Abstract
Patient: Male, Newborn
Final Diagnosis: Short bowel syndrome
Symptoms: Short bowel
Medication: —
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology • Geriatrics
Objective:
Unusual clinical course
Background:
Short bowel syndrome in infants is relatively rare. It consists of malabsorption caused by a congenital short bowel or extensive resection of a large part of the small intestine. The postoperative mortality rate is high and surviving patients develop many complications. Wernicke encephalopathy is caused by vitamin B1 (thiamin) deficiency. Delayed treatment may lead to irreversible neuron necrosis, gliosis, severe amnesia, Korsakoff psychosis, or even death.
Case Report:
We report the case of a premature infant with extremely low birth weight and short bowel syndrome. He was treated with early enteral nutrition combined with succus entericus reinfusion with no complications. Four months after discharge, he was diagnosed with Wernicke encephalopathy. He was treated with intravenous vitamin B1 (100 mg IV/d) and was administered oral vitamin B1 (20 mg 3 times daily) by his wet nurse. Vitamin B1 levels returned to normal after 4 days (69.8 nmol/L). Physical development was normal at the follow-up at a corrected age of 2 years.
Conclusions:
Preventive measures for Wernicke encephalopathy should be implemented in patients with long-term malnutrition or absorption disorders. The risk of vitamin B1 deficiency increases in patients receiving parenteral nutrition and medical staff should be aware of the importance of the vitamin B1 status.
MeSH Keywords: Short Bowel Syndrome, Thiamine Deficiency, Wernicke Encephalopathy
Background
Short bowel syndrome is malabsorption caused by a congenital short bowel or extensive resection of a large part of the small intestine. This condition is relatively rare in infants. Eighty percent of children with short bowel syndrome manifest this condition in the neonatal period, and the overall mortality rate for short bowel syndrome is 20% to 40% [1].
Wernicke encephalopathy is caused by vitamin B1 (thiamin) deficiency and mainly occurs in people with chronic alcohol abuse. However, it is also seen in other malnourished patients, such as those with starvation, severe vomiting during pregnancy, on long-term hemodialysis, liver failure, insufficient parenteral nutrition, and malignancies. The pathogenesis of Wernicke encephalopathy is associated with reduced energy metabolism in the brain due to decreased transketolase activity, local lactic acidosis, glutamate receptor-mediated neurotoxicity, and destruction of the blood-brain barrier [2]. The classic presentation is a triad of ophthalmoplegia, ataxia, and confusion; however, not all patients present with this triad [3]. Delayed treatment can lead to irreversible neuron necrosis, gliosis, severe amnesia, Korsakoff psychosis, or even death. In this case report, we present the treatment course of a premature extremely low birth weight infant who had short bowel syndrome accompanied by Wernicke encephalopathy to determine safe and effective treatment methods and assess clinical implications.
Case Report
A male infant was born at the gestational age of 29+3 weeks to a primigravid and primiparous mother. He was delivered by cesarean section due to severe pre-eclampsia in the mother. She had a 3-year history of hypertension and a family history of eclampsia. The neonate did not develop asphyxiation and had a birth weight of 1.258 kg, height of 41.5 cm, and a head circumference of 29 cm, which was below the 3rd percentile (3P). He had poor responses, systemic pallor, a capillary refill time of 2 s, irregular breathing, a distended abdomen, and borborygmus was absent. Blood tests showed a white blood cell count of 8.3×109/L (normal, 10–20×109/L); 61.2 g/L hemoglobin (normal, 145–220 g/L); a platelet count of 22×109/L (normal, 100–300×109/L); and C-reactive protein >160 mg/L (normal, ≤10 mg/L).
After delivery, the infant developed abdominal distension and was given parenteral nutritional support. An abdominal ultra-sound revealed intestinal malrotation. Gastroschisis repair, ileocecectomy, appendectomy, and ileostomy were carried out 2 days after birth. The jejunum and part of the ileum were resected. Dilated intestines were incised, and the ends were anastomosed. The terminal ileum was pulled out of the skin for enterotomy. Only 40 cm of the small intestine remained, and the ileocecal valve and the entire colon were retained. During surgery, an indwelling peripherally inserted central catheter was used for parenteral nutrition with 150 mL/kg/d of fluid volume with extra supplementation for volume lost during the ostomy. The maximum amount of amino acid added was 3.5 g/kg/d. Due to short bowel syndrome, cholestasis and liver function damage occurred in the course of the disease; therefore, total fat emulsion was controlled at 1 g/kg/d. The glucose rate was adjusted according to the blood glucose level. The glucose infusion rate ranged from 12 to 16 mg/kg/min when on total parenteral nutrition (TPN) based on his caloric growth needs of 9–115 kcal/kg/d. Electrolytes, trace elements, and free carnitine levels were monitored, and aggressive supplementation was included. In this patient, a jejunostomy (proximal fistula) and 1 distal mucous fistula were retained. The distal mucous fistula enabled use of the succus entericus reinfusion. Six days after surgery, enteral nutrition was established, and excretion appeared at the proximal fistula. Eleven days after surgery, approximately 150–300 mL liquid discharge was observed each day at the proximal fistula. This gradually increased, and enteral nutrition was changed to nasogastric tube feeding and the succus entericus reinfusion was restarted. Before the succus entericus reinfusion, distal fistula imaging was carried out to confirm patency of the distal intestine (Figure 1). The rein-fusion tube was a bendable and soft nasogastric tube (6–8 Fr) inserted to a depth of 5 cm. Succus entericus reinfusion was performed once every 3 h, matching a normal breastfeeding routine. The feeding and reinfusion rates were adjusted according to the patient’s tolerance to completely wean off the parenteral nutrition. During the feeding and reinfusion period, the infant’s vital signs, water, and electrolytes, and body mass were monitored. At 40 days after the operation, the succus entericus reinfusion was discontinued and the intestinal fistula was closed. His weight had increased to 3.4 kg. Succus entericus reinfusion enabled the infant to gradually decrease reliance on the TPN and tolerate oral formula feeding. He was not breastfed, as the mother had been prescribed medication for high blood pressure for the past 2 years. The infant was discharged after 67 days and was fed exclusively orally with an extensively hydrolyzed formula combined with high-energy, whole-protein formula milk (volume, 148 mL/kg/d; energy content, 124 kcal/kg/d, including 3.1 mg vitamin B1) for 187 days after delivery.
Figure 1.
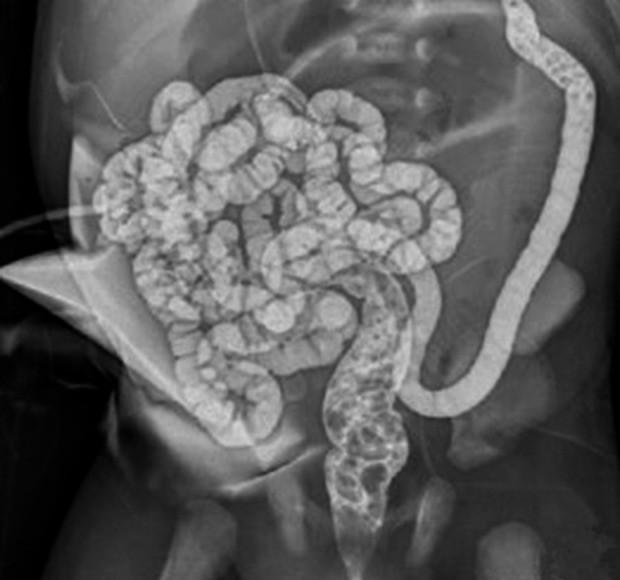
Distal fistula imaging (Before succus entericus reinfusion, distal fistula imaging was used to observe patency).
Four months after discharge, the patient developed moaning, a dazed look, low tension in the frontal suture, and facial pallor. Upon examination at the emergency room in the hospital, the patient was somnolent, and his Glasgow Coma score was 8. His head CT showed that the bilateral basal ganglia striatum, caudate nucleus head, and medial dorsal nucleus were symmetrical; there was reduced density, clear and regular borders, and figure-eight shape changes (Figure 2). Blood tests showed that the vitamin B1 level was 29.7 nmol/L (normal range, 41.5–108.9 nmol/L). Because there was no significant clinical manifestation of elevated intracranial pressure, tests for intracranial pressure and cerebrospinal fluid were not performed. Based on his medical history, other signs, classic head CT presentation, and short-term efficacy when intravenous vitamin B1 (100 mg IV/d) was used for 2 days, the patient was diagnosed with Wernicke encephalopathy.
Figure 2.
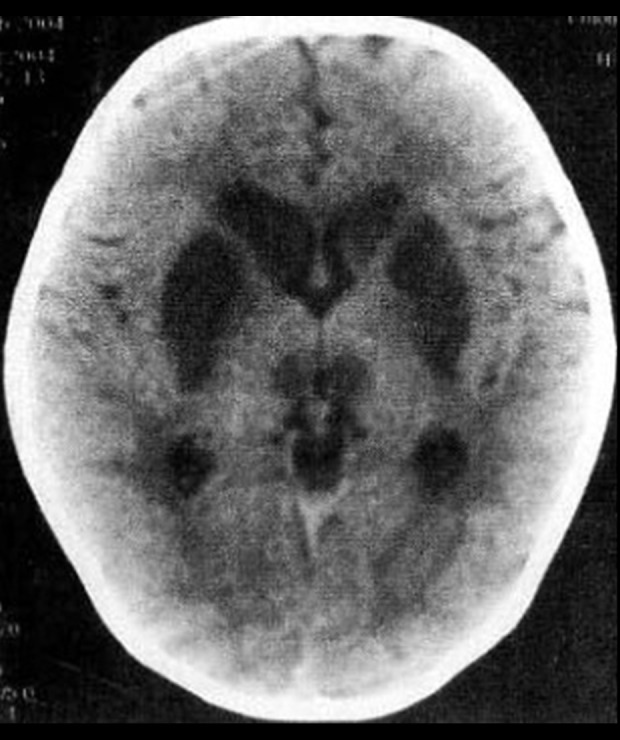
Bilateral basal ganglia striatum, caudate nucleus head, and medial dorsal nucleus were symmetrical, and the density was decreased.
He was prescribed penicillin and ceftriaxone (or cefotaxime sodium) for infection prophylaxis, and diazepam and/or pheno-barbital as antispasmodics, supported with oxygen inhalation and fluid supplementation. Intravenous vitamin B1 was added for treatment (100 mg IV/d) for the first 3 days and vitamin B1 was administered orally (20 mg/3 times daily) by his wet nurse for the next 5 days. The change in vitamin B1 levels was monitored daily to adjust the dosage accordingly. Vitamin B1 levels returned to normal after 4 days (69.8 nmol/L). During the course of treatment, liver function was examined. This patient had comorbid impaired liver function and underwent hepato-protective treatment for 1 week while correcting his electrolyte imbalance. Nebulization and sputum suction, turning over, and back patting were performed regularly to ensure that the patient’s respiratory tract was patent. Prophylactic vitamin B1 (20 mg/d) was administered orally until the age of 2 years.
After discharge on the 9th day of treatment, the patient was followed up and continued to be administered an extensively hydrolyzed formula combined with high-energy formula milk. At the corrected age of 14 months, his weight was above the 10th percentile (P10) for same-age children, while his height was still below P10. At the corrected age of 2 years, his height and weight were above P10 for same-age children. At 31 months, his height and weight were between P10 and P25 (normal). A cranial magnetic resonance image showed T2W hyperdense signals at the bilateral posterior cerebral ventricles, and adjacent to the trigone of the lateral ventricle, with a possibility that it was unmyelinated. Dual-energy X-ray imaging of bone mineral density showed a z-value of 0.2. The Griffiths Mental Development Scale at 40 months after birth showed that the locomotor peak was 38.1 (P10–P25), the performance peak was 40.0 (P25–P30), the personal-social peak was 39.5 (P5–P7), the eye-hand coordination peak was 30.0 (P2–P5), and the language peak was 15.2 (<PI).
Written consent was obtained from the parents of the infant for publication of this case report and all accompanying images, and it was put on record by the Ethics Committee.
Discussion
Neonatal short bowel syndrome refers to decreased small intestine absorption due to the exclusion or resection of a large part of the small intestine or a congenital short bowel, which cannot satisfy the nutritional requirements for normal growth and development of infants. Such patients require at least 42 days of parenteral nutrition support [4]. In a normal body, the small intestine regulates nutrient digestion and absorption; most nutrients are absorbed by the proximal end of the jejunum, while the terminal ileum is responsible for absorption of bile acid and vitamins. If the terminal ileum is resected, enterohepatic circulation is disrupted, thereby causing cholelithiasis and cholestasis. The loss of ileal function accelerates peristalsis and loss of succus entericus. Therefore, the shorter the intestinal length, the poorer the nutrient absorption, and the greater the dependence on parenteral nutrition and worse outcomes [5].
In this patient, the fistulas had not closed sufficiently after the double ostomy, and succus entericus reinfusion was employed where succus entericus from the proximal end was re-infused into the intestine in the distal end, which simulated a normal, intact digestive tract. This helped to reduce water and electrolyte loss and drive adaptive changes in the intestine, eventually creating conditions for fistula closure. In addition, the succus entericus reinfusion utilized the advantages of the distal small intestine to help drive enterohepatic circulation of bile, reduce the risk of cholelithiasis and cholestasis, ensure that the pediatric patient could be weaned off parenteral nutrition before fistula closure, reduce the risk of hepatic impairment and sepsis, and other complications, and improve the safety of the treatment [6]. Many studies have found that succus entericus reinfusion treatment of high enterotomy is safe and effective; however, some researchers have reported a risk for developing complications. Haddock reported that in 23 pediatric patients who underwent succus entericus rein-fusion, 4 developed related complications (intestinal bleeding, 1 patient; intestinal perforation, 3 patients), and 1 patient died due to intestinal perforation. The study suggested that there is a high safety risk in succus entericus reinfusion and that it is worth examining whether succus entericus reinfusion should be carried out when parenteral nutrition techniques have been continuously improving [7].
The results of the present study showed that this patient was weaned off parenteral nutrition after treatment, body mass was significantly increased when compared to that before reinfusion, and no related complications occurred. Therefore, the use of succus entericus reinfusion can benefit systemic nutrition in a pediatric patient and create conditions for recovery. Hence, this is an effective treatment for short bowel syndrome in neonates after an ostomy.
This pediatric patient developed Wernicke encephalopathy after discharge and was treated with vitamin B1. Intravenous vitamin B1 was added for treatment (100 mg IV/d) for the first 3 days and vitamin B1 was administered orally (20 mg/3 times daily) by his wet nurse for the next 5 days. Wernicke encephalopathy is effectively treated with vitamin B1 supplementation; however, there is currently no medical consensus on recommendations for dosage, administration of vitamin B1, and treatment time. Nevertheless, we suggest that the vitamin B1 dose be 200 mg per day until serum vitamin B1 levels return to normal, and more often through intravenous rather than intramuscular injection. Due to the younger age and lower weight of this patient, vitamin B1 was administered at 100 mg/d for 3 days. The literature suggests that any disease or condition that causes the loss of large amounts of vitamin B1 or interferes with vitamin B1 absorption can lead to Wernicke encephalopathy after only 2–3 weeks [3]. Wernicke encephalopathy has a better prognosis with early diagnosis and treatment. Delayed treatment can lead to irreversible neuron necrosis, gliosis, severe amnesia, Korsakoff psychosis, or even death. Full recovery is seen in less than 50% of such patients.
We searched the literature in PubMed with the keywords “short bowel syndrome” and “Wernicke encephalopathy.” We searched for all cases of short bowel syndrome with comorbid Wernicke encephalopathy and found 4 cases that fulfilled our search criteria [8–11]. These cases have been summarized in Table 1. In 1999, Vasconcelos et al. [12] reported the first case series of pediatric Wernicke encephalopathy. They concluded that overlooking vitamin B1 supplementation during parenteral nutrition is an important cause of non-alcoholic Wernicke encephalopathy. This review also showed that routine vitamin supplementation had not been administered when the Wernicke encephalopathy patients had received parenteral nutrition. In 1 case, the caregiver had poor treatment compliance [8].
Table 1.
Summary of cases with Wernicke’s encephalopathy with short bowel syndrome on total parenteral nutrition.
| Case | Age (yr/d) | Sex | Country | Year | Underlying disease | TPN | MRI finding | Thiamin level | Thiamin treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Bian et al. [11] | 75 yr | F | China | 2016 | SBS secondary to gastrectomy | PN dependent without thiamin | High T2 intensity in both mammillary bodies and bilateral thalamus | Undetectable | NA | Improvement within 10 d and complete resolution in 29 d |
| Santarpia et al. [10] | 20 yr | F | Italy | 2009 | SBS secondary to gastrectomy | TPN dependent without thiamin | Bilateral and symmetrical hyper-intensities in the subthalamus | Undetectable | 100 mg IV daily | Improvement within 15 d and complete resolution in 40 d |
| Benidir et al. [8] | 8 yr | F | Canada | 2014 | SBS secondary to gastroschisis | TPN dependent without thiamin supplementation for 3 months | Symmetric lesions of the basal ganglia, mammillary bodies and peri-aqueductal region of the midbrain | Undetectable | 100 mg IV daily | Complete resolution within 48 h |
| Roilides et al. [9] | 3 yr | M | Greece | 2019 | SBS secondary to NEC gastrectomy for double volvulus | PN dependent with 3.1 mg thiamin | Bilateral symmetric hyper-intense signals in the peri-aqueductal area and both medial thalami | 10 μg/L (normal levels, 28–85) | 25 mg IM daily | Improvement within 48 hr and complete resolution in 96 hr |
| Present case | 3 d | M | China | 2019 | SBS secondary to gastroschisis | PN dependent with 3.0 mg thiamin | Symmetric lesions of the basal ganglia, caudate nucleus head, thalamus medial margin symmetry | 19.7 nmol/L (normal levels, 41.5–108.9 nmol/L) | 100 mg IV daily | Improvement within 72 hr and complete resolution in 96 hr |
F – Female; IM – intramuscular; IV – intravenous; M – Male; MRI – magnetic resonance imaging; NA – not applicable; NEC – necrotizing enterocolitis; PN – parenteral nutrition; SBS – short bowel syndrome; TPN – total parenteral nutrition.
In our case, although parenteral nutrition was provided during multiple hospitalizations and attention was paid to energy supplementation and nutritional needs to ensure sufficient vitamin intake, our patient still developed Wernicke encephalopathy. This is similar to the case reported by Santarpia et al. [10]. This may be due to several reasons. First, the parents did not provide supplementation strictly according to the required dose. Even though the infant’s general condition had improved, fever, anorexia, vomiting, and other diseases may have led to vitamin B1 deficiency resulting from an insufficient dose [13]. Second, vitamin B1 is photosensitive. Improper storage of the vitamin B1 at home may have led to vitamin degradation and affected the treatment results. Lastly, the caregivers may have omitted vitamin supplementation during parenteral nutrition. Even though the caregivers denied this, the infant showed good clinical improvement upon vitamin supplementation. Medical staff may have also underestimated the importance of vitamin supplementation in this situation, as reflected in the case reports [8–11] (Table 1).
Short bowel syndrome may be accompanied by catheter-related complications and absorption-induced diarrhea, fluid and electrolyte imbalance, and deficiency in macronutrients and micronutrients, and requires meticulous and comprehensive nutritional assessment, monitoring, and treatment [14–16]. Wernicke encephalopathy has an atypical clinical presentation and poor prognosis [13]. In addition to patients with short bowel syndrome, the nutritional status of other patients should also be considered, especially those with long-term fasting or reduced food intake and those who have received parenteral nutrition after gastric surgery [17–19]. For example, Tozzo [17] described a patient who had undergone Roux-en-Y subtotal gastrectomy for gastric cancer and was reported to have developed Wernicke encephalopathy, followed by irreversible neurological symptoms. The caregivers in that case were almost sued by the patient’s family. Active supplementation of vitamin B1 and other nutrients can prevent Wernicke encephalopathy or other metabolic encephalopathies. Therefore, clinicians should be aware of Wernicke encephalopathy in order to establish an early diagnosis and plan for early prevention and intervention [20]. When necessary, aggressive supplementation with vitamin B1 and other nutrients can prevent occurrence of Wernicke encephalopathy or other metabolic encephalopathies. The physical development of our pediatric patient was normal at the follow-up of more than 1 year. However, his language, hand-eye coordination, and social interactions still lagged by varying degrees. While nutritional status is prioritized, the mental development of the patient should also be followed up for early detection and possible intervention.
Conclusions
In summary, succus entericus reinfusion is safe and effective, and has important clinical significance for pediatric patients with short bowel syndrome. A differential diagnosis of metabolic encephalopathy should be made when neuropsychiatric symptoms appear in patients with intestinal disorders. Important preventive measures for Wernicke encephalopathy should be implemented in patients with long-term malnutrition or absorption disorders. Aggressive vitamin B1 supplementation should be administered to patients at high risk or suspected of Wernicke encephalopathy to improve prognosis. Wernicke encephalopathy remains a severe complication of short bowel syndrome. The risk of vitamin B1 deficiency is increased in patients receiving parenteral nutrition and medical staff should be aware of the importance of the vitamin B1 status.
Footnotes
Department and institution where work was done
International School of Nursing, Huangshan Vocational Technical College, Huangshan, Anhui, P.R. China.
Conflicts of interest
None.
References:
- 1.Duro D, Kamin D, Duggan C. Overview of pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47(1):S33–36. doi: 10.1097/MPG.0b013e3181819007. [DOI] [PubMed] [Google Scholar]
- 2.Dhir S, Tarasenko M, Napoli E, Giulivi C. Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Front Psychiatry. 2019;10:207–18. doi: 10.3389/fpsyt.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvin R, Bråthen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–18. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 4.Rege AS, Sudan DL. Autologous gastrointestinal reconstruction: Review of the optimal nontransplant surgical options for adults and children with short bowel syndrome. Nutr Clin Pract. 2013;28(1):65–74. doi: 10.1177/0884533612460405. [DOI] [PubMed] [Google Scholar]
- 5.Pflug AM, Utiyama EM, Fontes B, et al. Continuous reinfusion of succus entericus associated with fistuloclysis in the management of a complex jejunal fistula on the abdominal wall. Int J Surg Case Rep. 2013;4(8):716–18. doi: 10.1016/j.ijscr.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calicis B, Parc Y, Caplin S, et al. Treatment of postoperative peritonitis of small-bowel origin with continuous enteral nutrition and succus entericus reinfusion. Arch Surg. 2002;137(3):296–300. doi: 10.1001/archsurg.137.3.296. [DOI] [PubMed] [Google Scholar]
- 7.Haddock CA, Stanger JD, Albersheim SG, et al. Mucous fistula refeeding in neonates with enterostomies. J Pediatr Surg. 2015;50(5):779–82. doi: 10.1016/j.jpedsurg.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Benidir AN, Laughlin S, Ng VL. Visual disturbances in total parenteral nutrition dependent liver transplant pediatric patient. Gastroenterology. 2014;146(5):e10–11. doi: 10.1053/j.gastro.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Roilides I, Vasilaki K, Xinias I, et al. Thiamine deficiency in a child with short bowel syndrome and review. Pediatr Gastroenterol Hepatol Nutr. 2019;22(5):493–99. doi: 10.5223/pghn.2019.22.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santarpia L, Alfonsi L, Pasanisi F, Contaldo F. Wernicke’s encephalopathy in a patient with short bowel syndrome on total parenteral nutrition: A case report. ESPEN J. 2009;4(5):e245–47. [Google Scholar]
- 11.Bian XJ, Wang H, Ge WH. Parenteral nutrition support and analysis of a case of Wernicke encephalopathy with short bowel syndrome. Pharmaceutical and Clinical Research. 2016;24:505–6. [Google Scholar]
- 12.Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke’s Encephalopathy. Pediatr Neurol. 1999;20(4):289–94. doi: 10.1016/s0887-8994(98)00153-2. [DOI] [PubMed] [Google Scholar]
- 13.Lallas M, Desai J. Wernicke encephalopathy in children and adolescents. World J Pediatr. 2014;10(4):293–98. doi: 10.1007/s12519-014-0506-9. [DOI] [PubMed] [Google Scholar]
- 14.Fuglestad MA, Thompson JS. Inflammatory bowel disease and short bowel syndrome. Surg Clin North Am. 2019;99(6):1209–21. doi: 10.1016/j.suc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Billiauws L, Joly F. Emerging treatments for short bowel syndrome in adult patients. Expert Rev Gastroenterol Hepatol. 2019;13(3):241–46. doi: 10.1080/17474124.2019.1569514. [DOI] [PubMed] [Google Scholar]
- 16.Billiauws L, Corcos O, Joly F. What’s new in short bowel syndrome? Curr Opin Clin Nutr Metab Care. 2018;21(4):313–18. doi: 10.1097/MCO.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 17.Tozzo P, Caenazzo L, Rodriguez D, Bolcato M. Delayed diagnosis of Wernicke encephalopathy with irreversible neural damage after subtotal gastrectomy for gastric cancer: A case of medical liability? Int J Surg Case Rep. 2017;30:76–80. doi: 10.1016/j.ijscr.2016.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong HJ, Park JW, Kim YJ, et al. Wernicke’s Encephalopathy after sleeve gastrectomy for morbid obesity – a case report. Ann Rehabil Med. 2011;35(40):583–86. doi: 10.5535/arm.2011.35.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landais A, Saint-Georges G. [Wernicke’s encephalopathy following sleeve gastrectomy for morbid obesity] Rev Med Interne. 2014;35:760–63. doi: 10.1016/j.revmed.2014.01.010. [in French] [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Kataria A, Kolla BP, et al. Wernicke encephalopathy – clinical pearls. Mayo Clin Proc. 2019;94(6):1065–72. doi: 10.1016/j.mayocp.2019.02.018. [DOI] [PubMed] [Google Scholar]


