrate is not taken up by cells and therefore the 1 part citrate solution is diluted only within the plasma compartment. A correction factor of 1.17 assumes a hematocrit value of 40%.
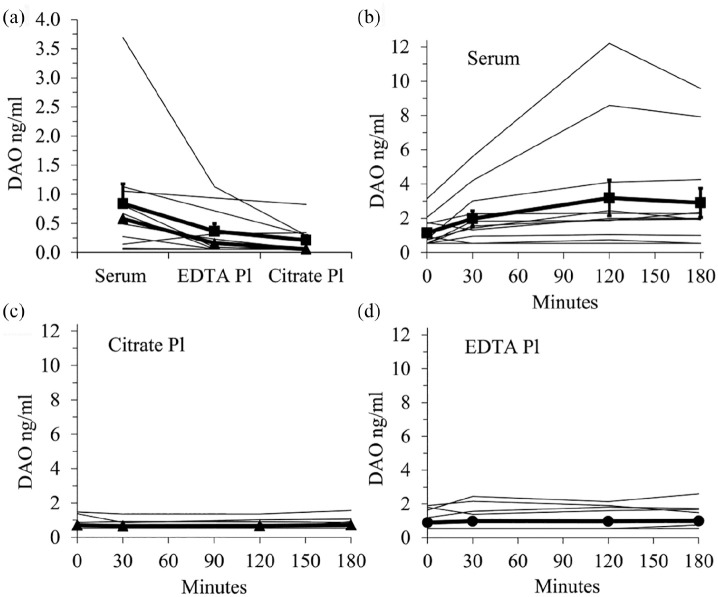
Figure 1.
Serum human DAO concentrations are higher as compared to citrate and EDTA plasma. (a) DAO concentrations in serum, citrate and EDTA plasma from 10 healthy volunteers were measured with a DAO ELISA. The mean of duplicates is shown. Serum tubes were left for 30 min before centrifugation. The mean (+/− SEM) and median are represented by filled squares and triangles respectively. In (b–d) serum, citrate, and EDTA plasma from 12 healthy volunteers (four subjects also included in Figure 1(a)) were prepared immediately or after 30, 120, and 180 min incubation at room temperature. The means (+/− SEM) are shown as bold lines.
DAO: diamine oxidase; Pl: plasma.
LPS administration
Fifteen healthy volunteers were enrolled in a cross-over study to receive either NaCl or Colistin sulfate.23 We analyzed the NaCl arm. Both groups received 2 ng/kg bodyweight of LPS (E. coli 0113 Reference Endotoxin, CC-RE Lot 3; National Institutes of Health, Bethesda, US). EDTA plasma was prepared at different time points and stored at −80°C. We did not perform a formal sample size calculation for the in vivo LPS study. The data from the 15 subjects in the NaCl arm were evaluated in an exploratory fashion.
G-CSF administration
Healthy volunteers received a single intravenous dose of 300 µg G-CSF (Neupogen® filgrastim).24 EDTA plasma was prepared at different time points and stored at −80°C.
In vivo bacteremia and high CRP Patient Cohorts
We tested EDTA plasma samples from three different patient subcohorts selected for high CRP and culture-proven bacteremia. All three cohorts were comprised mainly of non-surgical, non-intensive care ward inpatients. CRP and IL-6 were measured in the clinical chemistry laboratory of the Vienna General Hospital with standard methods. Determination of bacteremia was performed following standard procedures.
Human diamine oxidase ELISA
The development and the characterization of the human DAO ELISA were recently published.4 Recombinant human DAO, produced and characterized as described in Gludovacz et al.,25 was spiked into some bacteremia samples to analyze the presence of interfering substances.
Tryptase ELISA
Total serum tryptase concentrations were measured by the commercial fluoroimmunoenzyme assay (Thermo Scientific, Uppsala, Sweden) in the clinical chemistry laboratory of the Vienna General Hospital using an accredited procedure. The detection limit of the assay is 1 ng/mL and the coefficient of variation (CV) is 4.5% at 9 ng/mL.
Ethics
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. All patients and all healthy volunteers provided their informed consent before the collection of blood samples. The ethics approval numbers for the blood samples from healthy volunteers were EC-No.2030/2013 and EC-No.1810/2015, for the LPS study EC-No.1577/2014 and for the G-SCF Study EC-No.484/2006. For Sepsis Cohort_1 ethical approval was waived by the Ethics Committee of the Medical University of Vienna because the samples were collected during routine procedures and were completely and irreversibly anonymized. Ethics approval numbers for Sepsis Cohort_2 was EC-No. 8/2009 and Sepsis Cohort_3 EC-No. 518/2011.
Statistical analysis
Because a normal distribution of DAO concentrations in Figures 1 to 3 was only fulfilled in some samples, we used the non-parametric Friedman ANOVA followed by the conservative Nemenyi post-hoc significance testing method to determine significant differences between groups or pairs. Otherwise only standard descriptive statistical methods were used.
Figure 3.
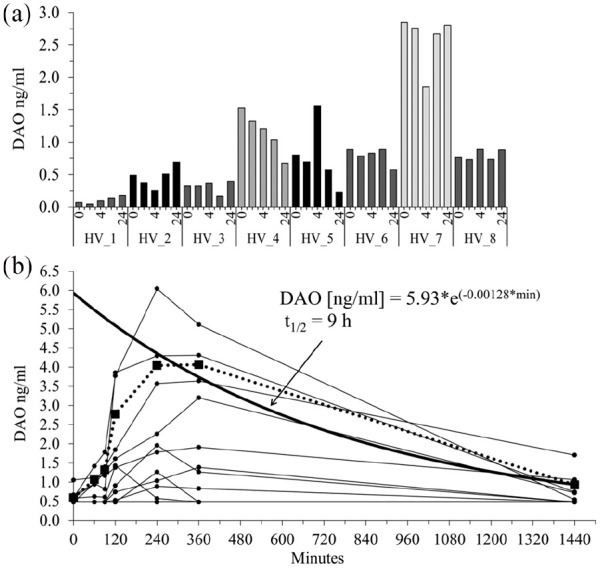
DAO concentrations were not increased after G-CSF but after LPS administration in healthy volunteers. (a) Eight healthy volunteers (HV) received a single intravenous bolus dose of 300 µg (4.2 µg/kg) G-CSF and EDTA plasma was prepared at different timepoints. (b) Fifteen healthy volunteers received a single LPS dose of 2 ng/kg (on average 158 ng LPS). Blood samples were drawn at multiple timepoints. The dotted line with the filled squares represents the mean of the four highest “LPS responders.” The means at 240, 360, and 1440 min were used to calculate the mono-exponential decay curve (bold line). The estimated DAO half-life is 9 h. DAO concentrations were measured with a DAO ELISA and the mean of duplicates are shown. DAO concentrations below the limit of detection (0.48 ng/mL) were set to 0.48 ng/mL.
Results
DAO concentrations are elevated in serum but not plasma
During the development of the human DAO ELISA we measured DAO in 10 serum, EDTA, and citrate plasma samples from the same subjects (Figure 1(a)). The P-value of the Friedman ANOVA was <0.01 and the post-hoc Nemenyi pairwise comparison showed that serum and citrate plasma were statistically significantly different (P = 0.014). We next incubated serum, EDTA and citrate plasma blood collection tubes for 0, 30, 120, and 180 min at room temperature before preparation of serum or plasma (Figure 1(b)–(d)). Only serum DAO concentrations were statistically highly significantly different in this time-series experiment. The P-value using Friedmann ANOVA was significant only with the serum samples (P < 0.01 versus 0.64 for EDTA and 0.63 for citrate plasma). Using the Nemenyi post-hoc test the P values comparing serum baseline (0 min) with 30, 120, and 180 min were 0.89, 0.005, and 0.018, respectively.
Incubation of EDTA blood collection tubes at room temperature or 4°C for 0, 3, 8, and 24 h revealed no significant DAO concentration increase (Table 1). Serum samples at room temperature released more DAO compared to 4°C. Most of the DAO antigen is released after 3 h with some additional increase after 24 h compared to 3 and 8 h. This implies that released DAO is stable in whole blood incubated at room temperature for several hours. Human DAO concentrations are not influenced, regardless whether serum was frozen after the 24 h incubation period or left in the refrigerator overnight (the two far right columns in Table 1).
Table 1.
EDTA anticoagulated whole blood prevented DAO release at 4°C and room temperature after incubation for as long as 24 h.
| Hours | EDTA RT | EDTA 4°C | Serum RT | Serum 4°C | Serum RT ON 4°C | Serum 4°C ON 4°C | |
|---|---|---|---|---|---|---|---|
| HV_1 | 0 | 1.5 | 2.7 | ||||
| 3 | 1.6 | 1.9 | 9.0 | 3.4 | |||
| 8 | 1.7 | 1.5 | 9.4 | 3.6 | |||
| 24 | 1.4 | 1.6 | 11.8 | 3.7 | 10.8 | 3.6 | |
| HV_2 | 0 | 0.7 | 0.0 | 0.8 | 0.0 | ||
| 3 | 0.7 | 0.5 | 3.0 | 0.7 | |||
| 8 | 0.8 | 0.5 | 3.3 | 0.8 | |||
| 24 | 1.0 | 0.5 | 4.3 | 0.9 | 4.1 | 0.9 | |
| HV_3 | 0 | 0.6 | 0.6 | ||||
| 3 | 0.6 | 0.5 | 1.8 | 0.5 | |||
| 8 | 0.6 | 0.5 | 1.9 | 0.6 | |||
| 24 | 0.6 | 0.5 | 2.8 | 0.6 | 2.9 | 0.6 | |
| HV_4 | 0 | 0.5 | 0.5 | ||||
| 3 | 0.5 | 0.5 | 1.8 | 0.5 | |||
| 8 | 0.5 | 0.5 | 1.7 | 0.5 | |||
| 24 | 0.5 | 0.5 | 1.8 | 0.5 | 1.8 | 0.5 | |
| HV_5 | 0 | 0.7 | 1.0 | ||||
| 3 | 1.0 | 0.5 | 3.8 | 0.9 | |||
| 8 | 0.9 | 0.5 | 4.2 | 1.0 | |||
| 24 | 1.0 | 0.6 | 5.7 | 1.2 | 6.0 | 1.3 |
EDTA and serum blood collections tubes from five healthy volunteers (HV) were incubated at room temperature (RT) and 4°C for 0, 3, 8, or 24 h before preparation of plasma and serum. The two columns on the right side were left overnight (ON) at 4°C before DAO ELISA measurements. The other samples were frozen overnight. Each point represents the mean of duplicates. Healthy volunteers in Tables 1 and 2 were only partially the same subjects.
Table 2.
Minimal DAO release in heparin anticoagulated whole blood after 24 h incubation at room temperature.
| Hours | Heparin RT | Heparin 4°C | Serum RT | Serum 4°C | |
|---|---|---|---|---|---|
| HV_1 | 0 | 0.5 | 4.6 | ||
| 3 | 0.7 | 0.5 | 12.9 | 7.2 | |
| 24 | 3.1 | 1.3 | 18.6 | 6.6 | |
| HV_2 | 0 | 0.5 | 1.2 | ||
| 3 | 0.5 | 0.5 | 4.8 | 1.7 | |
| 24 | 0.5 | 0.5 | 6.7 | 1.7 | |
| HV_3 | 0 | 0.5 | 0.5 | ||
| 3 | 0.5 | 0.5 | 0.5 | 0.5 | |
| 24 | 0.5 | 0.5 | 0.5 | 0.5 | |
| HV_4 | 0 | 0.5 | 0.5 | ||
| 3 | 0.5 | 0.5 | 1.4 | 0.7 | |
| 24 | 0.5 | 0.5 | 3.1 | 1.5 | |
| HV_5 | 0 | 0.5 | 1.8 | ||
| 3 | 0.6 | 0.5 | 7.5 | 4.2 | |
| 24 | 1.6 | 0.5 | 12.7 | 3.5 | |
| HV_6 | 0 | 0.5 | 1.9 | ||
| 3 | 0.5 | 0.7 | 7.2 | 3.8 | |
| 24 | 1.9 | 0.6 | 10.4 | 2.7 |
Heparin blood collection tubes did not release significant amounts of human DAO within 3 h, but after 24 h DAO was partially released in some subjects (Table 2). Nevertheless, DAO release in heparin was minimal compared to serum.
Heparin and serum blood collection tubes from six healthy volunteers (HV) were incubated at room temperature (RT) and 4°C for 0, 3 or 24 h before preparation of plasma and serum. All samples were frozen for at least overnight before DAO concentration measurements. Each point represents the mean of duplicates.
Neutrophil activation releases DAO into plasma at 22°C and 37°C
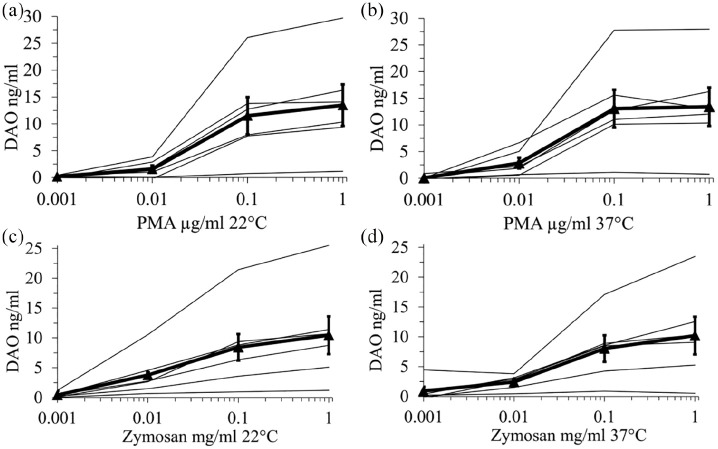
Based on a few publications about the presence of DAO in neutrophils we hypothesized that activation of neutrophils during blood coagulation might cause the increase of human DAO in serum samples. To test this hypothesis we stimulated 500 µL of heparin whole blood at 22°C and 37°C for 1 h with different PMA and zymosan concentrations. Human DAO concentrations increased statistically highly significantly with Friedmann ANOVA P-values of <0.001 under all four conditions (Figure 2). Using the Nemenyi post-hoc test the P-values comparing baseline with 0.1 and 1 µg/mL PMA were 0.038 and 0.001 for RT and 0.016 and 0.005 for 37°C. The corresponding P-values for 0.1 and 1 mg/mL zymosan were 0.01, 0.001 for RT and 0.016 and 0.001 for 37°C.
Figure 2.
Activation of neutrophils using PMA and zymosan dose-dependently increased DAO concentrations in heparin plasma. Heparin whole blood from six healthy volunteers was incubated for 60 min at room temperature (RT) and 37°C with different PMA (a and b) and zymosan (c and d) concentrations. After plasma preparation DAO concentrations were measured with a DAO ELISA and the mean of duplicates are shown. The bold line with filled triangles represents the mean (+/− SEM) of all subjects. The baseline values without the addition of PMA or zymosan have been subtracted from the other values for easier graphical presentation (log scale).
PMA: phorbol myristate acetate; DAO: diamine oxidase.
Using 15 ng/mL mean DAO concentration after neutrophil activation, assuming 50% release of DAO from the specific granules of neutrophils, ignoring any release from eosinophils and calculating with 4000 neutrophils/µl whole blood, each neutrophil contains about 4.4 fg DAO.
The PMA and zymosan data strongly suggest that the elevated DAO concentrations in serum are caused by neutrophil activation during blood coagulation.
LPS but not G-CSF increases DAO concentrations in healthy volunteers
In the next experiments we wanted to test whether DAO is also released in vivo after neutrophil activation. A single dose of 300 µg G-CSF injected intravenously into eight healthy volunteers did not cause any DAO antigen concentration increase when measured after 2, 4, 6, and 24 h (Figure 3(a)). A single dose of E. coli LPS at 2 ng/kg, however, induced a statistically highly significant release of DAO with a Friedmann ANOVA P-value of <0.001 (Figure 3(b)). Using the conservative Nemenyi post-hoc test, the P-values comparing baseline (0 min) with 240 and 360 min were 0.013 and 0.029, respectively. Other pairs showed no significant difference. Nevertheless, only four subjects responded strongly and six subjects did not show any DAO increase at any time point. The DAO peak seemed to occur about 4 to 6 h after LPS administration. The 4, 6, and 24 h data from the highest four responders were used to estimate the half-life of DAO using mono-exponential decay curve fitting. This calculation assumed that DAO was released at once from neutrophils and therefore the calculated half-life of 9 h might be considered a conservative (maximum) half-life estimate. The appropriate data to use a two-compartment model including the DAO release kinetic from neutrophils are not available.
Bacteremia patients do not show elevated DAO concentrations
If DAO is released after LPS administration from activated neutrophils, its concentrations might also be elevated in bacteremia patients and consequently it might contribute to the degradation of histamine released from activated basophils and mast cells or newly synthesized by activated neutrophils or other cells like macrophages. Therefore we tested DAO antigen concentrations in three different patient subcohorts with high-CRP and culture-proven bacteremia. A few key parameters of the three subcohorts are summarized in Table 3.
Table 3.
Key parameters of the selected bacteremia patients.
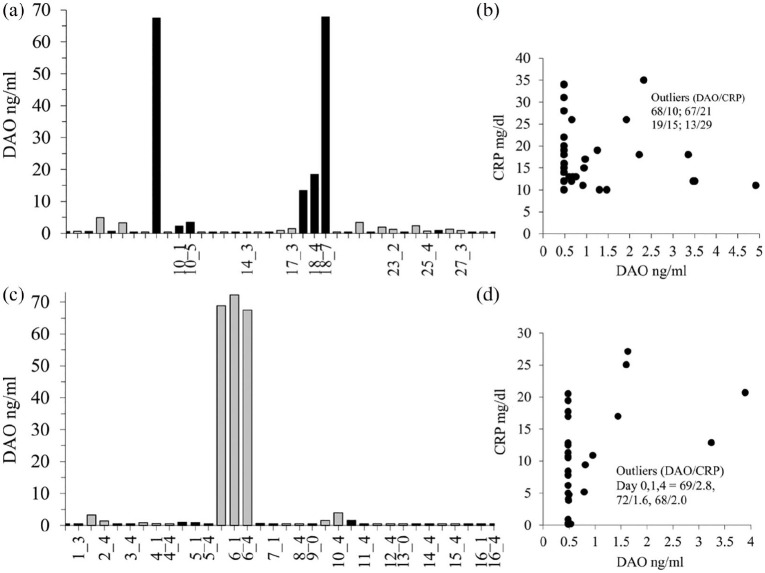
Human DAO concentrations were only relevantly elevated in 3 out of 55 (5.4%) independent EDTA plasma samples (Figure 4). All nine independent EDTA plasma samples from Subcohort_3 showed DAO values below 2 ng/mL at 2 to 3 different timepoints (data not shown). There was no correlation between DAO and CRP (Figure 4) or DAO with leukocyte counts (data not shown). There was also no correlation with the time of antibiotic administration (data not shown). Tryptase concentrations as a marker for mast cell activation were not elevated (<8 ng/mL) in the five samples of Subcohort_1 with DAO concentrations >5 ng/mL. We also spiked 20 ng/mL recombinant human DAO into 13 EDTA plasma samples from Subcohort_1 and recovered a mean (median, SD) of 24.4 (25.4, 3.04) ng/mL. Measurements of DAO antigen concentrations are not compromised in EDTA plasma from bacteremic patients.
Figure 4.
Only a few patients with bacteremia and high CRP-values showed elevated circulating DAO concentrations. (a) EDTA plasma DAO concentrations were measured in 30 different patients (Subcohort_1). For some patients multiple samples were available as indicated by the numbers on the x-axis. For example, 10_1 and 10_5 means a second sample of patient 10 after 1 and 5 days. (c) In Subcohort_2 EDTA plasma DAO concentrations were measured in 16 different patients with Staphylococcus aureus (n = 12) and epidermidis (n = 4) infections. For some patients multiple samples were available as indicated by the numbers on the x-axis. In (b and d) CRP versus DAO concentrations were plotted. DAO concentrations below the limit of detection (0.48 ng/mL) were set to 0.48 ng/mL.
The patient with the single high DAO concentration in Subcohort_1 (65 years old) suffered from an E. coli infection and was also diagnosed with Legionella pneumonia and urosepsis. The subject was bone marrow transplanted. The second high DAO patient (79 years old) with increasing DAO concentrations over 7 days suffered from Staphylococcus aureus bacteremia with diagnosed pneumonia and chronic kidney disease. DAO concentrations on Days 0, 1, and 7 (13, 19, 68 ng/mL) were negatively correlated with CRP (29, 15, 10 mg/dL) and IL-6 levels (97, 29, 17 pg/mL). The high DAO patient (84 years old) in Subcohort_2 was diagnosed with pneumonia and sepsis from Staphylococcus aureus and Enterococcus faecium. The CRP-values over the 4 days were below 3 mg/dL.
Discussion
Measurement of DAO concentrations in serum do not reflect the amount of circulating DAO but the amount of DAO released predominantly from neutrophils during blood coagulation. It has been known for decades that in vitro coagulation causes complement activation,28,29 but the precise mechanism is not clear.30,31 Serum DAO measurements cannot reflect the amount of active DAO present in the gastrointestinal tract and therefore we concur with the increasing scepticism towards using DAO measurements as a diagnostic tool for histamine intolerance. It is also unlikely that plasma DAO levels, which were below 0.5 ng/mL in 30% to 60% of subjects,4 could be a useful correlate for DAO activity or antigen concentrations in gastrointestinal tract epithelial cells. Serum DAO concentrations might reflect the propensity of neutrophils to release their specific granules, but can this be correlated with increased histamine sensitivity? An improved DAO ELISA with a lower limit of detection might show a correlation with histamine intolerance symptoms using plasma or even serum. It is conceivable that in histamine-intolerant subjects mast cells have a higher susceptibility to degranulate. The released mediators could prime neutrophils, which might be reflected in an increased rate of DAO release from neutrophils during serum preparation.
The G-CSF data are in agreement with published data showing that G-CSF mainly primes neutrophils, causing increased synthesis and release, but does not per se cause degranulation of primary or secondary (specific) granules.32
The peak of in vivo DAO release after 4 to 6 h following LPS administration is somewhat delayed compared to the peak of IL-6, IL-8, and TNF-alpha, which occurred after 2 to 3 h.23 All three cytokines are potent activators of neutrophils. We did not measure histidine decarboxylase mRNA expression or histamine concentrations but they are known to be induced after LPS administration, either directly or more likely indirectly. The release of DAO might be considered a counterregulatory activity to the pro-inflammatory action of LPS or histamine, but this is possibly only relevant in tissue and not plasma. Why is DAO not elevated in more bacteremic patients and what caused the strong increase of DAO in the three patients?
Human neutrophil lipocalin (HNL) is located together with DAO in the specific granules and is considered a marker for bacterial infections.33 Why is HNL elevated in many bacteremic patients approximately 3-fold in plasma and even more in serum but DAO is not? First, our septic patients were only included after a confirmed positive blood-culture test and this delayed inclusion into the studies for 24 h or more. In addition, our patients received antibiotics before inclusion, whereas Xu et al. were able to include the patients before antibiotic treatment started.33 The 24 to 48 h time window might have masked any temporary DAO increase.
The increase in HNL between viral infections, which are considered to have similar values to control subjects, and bacterial infections was approximately 4-fold in serum and 3-fold in plasma.33 A 3-fold increase in DAO might be below the resolution in such conditions. Normal concentrations of DAO are about 1 ng/mL with 30% to 60% of subjects having concentrations below 0.5 ng/mL.5 The amount of DAO in neutrophils might be not high enough to increase the circulating DAO concentrations. In the specific granules of neutrophils the HNL and lactoferrin concentrations are 100 to 1000-fold higher compared to DAO (410 fg and 3.2 pg vs 4.4 fg for DAO per neutrophil, respectively).34
Another explanation might be that the half-life of HNL is longer compared to DAO. The half-life of HNL in humans is not known. The half-life of DAO was stated as 27 h in one publication, but this value is derived from DAO measurements after parturition.35 We now know that DAO is synthesized and released only from fetal EVTs and they partially invade into the myometrium.5 Therefore, after parturition DAO producing EVTs are still present in the mother and might secrete DAO for a few more days. The measured half-life of DAO after parturition is thus a combination of continuing DAO expression and secretion from remaining EVTs and the “true” half-life in the plasma compartment. It is therefore likely shorter than the 27 h stated. Using the LPS data it can be estimated at a maximum 9 h, although it is likely to be less, because the release of DAO from neutrophils is another compartment influencing the “true” half-life in plasma. D’Agostino et al. perfused human plasma and placenta DAO into isolated rat liver and DAO activity was extracted with a half-life of about 10 min.36 Therefore, if the half-life of DAO in humans is less than a few hours, we might have missed the release of DAO from neutrophils.
We do not have a good explanation as to why three patients had high DAO concentrations. We are not aware of any data showing DAO mRNA upregulation, increased synthesis and DAO release in animal models or humans after LPS administration or during bacteremia. In general very little is known about the regulation of the human DAO promoter. It is conceivable that DAO was released from the heparin/heparan sulfate-sensitive storage sites in the gastrointestinal tract, but none of the patients received high molecular weight heparin and low molecular weight heparin is unlikely able to release DAO following subcutaneous administration.37 Massive mast cell activation might be an option to release DAO and increase plasma concentrations but tryptase levels were normal.17 Kidney damage might also be a possible explanation. Two of the three high DAO patients also had acute infections of the urinary tract system. Acute damage to the proximal tubular cells might release DAO into the circulation. The bilirubin values of these patients were all normal, arguing against a reduced clearance of DAO by the liver, but how quick human DAO is cleared from the circulation is unknown. All three patients were diagnosed with pneumonia, but several other patients with pneumonia or urosepsis had normal DAO concentrations.
Maximum in vitro neutrophil activation with zymosan and PMA using heparin anticoagulated whole blood from healthy volunteers increased DAO concentrations from a mean of about 1 to 15 ng/mL. We assumed 4000 neutrophils/µL. Although 15 ng/mL DAO are not able to degrade histamine rapidly, the histamine half-life will be about 25 min, the number of neutrophils for example in inflamed synovial fluid in a rat model increased 20 to 35-times.38 Kortekangas et al. published highly elevated synovial fluid leukocyte counts in humans with mean values of about 50,000/µL.39 Similar data were reported by Coutlakis et al.40 If the neutrophils highly enriched in synovial fluid or tissue are activated, DAO could reach concentrations of several hundred ng/mL, matching levels reached during pregnancy. Such high concentrations would rapidly degrade histamine with a half-life of less than 5 min. In addition, DAO could also oxidize the polyamines putrescine and spermidine released from apoptotic or dying cells. The concentrations of putrescine and spermidine in synovial fluid from patients with different underlying diseases were between 40 and 100 µM.41 Human DAO will readily oxidize both polyamines in this concentration range.25 The serum concentrations of putrescine and spermidine combined are below 1 µM.42
The role of DAO release from neutrophils during inflammation is unclear, but might be studied using a predominantly neutrophil-based inflammation model comparing wildtype with DAO knock-out mice. Hydrogen peroxide and possibly the reactive aldehydes released by DAO might contribute to the tissue damage induced by neutrophils directly or indirectly via myeloperoxidase-mediated radical formation. Diamine oxidase knock-out mice might show less neutrophil-induced damage. On the other hand, locally increased histamine concentrations in a DAO knock-out mouse background might induce more inflammation. The net effect is unpredictable.
Conclusion
Serum DAO measurements are not suitable to measure circulating DAO, but might reflect the propensity of neutrophils to degranulate. DAO is unlikely a suitable marker for bacterial infections, but its release from activated neutrophils into the synovial fluid or interstitial fluid in inflamed tissue could counteract the proinflammatory actions of histamine. The physiological or pathophysiological role of DAO in the specific granules of neutrophils and eosinophils is a black box, but is certainly worthy of further exploration.
Acknowledgments
We acknowledge with gratitude the willingness of all healthy volunteers and patients to participate in the different studies and the contributions of all staff members in the Department of Clinical Pharmacology. We are indebted to Sarah Ely for the final polish in the proper usage of the English language.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: In the publication we used blood samples and only blood samples from several different studies. In all cases except one (see below) approval was granted by the Ethics Committee of the Medical University of Vienna.
Healthy Volunteers: EC-No.2030/2013 and EC-No.1810/2015
G-SCF Study in healthy volunteers: EC-No.484/2006
LPS Study in healthy volunteers: EC-No.1577/2014
Sepsis Cohort_1: Ethical approval for this study was waived by the Ethics Committee of the Medical University of Vienna because the samples were collected during routine procedures and were completely and irreversibly anonymized.
Sepsis Cohort_2: EC-No. 8/2009
Sepsis Cohort_3: EC-No. 518/2011
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all healthy volunteers and patients before inclusion into the different studies.
Trial registration: Healthy Volunteers: Not applicable; Non-CTIMP
G-SCF Study in healthy volunteers: Eudract CT numbers: 2006-005582-18
LPS Study in healthy volunteers: EudraCT number 2014-002857-20
Sepsis Cohort_1: Not applicable; Non-CTIMP
Sepsis Cohort_2: Not applicable; Non-CTIMP
Sepsis Cohort_3: Not applicable; Non-CTIMP
ORCID iD: Thomas Boehm  https://orcid.org/0000-0002-8294-0797
https://orcid.org/0000-0002-8294-0797
References
- 1. Best CH, McHenry EW. (1930) The inactivation of histamine. The Journal of Physiology 70: 349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elmore BO, Bollinger JA, Dooley DM. (2002) Human kidney diamine oxidase: Heterologous expression, purification, and characterization. Journal of Biological Inorganic Chemistry 7: 565–579. [DOI] [PubMed] [Google Scholar]
- 3. Southren AL, Kobayashi Y, Sherman DH, et al. (1964) Diamine oxidase in human pregnancy: Plasma diamine oxidase in nonpregnant and normal pregnant patients. American Journal of Obstetrics & Gynecology 89: 199–203. [DOI] [PubMed] [Google Scholar]
- 4. Boehm T, Pils S, Gludovacz E, et al. (2017) Quantification of human diamine oxidase. Clinical Biochemistry 50: 444–451. [DOI] [PubMed] [Google Scholar]
- 5. Velicky P, Windsperger K, Petroczi K, et al. (2018) Pregnancy-associated diamine oxidase originates from extravillous trophoblasts and is decreased in early-onset preeclampsia. Scientific Reports. Epub ahead of print 20 April DOI: 10.1038/s41598-018-24652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeiger RS, Twarog FJ, Colten HR. (1976) Histaminase release from human granulocytes. Journal of Experimental Medicine 144: 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herman JJ, Rosner IK, Davis AE, et al. (1979) Complement-dependent histaminase release from human granulocytes. Journal of Clinical Investigation 63: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melamed J, Medicus RG, Arnaout MA, et al. (1983) Induction of granulocyte histaminase release by particle-bound complement C3 cleavage products (C3b, C3bi) and IgG. Journal of Immunology 131(1): 439–444. [PubMed] [Google Scholar]
- 9. Schayer RW. (1960) Relationship of induced histidine decarboxylase activity and histamine synthesis to shock from stress and from endotoxin. American Journal of Physiology 198: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 10. Xu X, Zhang H, Song Y, et al. (2012) Strain-dependent induction of neutrophil histamine production and cell death by Pseudomonas aeruginosa. Journal of Leukocyte Biology 91(2): 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segel GB, Halterman MW, Lichtman MA. (2011) The paradox of the neutrophil’s role in tissue injury. Journal of Leukocyte Biology 89(3): 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hinshaw LB, Jordan MM, Vick JA. (1961) Histamine release and endotoxin shock in the primate. Journal of Clinical Investigation 40: 1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brackett DJ, Hamburger SA, Lerner MR, et al. (1990) An assessment of plasma histamine concentrations during documented endotoxic shock. Agents and Actions 31(3–4): 263–274. [DOI] [PubMed] [Google Scholar]
- 14. Neugebauer E, Rixen D, Garcia-Caballero M, et al. (1994) Time sequence of histamine release and formation in rat endotoxic shock. Shock 1(4): 299–306. [DOI] [PubMed] [Google Scholar]
- 15. Neugebauer E, Lorenz W, Rixen D, et al. (1996) Histamine release in sepsis: a prospective, controlled, clinical study. Critical Care Medicine 24(10): 1670–1677. [DOI] [PubMed] [Google Scholar]
- 16. Jacobs R, Kaliner M, Shelhamer JH, et al. (1989) Blood histamine concentrations are not elevated in humans with septic shock. Critical Care Medicine 17(1): 30–35. [DOI] [PubMed] [Google Scholar]
- 17. Boehm T, Reiter B, Ristl R, et al. (2019) Massive release of the histamine-degrading enzyme diamine oxidase during severe anaphylaxis in mastocytosis patients. Allergy 74(3): 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Music E, Korosec P, Silar M, et al. (2013) Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wiener Klinische Wochenschrift 125(9–10): 239–243. [DOI] [PubMed] [Google Scholar]
- 19. Manzotti G, Breda D, Di Gioacchino M, et al. (2016) Serum diamine oxidase activity in patients with histamine intolerance. International Journal of Immunopathology and Pharmacology 29(1): 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwelberger HG. (2009) Histamine intolerance: Overestimated or underestimated? Inflammation Research 58 Suppl 1: 51–52. [DOI] [PubMed] [Google Scholar]
- 21. Reese I, Ballmer-Weber B, Beyer K, et al. (2017) German guideline for the management of adverse reactions to ingested histamine. Allergo Journal International 26(2): 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tufvesson G, Tryding N. (1969) Determination of diamine oxidase activity in normal human blood serum. Scandinavian Journal of Clinical and Laboratory Investigation 24: 163–168. [DOI] [PubMed] [Google Scholar]
- 23. Matzneller P, Strommer S, Drucker C, et al. (2017) Colistin reduces LPS-triggered inflammation in a human sepsis model in vivo: A randomized controlled trial. Clinical Pharmacology and Therapeutics 101(6): 773–781. [DOI] [PubMed] [Google Scholar]
- 24. Schoergenhofer C, Schwameis M, Wohlfarth P, et al. (2017) Granulocyte colony-stimulating factor (G-CSF) increases histone-complexed DNA plasma levels in healthy volunteers. Clinical Experimental Medicine 17(2): 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gludovacz E, Maresch D, Bonta M, et al. (2016) Characterization of recombinant human diamine oxidase (rhDAO) produced in Chinese Hamster Ovary (CHO) cells. Journal of Biotechnology 227: 120–130. [DOI] [PubMed] [Google Scholar]
- 26. Schwameis M, Steiner MM, Schoergenhofer C, et al. (2015) D-dimer and histamine in early stage bacteremia: A prospective controlled cohort study. European Journal of Internal Medicine 26: 782–786. [DOI] [PubMed] [Google Scholar]
- 27. Ratzinger F, Schuardt M, Eichbichler K, et al. (2013) Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS One 8(12): e82946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mollnes TE, Garred P, Bergseth G. (1988) Effect of time, temperature and anticoagulants on in vitro complement activation: Consequences for collection and preservation of samples to be examined for complement activation. Clinical and Experimental Immunology 73(3): 484–488. [PMC free article] [PubMed] [Google Scholar]
- 29. Plow EF. (1982) Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. Journal of Clinical Investigation 69(3): 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wachtfogel YT, Kucich U, James HL, et al. (1983) Human plasma kallikrein releases neutrophil elastase during blood coagulation. Journal of Clinical Investigation 72(5): 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plow EF, Plescia J. (1988) Neutrophil secretion during blood coagulation: evidence for a prekallikrein independent pathway. Thrombosis and Haemostasis 59(3): 360–363. [PubMed] [Google Scholar]
- 32. Balazovich KJ, Almeida HI, Boxer LA. (1991) Recombinant human G-CSF and GM-CSF prime human neutrophils for superoxide production through different signal transduction mechanisms. Journal of Laboratory and Clinical Medicine 118(6): 576–584. [PubMed] [Google Scholar]
- 33. Xu SY, Pauksen K, Venge P. (1995) Serum measurements of human neutrophil lipocalin (HNL) discriminate between acute bacterial and viral infections. Scandinavian Journal of Clinical and Laboratory Investigation 55(2): 125–131. [DOI] [PubMed] [Google Scholar]
- 34. Xu S, Höglund M, Venge P. (1996) The effect of granulocyte colony-stimulating factor (G-CSF) on the degranulation of secondary granule proteins from human neutrophils in vivo may be indirect. British Journal of Haematology 93(3): 558–568. [DOI] [PubMed] [Google Scholar]
- 35. Carrington ER, Frishmuth GJ, Oesterling MJ, et al. (1972) Gestational and postpartum plasma diamine oxidase values. Obstetrics and Gynecology 39(3): 426–430. [PubMed] [Google Scholar]
- 36. D’Agostino L, Ciacci C, Capuano G, et al. (1986) Metabolic fate of plasma diamine oxidase: Evidence of isolated and perfused rat liver uptake. Digestion 34: 243–250. [DOI] [PubMed] [Google Scholar]
- 37. Biebl M, Klocker J, Perkmann R, et al. (2003) Effects of unfractionated and low molecular weight heparins on diamine oxidase release. Inflammation Research 52 Suppl 1: S63–64. [DOI] [PubMed] [Google Scholar]
- 38. Issekutz AC, Issekutz TB. (1991) Quantitation and kinetics of polymorphonuclear leukocyte and lymphocyte accumulation in joints during adjuvant arthritis in the rat. Laboratory Investigation 64(5): 656–663. [PubMed] [Google Scholar]
- 39. Kortekangas P, Aro HT, Tuominen J, et al. (1992) Synovial fluid leukocytosis in bacterial arthritis vs. reactive arthritis and rheumatoid arthritis in the adult knee. Scandinavian Journal of Rheumatology 21(6): 283–288. [DOI] [PubMed] [Google Scholar]
- 40. Coutlakis PJ, Roberts WN, Wise CM. (2002) Another look at synovial fluid leukocytosis and infection. Journal Clinical Rheumatology 8(2): 67–71. [DOI] [PubMed] [Google Scholar]
- 41. Yukioka K, Wakitani S, Yukioka M, et al. (1992) Polyamine levels in synovial tissues and synovial fluids of patients with rheumatoid arthritis. Journal of Rheumatology 19(5): 689–692. [PubMed] [Google Scholar]
- 42. Loeser C, Wunderlich U, Foelsch UR. (1988) Reversed-phase liquid chromatographic separation and simultaneous fluorimetric detection of polyamines and their monoacetyl derivatives in human and animal urine, serum and tissue samples: An improved, rapid and sensitive method for routine application. Journal of Chromatography 430(2): 249–262. [DOI] [PubMed] [Google Scholar]