Abstract
Background
High social risk, as quantified by the social determinants of health (SDH), may lead to disability. This association has not been well explored in remote settings. Using the three Villages Study cohort, we assessed the association between SDH and disability among stroke-free older adults living in a rural Ecuadorian community.
Methods
SDH were measured by the use of the Gijon Scale and disability by the Functional Activities Questionnaire. All participants had a brain MRI to assess subclinical biomarkers of cerebral small vessel disease. Multivariate models were fitted to assess the association between components of SDH and disability, after adjusting for covariates of interest.
Results
The mean age of 478 enrolled individuals was 70.1 ± 8 years (59% women). High social risk was observed in 220 (46%) individuals and disability in 222 (46%). There was an almost direct linear relationship between SDH and disability, after taking into account the effect of age. A generalized linear model, adjusted for all included covariates, showed an independent association between social risk and disability (P < .001). In addition, multivariate models showed that independent SDH components more strongly associated with disability were worse support networks and social relationships. In contrast, the single SDH component not associated with disability was the economic status.
Conclusions
This study showed a robust association between SDH and disability. Economic needs were surpassed by other components of SDH. This knowledge will help to develop strategies for the control of factors that may be in the path for disability among older adults living in rural settings.
Keywords: social determinants of health, disability, functional impairment, rural communities, population study
Introduction
According to the World Health Organization (WHO), “health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”.1 In this view, the so-called “Social Determinants of Health (SDH)” are of fundamental importance for the healthy status and functional independence of individuals with or without chronic diseases. These determinants may also be in the path for the occurrence and progression of several morbid conditions.2 Recent studies have suggested a link between SDH and cardiovascular diseases, including stroke.3-5 Accordingly, a high social risk not only increases the prevalence of cardiovascular risk factors and increases mortality after an event, but also increase disability among stroke survivors by means of several psychological, behavioral and biological mechanisms.6-8
The SDH include situations in which persons are born, grow, live, work and age, as well as the adequacy of medical care.9 While these determinants have been associated with disability, the relationship between social risk and functional impairment is complex and probably bidirectional. As the social risk increases so too does functional impairment and the development of disability may lead to an increase in social risk.10
While poverty and disparities in health care are contributors to higher social risk, SDH assessment varied across studies, and it has been difficult to standardize which determinants better assess the actual social risk in different settings. In any case, SDH vary according to the level of development of a given community and their assessment must be adapted consequently.11-14
The study of the association between SDH and disability in individuals living in diverse remote rural settings provides an optimal scenario to better understand their particular needs, the factors responsible for such association, and the application of region-specific interventions intended to reduce functional impairment among older adults living in underserved communities. Taking the opportunity of an already established clinical cohort of community-dwelling older adults living in 3 neighboring rural villages located in coastal Ecuador.15,16 the present study assessed the association between SDH and disability in stroke-free older adults living in these villages.
Methods and Materials
Study population: Residents of the 3 studied villages—Atahualpa, El Tambo, and Prosperidad—are homogeneous regarding ethnicity, overall lifestyles and cardiovascular risk factors, as detailed elsewhere.15 Phenotypic characteristics of these people suggest an Amerindian ancestry, almost all men work as artisan carpenters and most women are homemakers. The villages have electricity but some houses do not have pipe water, most streets are not paved, and many houses are made of cane or wood. Locals often mobilize by walking or bicycle riding. The above-mentioned consistencies provide optimal grounds for the conduction of this kind of population-based studies and reduce the possibility of hidden confounders or marked disparities causing biases in the assessment of SDH. Only Atahualpa has a Public Health Center. Instead, the other 2 villages have to rely on a small Public Hospital located in a neighboring city (Ancón).
Study design: All residents aged ≥60 years of the 3 above-mentioned villages were identified during door-to-door surveys, and those who signed a comprehensive informed consent were enrolled. Field instruments to assess variables of interest were administered by a physician trained to guarantee uniformity in data collection. Individuals with a suspected stroke were identified during the surveys (using a validated field instrument) and then, a certified neurologist confirmed the diagnosis with the aid of MRI (performed in all the study population).17,18 Subjects with an overt stroke were excluded from analysis. Multivariate models were used to assess the association between SDH—and their individual components—and disability, after adjusting for relevant covariates. The study followed the recommendations of the standards for reporting of observational studies in epidemiology (STROBE) guidelines,19 and was approved by the I.R.B. of our Institution.
Social determinants of health: The Gijon Scale was used to measure the SDH. This is a validated field instrument—originally constructed in Spanish—that assesses 5 risk situations and social problems, which include family situation, economic status, housing, social relationships and support networks.12 Each section (or component) of the Gijon scale has 5 questions that are weighted on a 1 to 5 scale, for a maximal score of 25. A total score of <10 points indicates no social risk. This scale was used because it is appropriate to the study population and has been recommended by the Minister of Health of our country.20
Measurement of disability: The Functional Activities Questionnaire (FAQ), developed by Pfeffer et al,21 and subsequently validated in Spanish,22 was used to evaluate the ability to perform activities of daily living. The FAQ is composed of 10 questions, each rated on a four-point Likert scale that ranges from 0 (normal) to 3 (totally dependent). The maximum total score is 30 points, with a cutoff score of ≥9 demonstrating impaired functionality.
Cerebral small vessel disease (cSVD) assessment and rating: Neuroimaging biomarkers of cSVD were identified. These include white matter hyperintensities (WMH) of presumed vascular origin, deep cerebral microbleeds (CMB), lacunes of presumed vascular origin and enlarged basal ganglia perivascular spaces (BG-PVS). MRIs readings followed research standards for cSVD.23 Accordingly, points were assigned to WMH if they were graded as moderate-to-severe, to CMB and lacunes (respectively) if there was at least 1 lesion located deep in the brain and to enlarged BG-PVS if >10 of these lesions were present in a single slice in 1 side of the brain.24
Clinical covariates investigated: Demographics, level of education, cardiovascular risk factors, frailty status, sleep quality and symptoms of depression were selected as covariates, as they may have an influence on the association between SDH and disability. The American Heart Association (AHA) criteria were used to determine smoking status, physical activity, diet, the body mass index, blood pressure, fasting glucose, and total cholesterol blood levels.25 Frailty, sleep quality, and symptoms of depression were respectively assessed by the use of the Edmonton Frail Scale (EFS), the Pittsburgh Sleep Quality Index (PSQI) and the depression axis of the depression-anxiety-stress scale-21 (DASS-21), as previously described.26-28
Statistical analysis: STATA version 16 (College Station, TX, USA) was used for data analyses. In univariate analyses, continuous variables were compared by linear models and categorical variables by means of the x2 or Fisher exact test as appropriate. A fully adjusted generalized linear model was fitted to calculate the association between the total SDH score and disability (as the dependent variable). In addition, multivariate generalized linear models were fitted to calculate the association of individual components of SDH and disability.
Results
Brain MRIs were performed in 590 (83%) out of 712 community-dwelling individuals aged ≥60 years identified during door-to-door surveys. Of the remaining 122 individuals, 53 refused the practice of MRI, 14 were bedridden and could not be transported to Guayaquil, 12 experienced claustrophobia during the exam, and one had an implanted pacemaker. In addition, 42 persons had died or emigrated between enrollment and the invitation for MRI. Of the 590 individuals with an MRI, 62 were excluded because of an overt stroke and 50 either declined further participation, emigrated or died between MRI and the interviews for assessment of SDH and disability, leaving 478 individuals enrolled in the present study.
There were several differences in demographics and cardiovascular risk factors across participants and those individuals that were not included in this study (Table 1). Such differences were mostly the result of the inclusion criteria, since patients with an overt stroke and those bedridden were excluded.
Table 1.
Differences in Demographics and Cardiovascular Factor Across Participants and Individuals Excluded for this Study.
| Variable | Excluded (n = 234) | Included (n = 478) | P value |
|---|---|---|---|
| Age, years (mean ± SD) | 73.4 ± 9.5 | 70.1 ± 8 | <.001* |
| Women, n (%) | 113 (48) | 283 (59) | .006* |
| Primary school education, n (%) | 202 (86) | 369 (77) | .004* |
| Current smoker, n (%) | 5 (2) | 17 (4) | .304 |
| Body mass index ≥30 kg/m2, n (%) | 51 (22) | 120 (25) | .332 |
| Poor physical activity, n (%) | 56 (24) | 43 (9) | <.001* |
| Poor diet, n (%) | 18 (8) | 51 (11) | .207 |
| Blood pressure ≥140/90 mmHg, n (%) | 130 (56) | 187 (39) | <.001* |
| Fasting glucose ≥126 mg/dL, n (%) | 88 (38) | 129 (27) | .004* |
| Total cholesterol ≥240 mg/dL, n (%) | 19 (8) | 61 (13) | .065 |
Statistically significant result.
The mean age of participants was 70.1 ± 8 years, 283 (59%) were women, and 369 (77%) had primary school education only. Seventeen subjects (4%) were current smokers, 120 (25%) had a body mass index ≥30 kg/m2, 43 (9%) had poor physical activity, 51 (11%) had a poor diet, 187 (39%) had blood pressure ≥140/90 mmHg, 129 (27%) had fasting glucose ≥126 mg/dL, and 61 (13%) had total cholesterol levels ≥240 mg/dL. One-hundred individuals (21%) were frail according to the EFS (≥7 points), 197 (41%) had a poor sleep quality according to the PSQI (≥5 points), and 60 (13%) had symptoms of depression according to the DASS-21 (≥5 points). Moderate-to-severe WMH were noticed in 116 individuals (24%), deep CMB in 29 (6%), silent lacunes in 32 (7%), and >10 enlarged BG-PVS in 126 (26%).
Measurements of SDH indicated social risk in 220 (46%) individuals. The mean ± SD score of the Gijon scale was 9.8 ± 2.8 points. Mean values for individual components of SDH were 1.7 ± 1.1 points for family situation, 3.5 ± 1.2 points for economic status, 1.7 ± 0.8 points for housing, 1.7 ± 1 points for social relationships and 1.1 ± 0.6 points for support networks. The mean FAQ score for the entire sample was 9.3 ± 6.7 points, with 222 (46%) having impaired functionality (disability). A locally weighted scatterplot smoothing (LOWESS) graph showed an almost direct linear relationship between SDH and disability, after taking into account the effect of age (Figure 1).
Figure 1.
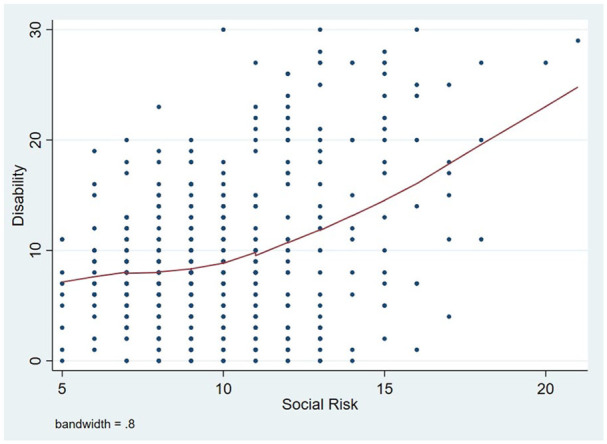
Locally weighted scatterplot smoothing (LOWESS) graph showed an almost direct linear relationship between social determinants of health and disability, after taking into account the effect of age.
Table 2 shows several differences in the investigated covariates across individuals with and without social risk and disability (univariate analysis). Individuals with social risk were older, more often female, and had more often primary school education, worse physical activity, poor diet, and high blood pressure than those without social risk. In addition, these individuals were more often frail, had worse sleep quality, more often moderate-to-severe WMH and >10 enlarged BG-PVS than those without social risk. On the other hand, disability was more frequent with increased age, in those with primary school education only, as well as in individuals with poor physical activity, high blood pressure, frail status, symptoms of depression, moderate-to-severe WMH, and silent lacunes.
Table 2.
Characteristics of Atahualpa, El Tambo and Prosperidad Residents Aged ≥60 Years Across Categories of Social Determinants of Health and Disability (Univariate Analyses).
| Variable | Total series (n = 478) | Social determinants of health |
Functional impairment (disability) |
||||
|---|---|---|---|---|---|---|---|
| No social risk (n = 258) | Social risk (n = 220) | P value | Not disabled (n = 256) | Disabled (n = 222) | P value | ||
| Age, years (mean ± SD) | 70.1 ± 8 | 68.6 ± 6.7 | 71.9 ± 9 | <.001* | 67.4 ± 6.2 | 73.2 ± 8.7 | <.001* |
| Women, n (%) | 283 (59) | 138 (53) | 145 (66) | .006* | 142 (55) | 141 (64) | .074 |
| Primary school education, n (%) | 369 (77) | 182 (70) | 187 (85) | <.001* | 186 (73) | 183 (82) | .011* |
| Current smoker, n (%) | 17 (4) | 13 (5) | 4 (2) | .081 | 13 (5) | 4 (2) | .081 |
| Body mass index ≥30 kg/m2, n (%) | 120 (25) | 60 (23) | 60 (27) | .313 | 61 (24) | 59 (27) | .489 |
| Poor physical activity, n (%) | 43 (9) | 10 (4) | 33 (15) | <.001* | 13 (5) | 30 (14) | .001* |
| Poor diet, n (%) | 51 (11) | 11 (4) | 40 (18) | <.001* | 31 (12) | 20 (9) | .274 |
| Blood pressure ≥140/90 mmHg, n (%) | 187 (39) | 90 (35) | 97 (44) | .039* | 80 (31) | 107 (48) | <.001* |
| Fasting glucose ≥126 mg/dL, n (%) | 129 (27) | 73 (28) | 56 (25) | .485 | 66 (26) | 63 (28) | .523 |
| Total cholesterol ≥240 mg/dL, n (%) | 61 (13) | 39 (15) | 22 (10) | .095 | 38 (15) | 23 (10) | .143 |
| Frail status, n (%) | 100 (21) | 31 (12) | 69 (31) | <.001* | 25 (10) | 75 (34) | <.001* |
| Poor sleep quality, n (%) | 197 (41) | 81 (31) | 116 (53) | <.001* | 100 (39) | 97 (44) | .305 |
| Symptoms of depression, n (%) | 60 (13) | 26 (10) | 34 (15) | .077 | 21 (8) | 39 (18) | .002* |
| Moderate-to-severe WMH, n (%) | 116 (24) | 43 (17) | 73 (33) | <.001* | 48 (19) | 68 (31) | .003* |
| Deep cerebral microbleeds, n (%) | 29 (6) | 15 (6) | 14 (6) | .802 | 12 (5) | 17 (8) | .175 |
| Silent lacunes, n (%) | 32 (7) | 14 (5) | 18 (8) | .229 | 11 (4) | 21 (9) | .024* |
| >10 enlarged BG-PVS, n (%) | 126 (26) | 52 (20) | 74 (34) | <.001* | 61 (24) | 65 (29) | .177 |
Abbreviations: BG-PVS: basal ganglia-perivascular spaces; WMH: white matter hyperintensities.
Statistically significant result.
A fully-adjusted generalized linear model showed a significant (and independent) association between social risk and disability (β: 0.44; 95% C.I.: 0.26-0.62; P < .001). In this multivariate model, increasing age, poor diet, frail status, symptoms of depression, and the presence of moderate-to-severe WMH, silent lacunes, and >10 enlarged BG-PVS remained as significant covariates (Table 3). Age was the most influential variable in the above-mentioned association (t = 8.84). Other covariates with high influence included frail status, symptoms of depression, and the presence of moderate-to-severe WMH. To better understand the effect of age in the association between SDH and disability, we constructed a contour plot with Shepard interpolation.29 The plot showed several clusters of individuals aged 80 years or older only at higher levels of disability but at different levels of SDH scores, suggesting no significant effect of age in the above-mentioned association (Figure 2).
Table 3.
Fully-Adjusted Generalized Linear Model Showing the Independent Association Between the Social Determinants of Health and Disability (as the Dependent Variable).
| Disability | β coefficient | 95% C.I. | t statistic | P value |
|---|---|---|---|---|
| Social determinants of health | 0.442 | 0.258 to 0.625 | 4.75 | <.001* |
| Age | 0.315 | 0.245 to 0.385 | 8.84 | <.001* |
| Female gender | 0.448 | –0.585 to 1.482 | 0.85 | .394 |
| Primary school education | 0.024 | –1.138 to 1.185 | 0.04 | .968 |
| Current smoker | –0.080 | –2.685 to 2.524 | −0.06 | .952 |
| Body mass index ≥30 kg/m2 | −0.206 | –1.344 to 0.931 | −0.36 | .722 |
| Poor physical activity | 1.206 | –0.555 to 2.967 | 1.35 | .179 |
| Poor diet | −2.484 | –4.054 to −0.914 | −3.11 | .002* |
| Blood pressure ≥140/90 mmHg | 0.933 | –0.048 to 1.915 | 1.87 | .062 |
| Fasting glucose ≥126 mg/dL | 0.979 | –0.079 to 2.037 | 1.82 | .070 |
| Total cholesterol ≥240 mg/dL | −0.487 | –1.904 to 0.929 | −0.68 | .499 |
| Frail status | 2.754 | 1.450 to 4.058 | 4.15 | <.001* |
| Poor sleep quality | −0.688 | –1.675 to 0.299 | −1.37 | .172 |
| Symptoms of depression | 2.233 | 0.774 to 3.691 | 3.01 | .003* |
| Moderate-to-severe WMH | 1.870 | 0.612 to 3.128 | 2.92 | .004* |
| Deep cerebral microbleeds | 2.104 | −0.055 to 4.263 | 1.02 | .056 |
| Silent lacunes | 2.157 | 0.134 to 4.179 | 2.10 | .037* |
| >10 enlarged BG-PVS | −1.689 | −2.916 to −0.462 | −2.71 | <.001* |
Abbreviations: BG-PVS: basal ganglia-perivascular spaces; WMH: white matter hyperintensities.
Statistically significant result.
Figure 2.
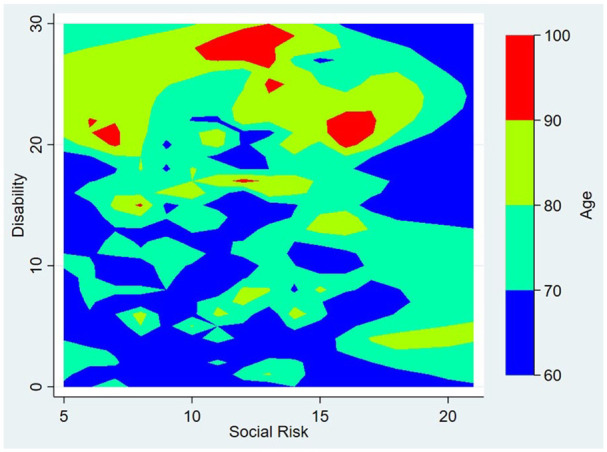
Contour plot with Shepard interpolation showing several clusters of individuals aged 80 years or older only at higher levels of disability but at different levels of social determinants of health scores, suggesting no significant effect of age in this association.
In addition, generalized linear models were fitted to calculate the association between the 5 individual components of SDH and disability, after adjusting for the aforementioned covariates (Table 4). In these models, the components more strongly associated with disability were the presence of worse support networks (OR: 2.64; 95% C.I.: 1.77-3.50) and social relationships (OR: 1.79; 95% C.I.: 1.28-2.31). In contrast, the single component not associated with disability was the economic status (OR: –0.41; 95% C.I.: –0.83-0.01).
Table 4.
Univariate Analyses and Generalized Linear Models Showing Associations Between Individual Components of Social Determinants of Health and Disability, After Adjusting for all Covariates.
| Total series (n = 478) | Not disabled (n = 256) | Disabled (n = 222) | Significance (univariate) | Significance (multivariate models) | |
|---|---|---|---|---|---|
| Family situation | 1.8 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.1 | P = .003* | OR: 0.48; 95% C.I.: 0.05-0.92; P = .029* |
| Economic status | 3.5 ± 1.2 | 3.6 ± 1.3 | 3.5 ± 1.1 | P = .368 | OR: −0.41; 95% C.I.: −0.83-0.01; P = .054 |
| Housing | 1.7 ± 0.8 | 1.6 ± 0.8 | 1.8 ± 0.9 | P = .010* | OR: 0.99; 95% C.I.: 0.43-1.56; P = .001* |
| Social relationships | 1.7 ± 1 | 1.4 ± 0.8 | 1.9 ± 1.2 | P < .001* | OR: 1.79; 95% C.I.: 1.28-2.31; P < .001* |
| Support networks | 1.1 ± 0.6 | 1 ± 0.3 | 1.2 ± 0.7 | P < .001* | OR: 2.64; 95% C.I.: 1.77-3.50; P < .001* |
Statistically significant result.
Discussion
This study, conducted in a population-based cohort of community-dwelling stroke-free older adults living in 3 rural villages of coastal Ecuador, showed a robust and independent association between SDH (and most of their individual components) and disability. Interestingly, the single individual component of SDH not associated with disability was the economic status of individuals, which suggest that—at the rural level—economic needs that might lead to disability are largely surpassed by other components of SDH, in particular by social relationships and support networks. Similar findings have been reported from a developed country, where the association between a higher income and a healthy status was less important among rural women than in their urban counterparts.14 Disparities across men and women regarding social risk are notable among people living in some rural areas of developed countries.30 However, this seems not to be important in the rural population enrolled in the present study, where gender did not remain as a significant independent covariate in multivariate models assessing the association between SDH and disability.
The role of social relationships and support networks to reduce loneliness and its impact on impaired functionality among older adults has not been well studied in remote rural communities. The feeling of loneliness among older adults is currently a public health problem in the developed world, especially in large urban centers,31,32 and might also be affecting older adults living in some remote rural settings.33 Loneliness often leads to depression, disturbed sleep and increased expression of cardiovascular risk factors, which, in turn, may facilitate the development of disability.34,35 However, the scenario is still less noticeable in other rural communities. As previously noticed, migration rate is low among individuals living in the current study population. This results in large families living in the same village and often in the same house or only a few blocks apart. In these settings, it is common for older adults to have more than 1 caregiver, which reduce the risk of burnout that results in functional impairment in the elder and an impaired quality of life of both the elder and the caregiver.36
To our knowledge, there are no study systematically addressing the role of subclinical cSVD on the association between SDH and disability in rural settings, and this is a novel aspect of the present study, particularly because damage of the cerebral microvasculature has been associated with functional impairment in studies conducted in the developed world,37 and this condition is prevalent in the study population.38 The presence of moderate-to-severe WMH and >10 enlarged BG-PVS were significantly associated with SDH, and moderate-to-severe WMH and silent lacunes were significantly associated with disability in univariate analyses. In addition, almost all biomarkers of cSVD (with the exception of CMB) remained as independently significant covariates in the multivariate model. This suggests an important influence of subclinical brain damage of vascular origin in the association between SDH and disability. The presence of cSVD biomarkers should be considered as potential targets for prevention in apparently healthy individuals at risk.
The cross-sectional design precludes the evaluation of the direction of the relationship between SDH and disability is a limitation of this study. Another potentially limitation is that we did not explore poor cognitive performance, which may be associated with disability. However, assessment of cognitive frailty was included among the investigated covariates. While a frail status remained independently significant in the regression model assessing the association between SDH and disability, it was not important enough to make this association to vanish in the multivariate model. The above-mentioned shortcomings of the present study were compensated by the population-based design with unbiased enrollment of individuals and the systematic assessment of the main investigated variables by means of validated field instruments. Also, the routine practice of MRI allowed to assess the role of subclinical brain damage of vascular origin in the studied association. Assessment of SDH by the use of a field instrument that is appropriate for remote rural settings—the Gijon scale—is another strength of the present study, since the selection of a given scale must take into account the specific needs and characteristics of the study population.
Knowledge on the burden of social risk, disability and the association between them is indispensable for the development of cost-effective preventive policies for controlling modifiable factors that are in the path of functional impairment among older adults living in resource-poor communities. Further multicentric studies using similar protocols are needed to confirm these findings.
Footnotes
Authors’ contributions: OHD: study design, imaging readings, manuscript drafting; RMM: statistical analyses; BYR: data collection and analysis; VJD: imaging readings, significant intellectual contribution to manuscript content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study supported by Universidad Espíritu Santo – Ecuador. The sponsor had no role in the design of the study, nor in the collection, analysis, and interpretation of data.
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
ORCID iD: Oscar H. Del Brutto  https://orcid.org/0000-0003-1917-8805
https://orcid.org/0000-0003-1917-8805
References
- 1. https://www.who.int/governance/eb/who_constitution_en.pdf
- 2. Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic disease. Am J Prev Med. 2017;52:S5–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease. A scientific statement from the American heart association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 5. Ferrario MM, Veronesi G, Kee F. Determinants of social inequalities in stroke incidence across Europe: a collaborative analysis of 126 635 individuals from 48 cohort studies. J Epidemiol Community Health. 2017;71:1210–1216. [DOI] [PubMed] [Google Scholar]
- 6. Abera SF, Gebru AA, Biesalski HK, et al. Social determinants of adult mortality from non-communicable diseases in northern Ethiopia, 2009-2015: evidence from health and demographic surveillance site. PloS One. 2017;12:e0188968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goh HT, Tan MP, Mazlan M, Abdul-Latif L, Subramanian P. Social participation determines quality of life among urban-dwelling older adults with stroke in a developing country. J Geriatr Phys Ther. 2019;42:E77–E84. [DOI] [PubMed] [Google Scholar]
- 8. Quiel L, Moreno Velásquez I, Gómez B, Motta J, Herrera-Ballesteros V. Social determinants and cardiovascular disease mortality in Panamá, 2012-2016. BMC Public Health. 2019:19:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marmot M, Friel S, Bell R, Houwelling TA, Taylor S, Commission on social determinants of health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372:1661–1669. [DOI] [PubMed] [Google Scholar]
- 10. Frier A, Barnett F, Devine S, Barker S. Understanding disability and the “social determinants of health”: how does disability affect peoples’ social determinants of health? Disabil Rehabil. 2016;40:538–547. [DOI] [PubMed] [Google Scholar]
- 11. Bourne PA. Social determinants of self-evaluated good health status of rural men in Jamaica. Rural Remote Health. 2009;9:1280. [PubMed] [Google Scholar]
- 12. Cabrera González D, Díaz Palacios E, Salamea Garcia A, et al. Evaluación de la fiabilidad y validez de una escala de valoración social en el adulto. Aten Primaria. 1999;23:434–440. [PubMed] [Google Scholar]
- 13. Riva M, Bambra C, Curtis S, Gauvin L. Collective resources or local social inequalities? Examining the social determinants of mental health in rural areas. Eur J Public Health. 2011;21:197–203. [DOI] [PubMed] [Google Scholar]
- 14. Wanless D, Mitchell BA, Wister AV. Social determinants of health for older women in Canada: does rural-urban residency matter? Can J Aging. 2010;29:233–247. [DOI] [PubMed] [Google Scholar]
- 15. Del Brutto OH, Mera RM, Peralta LD, et al. Cardiovascular health status among community-dwelling Ecuadorian natives living in neighboring rural communities: the Three Villages Study. J Community Health. 2020;45:154–160. [DOI] [PubMed] [Google Scholar]
- 16. Del Brutto OH, Mera RM, Recalde BY, et al. Association between pulsatile components of blood pressure and severe tooth loss in rural Ecuador: The Three Villages Study. J Prim Care Community Health. 2020;11:215013272092867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Brutto OH, Idrovo L, Mosquera A, et al. Validación de un cuestionario para la detección del ictus en poblaciones hispanoparlantes. Rev Neurol. 2004;39:301–304. [PubMed] [Google Scholar]
- 18. Del Brutto OH, Santamaría M, Zambrano M, et al. Stroke in rural coastal Ecuador: a Community-based survey. Int J Stroke. 2014;9:365–366. [DOI] [PubMed] [Google Scholar]
- 19. von Elm E, Altman G, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1437–1457. [DOI] [PubMed] [Google Scholar]
- 20. Ministerio de Salud Pública del Ecuador. Guías clínicas geronto-geriátricas de atención primaria de salud para el adulto mayor. Quito, Ecuador, Septiembre 2008. https://vicenteayalabermeo.files.wordpress.com/2011/04/guc3adas-adulto-mayor.pdf.
- 21. Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 22. Herrera MS, Saldías P, Testa N. Validación de un test breve para el diagnóstico de capacidad funcional en adultos mayores en Chile. Rev Med Chile. 2014;142:1128–1135. [DOI] [PubMed] [Google Scholar]
- 23. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2013;12:822–838. [DOI] [PubMed] [Google Scholar]
- 24. Huo YC, Li Q, Zhang WY, et al. Total cerebral disease burden predicts functional outcome in patients with acute ischemic stroke. Front Neurol. 2019;10:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lloyd-Jones D, Hong Y, Labarthe F, et al. Defining and setting national goals for cardiovascular health promotion. The American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 26. Del Brutto OH, Mera RM, Zambrano M, Sedler MJ. Influence of frailty on cognitive decline: a population-based cohort study in rural Ecuador. J Am Med Dir Assoc. 2019;20:213–216. [DOI] [PubMed] [Google Scholar]
- 27. Del Brutto OH, Mera RM, Zambrano M, Lama J, Del Brutto VJ, Castillo PR. Poor sleep quality and silent markers of cerebral small vessel disease: a population-based study in community-dwelling older adults (The Atahualpa Project). Sleep Med. 2015;16:428–431. [DOI] [PubMed] [Google Scholar]
- 28. Del Brutto OH, Mera RM, Del Brutto VJ, et al. Influence of depression, anxiety and stress on cognitive performance in community-dwelling older adults living in rural Ecuador: Results of the Atahualpa Project. Geriatr Gerontol Int. 2015;15:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dell’Accio F, Di Tommaso F. Scattered data interpolation by Shepard’s like methods: classical results and recent advances. Dolomites Research Notes on Approximation 2016;9:32–44. [Google Scholar]
- 30. Zhang Y, Shi W, Huang Z, Gao D, Guo Z, Chongsuvivatwong V. Gender and ethnic health disparities among the elderly in rural Guangxi, China: estimating quality-adjusted life expectancy. Glob Health Action. 2016;9:32261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez CJ, Elkind MS, Clemow L, et al. Association between social isolation and left ventricular mass. Am J Med. 2011;124:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Udell JA, Steg PG, Scirica BM, et al. Living alone and cardiovascular risk in outpatients at risk of or with atherothrombosis. Arch Int Med. 2012;172:1086–1095. [DOI] [PubMed] [Google Scholar]
- 33. Del Brutto OH, Tettamanti D, Del Brutto VJ, Zambrano M, Montalván M. Living alone and cardiovascular health status in residents of a rural village of coastal Ecuador. Environ Health Prev Med. 2013;18:422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Domènech-Abella J, Lara E, Rubio-Valera M, et al. Loneliness and depression in the elderly: the role of social network. Soc Psychiatry Psychiatr Epidemiol. 2017;52:381–390. [DOI] [PubMed] [Google Scholar]
- 35. Valtorta NK, Kanaan M, Cilbody S, Hanratty B. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur J Prev Cardiol. 2018;25:1387–1396. [DOI] [PubMed] [Google Scholar]
- 36. Rote S, Angel J, Hinton L. Characteristics and consequences of family support in Latino dementia care. J Cross Cult Gerontol. 2019;34:337–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Del Brutto OH, Mera RM, Recalde BY, Del Brutto VJ. Cerebral small vessel disease in community-dwelling older adults living in remote rural settings. J Neurol Sci. 2020;416:117016. [DOI] [PubMed] [Google Scholar]


