Abstract
Background:
Pancreatic adenocarcinoma is the fourth leading cause of cancer-related death. In cases with metastasis, the combination of 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) or gemcitabine-based chemotherapy regimens are considered the standard of care. However, the optimal sequence of these regimens is unclear.
Methods:
This retrospective study initially evaluated 186 patients with locally advanced/metastatic pancreatic cancer at three Italian institutions between February 2013 and October 2019. All patients had progressed after receiving gemcitabine-based first-line chemotherapy and were subsequently offered second-line FOLFIRINOX, FOLFOX-6, or FOLFIRI treatment. This study evaluated progression-free survival (PFS), overall survival from the start of second-line treatment (OS2), overall survival from the start of first-line treatment (OS1), and safety outcomes.
Results:
A total of 77 patients received ⩾4 cycles of second-line chemotherapy and were considered eligible: 15 patients received FOLFIRINOX, 32 patients received FOLFOX-6, and 30 patients received FOLFIRI. The FOLFIRINOX group had median PFS of 26.29 weeks and median OS2 of 47.86 weeks, while the FOLFIRI group had median PFS of 10.57 weeks and median OS2 of 25.00 weeks (p = 0.038). No significant differences were observed between the FOLFIRINOX and FOLFOX-6 groups in terms of PFS (26.29 weeks versus 23.07 weeks) or OS2 (47.86 weeks versus 42.00 weeks). The most common grade 3–4 toxicities were anemia, neutropenia, and thrombocytopenia, which occurred more frequently in the FOLFIRINOX and FOLFOX-6 groups.
Conclusion:
Relative to the FOLFIRI regimen, the FOLFIRINOX regimen had a favorable toxicity profile and better survival outcomes. No significant differences were observed relative to the FOLFOX-6 regimen.
Keywords: FOLFIRINOX, gemcitabine, pancreatic adenocarcinoma, safety, second line
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is estimated to be the eighth most commonly diagnosed cancer among American women and the tenth most commonly diagnosed cancer among American men, and in 2020 was estimated to be the fourth leading cause of cancer-related death.1 The 5-year survival rate for all stages is approximately 9%.1 Most cases are diagnosed at the locally advanced or metastatic stages, where chemotherapy is the standard of care.1 Gemcitabine monotherapy was the only treatment option for many years,2 although two poly-chemotherapeutic schemes have recently been approved for metastatic pancreatic cancer: 5-fluorouracil (5-FU), irinotecan, and oxaliplatin (FOLFIRINOX) and gemcitabine plus nab-paclitaxel.3,4 The multicenter phase III PRODIGE4/ACCORD11 trial randomized chemotherapy-naïve patients with advanced PDAC to receive gemcitabine monotherapy or FOLFIRINOX, which revealed that FOLFIRINOX provided a consistent improvement in overall survival (OS: 11.1 months versus 6.8 months, p < 0.001).3 Furthermore, FOLFIRINOX provided improvements in progression-free survival (PFS: 6.4 months versus 3.3 months, p < 0.001) and the objective response rate (ORR: 31.6% versus 9.4%, p < 0.001).3 The multicenter randomized phase III MPACT trial provided another milestone in the treatment of advanced pancreatic cancer, which compared first-line gemcitabine monotherapy with gemcitabine plus nab-paclitaxel. The combination of gemcitabine plus nab-paclitaxel provided significant improvements in OS [8.5 months versus 6.7 months, hazard ratio (HR): 0.72; p < 0.001] and in PFS (5.5 months versus 3.7 months, HR: 0.69; p < 0.001).4
These results led to the approval of both schemes as first-line options for treating metastatic PDAC. However, no prospective randomized trials have compared FOLFIRINOX with gemcitabine plus nab-paclitaxel, and we are aware of data only from retrospective analyses that revealed similar survival outcomes but different toxicity profiles.5–7 Thus, the treatment for patients with metastatic PDAC should be guided by the patient’s characteristics (e.g., performance status and comorbidities) and the anticipated drug-related adverse events.
While both schemes are universally approved as first-line treatment, there is no standard of care for second-line treatment of advanced PDAC. Fluoropyrimidine-based regimens are the main chemotherapeutic regimens that are used for patients who progress during first-line gemcitabine-based treatment, and these regimens improve survival outcomes relative to best supportive care.8 However, there are no randomized phase III trials comparing FOLFIRINOX with other fluoropyrimidine-based regimens for patients who experience progression during first-line gemcitabine-based treatment. Therefore, this retrospective study aimed to evaluate the efficacy and safety outcomes of second-line FOLFIRINOX, FOLFOX-6, or FOLFIRI treatment for patients with advanced pancreatic cancer who experienced progression during first-line gemcitabine-based treatment.
Methods
Study design
This multicenter retrospective study evaluated patients who were treated between February 2013 and October 2019 at three Italian hospitals: Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II; Medical Oncology Unit, IRCCS Istituto Tumori Giovanni Paolo II of Bari; and Unit of Oncology 2, University Hospital of Pisa. Medical records were searched to identify patients with advanced pancreatic cancer who experienced progression during first-line gemcitabine-based treatment and subsequently received second-line treatment using FOLFIRINOX, FOLFOX-6, or FOLFIRI. The retrospective study protocol was approved by the institutional review board at the main study site (Federico II University Hospital Institutional Ethics Committee, Naples; approval number: 160/19). The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. All patients had provided written informed consent for the research use of their anonymized data.
Inclusion and exclusion criteria
The inclusion criteria were: ⩾18 years old, a pathologically confirmed diagnosis of locally advanced or metastatic PDAC between February 2013 and October 2019, disease progression during first-line gemcitabine-based treatment according to version 1.1 of the Response Evaluation Criteria In Solid Tumors (RECIST 1.1), an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, and able to start a fluoropyrimidine-based poly-chemotherapy regimen. The exclusion criteria were: an ECOG PS of >2, death before the start of second-line treatment, used of a second-line single-drug treatment, or non-completion of ⩾4 cycles of the chosen second-line chemotherapy regimen.
Treatments
The first-line treatments involved gemcitabine as monotherapy or with other chemotherapeutic drugs at standard or reduced doses. The gemcitabine monotherapy was administered intravenously (IV) at 1000 mg/m2 on days 1, 8, and 15 of a 28-day cycle. The gemcitabine plus oxaliplatin regimen involved gemcitabine administered IV at 1000 mg/m2 plus oxaliplatin at 100 mg/m2 on days 1 and 15 of a 28-day cycle. The gemcitabine plus nab-paclitaxel regimen involved gemcitabine administered IV at 1000 mg/m2 and nab-paclitaxel at 125 mg/m2 on days 1, 8, and 15 of a 28-day cycle. The gemcitabine plus capecitabine regimen involved gemcitabine administered IV at 1000 mg/m2 on days 1 and 8 plus capecitabine at 1660 mg/m2 on days 1–14 of a 21-day cycle.
Patients who experienced progression during first-line treatment subsequently received second-line fluoropyrimidines-based chemotherapy (FOLFIRINOX, FOLFOX-6, or FOLFIRI). The FOLFIRINOX regimen involved oxaliplatin at 85 mg/m2, irinotecan at 180 mg/m2, leucovorin at 400 mg/m2, 5-FU bolus at 400 mg/m2, and a continuous 46-h IV infusion of 5-FU at 2400 mg/m2 on day 1 of a 14-day cycle. The FOLFOX-6 regimen involved oxaliplatin at 85 mg/m2, leucovorin at 200 mg/m2, 5-FU bolus at 400 mg/m2, and a continuous 46-h IV infusion of 5-FU at 2400 mg/m2 on day 1 of a 14-day cycle. The FOLFIRI regimen involved irinotecan at 180 mg/m2, leucovorin at 200 mg/m2, 5-FU bolus at 400 mg/m2, and a continuous 46-h IV infusion of 5-FU at 2400 mg/m2 on day 1 of a 14-day cycle. Filgrastim was not routinely recommended as primary prophylaxis.
Data collection
The patients’ medical records were reviewed to collect baseline data regarding age, sex, ECOG PS, carbohydrate antigen 19-9 (CA19-9) concentration, tumor stage, and tumor site (head/neck versus body/tail). The original radiology reports were reviewed to collect data regarding treatment responses. The treatment response was evaluated every 4–6 cycles using computed tomography (CT) and/or magnetic resonance imaging (MRI). A normal baseline serum CA19-9 concentration was defined as ⩽37 U/mL, and testing was performed at baseline, at the start of second-line treatment, at the time of the best response assessment, and at the time of disease progression.
Survival outcomes
The various outcomes were compared for FOLFIRINOX versus FOLFOX-6, FOLFIRINOX versus FOLFIRI, and FOLFOX-6 versus FOLFIRI. The PFS interval was calculated from the start of second-line treatment (FOLFIRINOX, FOLFOX-6, or FOLFIRI) to the first instance of disease progression or death because of any cause. The OS1 interval was calculated from the start of first-line chemotherapy to death because of any cause. The OS2 interval was calculated from the start of second-line chemotherapy to death because of any cause. Radiological tumor responses were assessed using the RECIST 1.1 criteria. Toxicities were assessed at the start of each treatment cycle and graded according to version 4.0 of the Common Terminology Criteria for Adverse Events.
Statistical analysis
The Kaplan–Meier method and log-rank test were used to compare time-to-event outcomes (OS and PFS). Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. A Cox proportional hazards model was used to evaluate OS and PFS, and the results were reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Differences were considered statistically significant at p-values of < 0.05. All statistical analyses and graphical plotting were performed using GraphPad Prism software (version 8.0; San Diego, CA, USA) and RStudio software (Integrated Development for R; RStudio, Inc., Boston, MA, USA).
Results
Patient and treatment characteristics
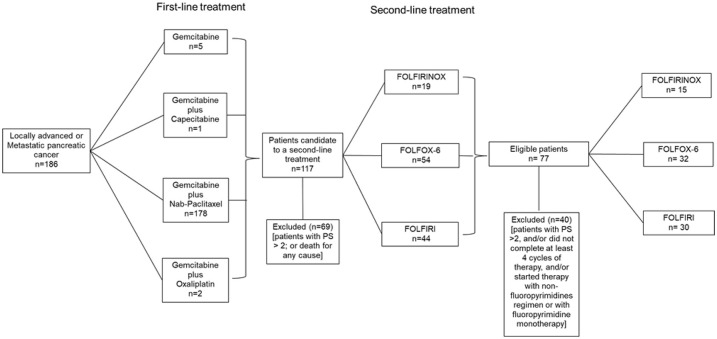
Between February 2013 and October 2019, 186 patients with locally advanced or metastatic PDAC were treated at the study centers. The first-line treatments involved gemcitabine monotherapy (5 patients), gemcitabine plus capecitabine (1 patient), gemcitabine plus nab-paclitaxel (178 patients), and gemcitabine plus oxaliplatin (2 patients). After disease progression, 69 patients (37%) were unable to receive second-line treatment because of premature death or poor performance status (ECOG PS of > 2). Second-line chemotherapy was offered to 117 patients (62%) at the discretion of the attending physician, which involved the FOLFIRINOX regimen (19 patients), the FOLFOX-6 regimen (54 patients), or the FOLFIRI regimen (44 patients). However, the present study only included patients who had completed ⩾4 chemotherapy cycles, which resulted in the FOLFIRINOX group including 15 patients, the FOLFOX-6 group including 32 patients, and the FOLFIRI group including 30 patients (Figure 1). Each treatment was continued until disease progression, unacceptable toxicity, or death because of any cause.
Figure 1.
Patient selection flowchart.
PS, performance status.
Most of the included patients (83%) had metastatic disease at the diagnosis, although seven patients (9%) had previously received neoadjuvant treatment and 10 patients (13%) had previously received adjuvant treatment. Table 1 shows the patients’ baseline demographic and disease characteristics. Almost all of the included patients (98.7%) had received first-line gemcitabine plus nab-paclitaxel, and only one patient in the FOLFOX-6 group had received first-line gemcitabine plus capecitabine (Supplemental Table S1).
Table 1.
Characteristic of patients at baseline.
| FOLFIRINOX n (%) |
FOLFOX-6 n (%) |
FOLFIRI n (%) |
p value | |
|---|---|---|---|---|
| Patients (n) | 15 | 32 | 30 | |
| Sex | 0.44 | |||
| • Male | 6 (40%) | 17 (53%) | 18 (60%) | |
| • Female | 9 (60%) | 15 (46%) | 12 (40%) | |
| Age | 0.01 | |||
| • Median–year | 56.2 | 63.1 | 65.2 | |
| • >65 year | 2 (13%) | 12 (37%) | 18 (60%) | |
| Neo-adjuvant chemotherapy | 0.45 | |||
| • Yes | 0 (0%) | 3 (9%) | 4 (13%) | |
| • No | 15 (100%) | 29 (90%) | 26 (86%) | |
| Adjuvant chemotherapy | 0.18 | |||
| • Yes | 0 (0%) | 4 (12%) | 6 (20%) | |
| • No | 15 (100%) | 28 (87%) | 24 (80%) | |
| ECOG I line | 0.40 | |||
| • 0 | 13 (86%) | 17 (53%) | 17 (56%) | |
| • 1 | 2 (13%) | 15 (46%) | 13 (43%) | |
| • 2 | 0 (0%) | 0 (0%) | 0 (0%) | |
| ECOG II line | 0.0002 | |||
| • 0 | 11 (73%) | 8 (25%) | 4 (13%) | |
| • 1 | 4 (26%) | 21 (65%) | 25 (83%) | |
| • 2 | 0 (0%) | 3 (9%) | 1 (3%) | |
| Biliary stent | 0.52 | |||
| • Yes | 3 (20%) | 3 (9%) | 3 (10%) | |
| • No | 12 (80%) | 29 (90%) | 27 (90%) | |
| Stage at diagnosis | 0.79 | |||
| • II–III | 1 (6%) | 3 (9%) | 4 (13%) | |
| • IV | 14 (93%) | 29 (90%) | 26 (86%) | |
| Tumor site | 0.66 | |||
| • Head/neck | 9 (60%) | 15 (46%) | 17 (56%) | |
| • Body/tail | 6 (40%) | 17 (53%) | 13 (43%) | |
| Baseline CA19.9 level | 0.80 | |||
| • </= 37 U/mL | 1 (6%) | 5 (15%) | 3 (10%) | |
| • > 37 U/mL | 12 (80%) | 26 (81%) | 24 (80%) | |
| • N/A | 2 (13) | 1 (3%) | 3 (10%) | |
| BR CA19.9 level | 0.61 | |||
| • </= 37 U/mL | 1 (6%) | 5 (15%) | 2 (6%) | |
| • > 37 U/mL | 11 (73%) | 24 (75%) | 25 (83%) | |
| • N/A | 3 (20%) | 3 (9%) | 3 (10%) | |
| PD CA19.9 level | 0.83 | |||
| • </= 37 U/mL | 0 (0%) | 2 (6%) | 3 (10%) | |
| • > 37 U/mL | 9 (60%) | 19 (59%) | 22 (73%) | |
| • N/A | 6 (40%) | 11 (34%) | 5 (16%) |
BR, best response; ECOG, Eastern Cooperative Oncology Group; N/A, not available; PD, progressive disease.
The main reasons for treatment discontinuation were disease progression and death because of any cause: 26% of patients in the FOLFIRINOX group, 40% of patients in the FOLFOX-6 group, and 73% of patients in the FOLFIRI group. In the FOLFIRINOX group, the 15 patients completed a total of 140 cycles, with a median treatment of 10 cycles (range: 6–16 cycles) and 81% of the patients received chemotherapy at 80% of the standard dose. In the FOLFOX-6 group, the 32 patients completed a total of 237 cycles, with a median treatment of 8.4 cycles (range: 4–18 cycles) and 91% of the patients received chemotherapy at 80% of the standard dose. In the FOLFIRI group, the 30 patients completed a total of 185 cycles, with a median treatment of 6.1 cycles (range: 4–17 cycles) and 80% of the patients received chemotherapy at 80% of the standard dose.
Efficacy
At the time of the analysis (31 October 2019), 68 patients had experienced a cancer-related event (local or distant progression) or death. The PFS analyses were based on 14 cancer-related events (93%) in the FOLFIRINOX group, 29 events (90%) in the FOLFOX-6 group, and 30 cancer-related events (100%) in the FOLFIRI group. The OS analyses were based on 10 events (66%) in the FOLFIRINOX group, 21 events (65%) in the FOLFOX-6 group, and 25 events (83%) in the FOLFIRI group. Patients without events at the time of analysis were censored based on the last informative follow-up.
The median PFS intervals, calculated from the start of second-line chemotherapy, were 26.29 weeks in the FOLFIRINOX group versus 23.07 weeks in the FOLFOX-6 group (p = 0.29) (Figure 2a) and 10.57 weeks in the FOLFIRI group (HR: 0.33, 95% CI: 0.18–0.60; p = 0.0001) (Figure 2b). There was no significant difference in the median OS1 intervals, calculated from the start of first-line chemotherapy, which were 103.7 weeks in the FOLFIRINOX, 76.29 weeks in the FOLFOX-6 group (Figure 3a), and 66.29 weeks in the FOLFIRI group (Figure 3b). The median OS2 intervals, calculated from the start of second-line chemotherapy, were 47.86 weeks in the FOLFIRINOX group versus 42.00 weeks in the FOLFOX-6 group (p = 0.37) (Figure 4a) and 25.00 weeks in the FOLFIRI group (HR: 0.48, 95% CI: 0.25–0.93; p = 0.038) (Figure 4b). The median PFS intervals were 23.07 weeks in the FOLFOX-6 group and 10.57 weeks in the FOLFIRI group (HR: 0.43, 95% CI: 0.25–0.75; p = 0.0005) (Figure 5a). The median OS2 intervals were 42.00 weeks in the FOLFOX-6 group and 25.00 weeks in the FOLFIRI group (p = 0.17) (Figure 5b).
Figure 2.
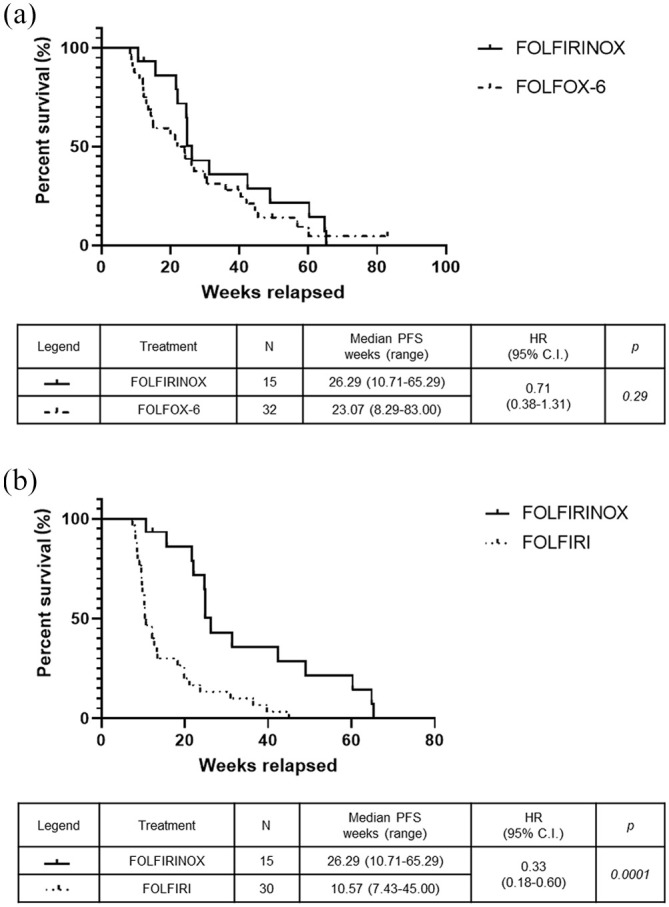
Kaplan–Meier PFS curves after starting second-line FOLFIRINOX, FOLFOX-6, or FOLFIRI. PFS for FOLFIRINOX versus FOLFOX-6 (a) and FOLFIRINOX versus FOLFIRI (b).
PFS, progression-free survival.
Figure 3.
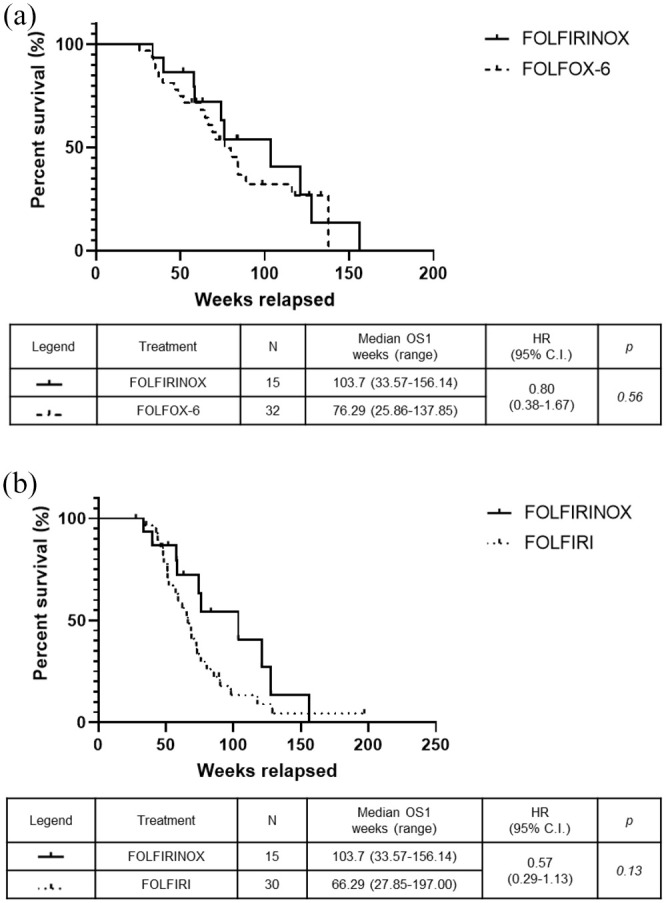
Kaplan–Meier OS1 for patients who received second-line FOLFIRINOX, FOLFOX-6, or FOLFIRI. OS1 for FOLFIRINOX versus FOLFOX-6 (a) and FOLFIRINOX versus FOLFIRI (b).
OS1, overall survival curves from the start of first-line treatment.
Figure 4.
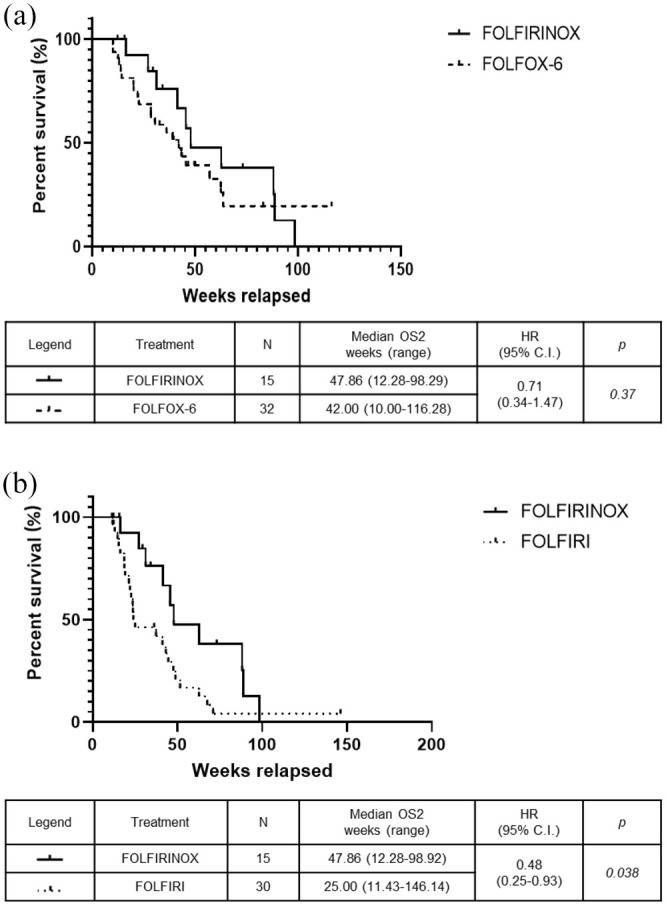
Kaplan–Meier OS2 using FOLFIRINOX, FOLFOX-6, or FOLFIRI. OS2 for FOLFIRINOX versus FOLFOX-6 (a) and FOLFIRINOX versus FOLFIRI (b).
OS2, overall survival curves from the start of second-line treatment.
Figure 5.
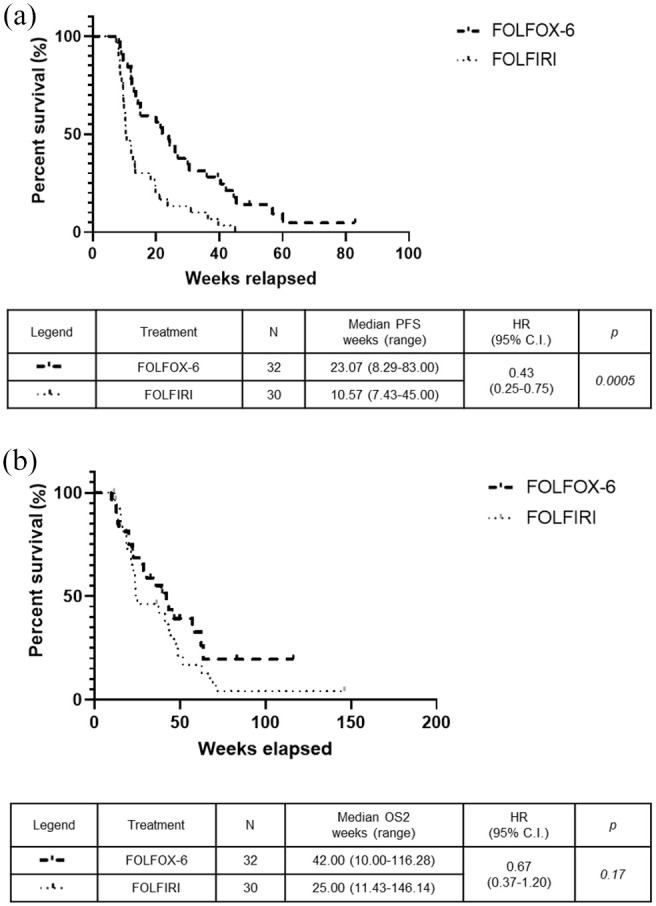
Kaplan–Meier curves for patients who received second-line FOLFOX-6 or FOLFIRI. The FOLFOX-6 and FOLFIRI groups were compared in terms of PFS (a) and OS2 (b).
OS2, overall survival curves from the start of second-line treatment; PFS, progression-free survival.
The disease control rate (DCR) was significantly higher in the FOLFIRINOX group (78%) than in the FOLFOX-6 group (59%) and in the FOLFIRI group (26%) (p = 0.004) (Table 2). The FOLFIRINOX group had a significantly better DCR than the FOLFIRI group (p = 0.004) but not relative to the FOLFOX-6 group (p = 0.51) (data not shown). The ORR was significantly higher in the FOLFIRINOX group (46%) than in the FOLFOX-6 group (18%) and in the FOLFIRI group (13%, p = 0.03) (Table 2). The FOLFIRINOX group had a significantly better ORR than the FOLFIRI group (p = 0.02) and tended to have a better ORR than the FOLFOX-6 group (p = 0.08). The FOLFOX-6 group had a significantly higher DCR than the FOLFIRI group (p = 0.01) (data not shown).
Table 2.
Responses to treatment.
| Variable | FOLFIRINOX (n = 15) |
FOLFOX-6 (n = 32) |
FOLFIRI (n = 30) |
p value |
|---|---|---|---|---|
| Response – n. (%) | ||||
| • Complete response | 0 (0%) | 0 (0%) | 0 (0%) | |
| • Partial response | 7 (46%) | 6 (18%) | 4 (13%) | |
| • Stable disease | 4 (26%) | 13 (40%) | 4 (13%) | |
| • Progressive disease | 3 (20%) | 13 (40%) | 22 (73%) | |
| • Could not be evaluated | 1 (6%) | 0 (0%) | 0 (0%) | |
| Rate of objective response | 0.033 | |||
| • n. (%) | 7 (46%) | 6 (18%) | 4 (13%) | |
| Rate of disease control | 0.004 | |||
| • n. (%) | 11 (78%) | 19 (59%) | 8 (26%) |
None of the patients’ characteristics independently influenced the outcomes in terms of PFS (Supplemental Figure S1), OS1 (Supplemental Figure S2), or OS2 (Supplemental Figure S3).
OS1, overall survival curves from the start of first-line treatment; OS2, overall survival curves from the start of second-line treatment; PFS, progression-free survival.
Adverse events
The most common grade 2 adverse events were anemia (FOLFIRINOX: 33%, FOLFOX-6: 22%, FOLFIRI: 20%), asthenia (FOLFIRINOX: 46%, FOLFOX-6: 56%, FOLFIRI: 36%), and neuropathy (FOLFIRINOX: 33%, FOLFOX-6: 28%, FOLFIRI: 16%). The most common grade 3–4 toxicities were neutropenia (FOLFIRINOX: 20%, FOLFOX-6: 6%, FOLFIRI: 0%) and thrombocytopenia (FOLFIRINOX: 6%, FOLFOX-6: 6%, FOLFIRI: 0%). There were no significant inter-group differences in terms of the adverse events (Table 3). Febrile neutropenia was not observed and none of the patients required secondary prophylaxis using GCS-F, although one patient each in the FOLFOX-6 and FOLFIRI groups received primary GCS-F prophylaxis. Hematological toxicity was the main reason for dose reductions and treatment delays.
Table 3.
Adverse events.
| Toxicities | FOLFIRINOX n. patients (%) |
FOLFOX-6 n patients (%) |
FOLFIRI n patients (%) |
p value |
|---|---|---|---|---|
| Anemia | 0.63 | |||
| • G2 | 5 (33%) | 7 (22%) | 6 (20%) | |
| • G3–G4 | 1 (6%) | 0 (0%) | 0 (0%) | |
| Neutropenia | 0.07 | |||
| • G2 | 0 (0%) | 4 (12%) | 2 (6%) | |
| • G3-G4 | 3 (20%) | 2 (6%) | 0 (0%) | |
| Thrombocytopenia | 0.57 | |||
| • G2 | 2 (13%) | 2 (6%) | 2 (6%) | |
| • G3–G4 | 1 (6%) | 2 (6%) | 0 (0%) | |
| Neuropathy | 0.99 | |||
| • G2 | 5 (33%) | 9 (28%) | 5 (16%) | |
| • G3–G4 | 1 (6%) | 1 (3%) | 0 (0%) | |
| Nausea | 0.09 | |||
| • G2 | 0 (0%) | 7 (22%) | 2 (6%) | |
| • G3–G4 | 1 (6%) | 0 (0%) | 0 (0%) | |
| Vomiting | 1 | |||
| • G2 | 2 (13%) | 3 (9%) | 0 (0%) | |
| • G3–G4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Elevated AST/ALT | 1 | |||
| • G2 | 1 (6%) | 1 (3%) | 0 (0%) | |
| • G3–G4 | 1 (6%) | 1 (3%) | 0 (0%) | |
| Diarrhea | 1 | |||
| • G2 | 2 (13%) | 5 (15%) | 4 (13%) | |
| • G3–G4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Asthenia | 0.81 | |||
| • G2 | 7 (46%) | 18 (56%) | 11 (36%) | |
| • G3–G4 | 0 (0%) | 2 (6%) | 2 (6%) |
Discussion
There is no consensus regarding second-line treatment for advanced pancreatic adenocarcinoma, although fluoropyrimidine-based regimens are typically offered to patients who experience progression during first-line gemcitabine-based treatment. The choice of second-line treatment is based on several variables, including age, performance status, comorbidities, best response to first-line chemotherapy, and toxicities at the end of the previous treatment. Patients with a good performance status typically receive a poly-chemotherapy regimen that includes combinations of 5-FU plus oxaliplatin or irinotecan, which provide favorable survival benefits.9–15 Thus, given the lack of consensus regarding second-line treatment, we retrospectively evaluated the survival outcomes of patients with metastatic PDAC that progressed during first-line gemcitabine-based chemotherapy and was subsequently treated using FOLFOX-6, FOLFIRI, or FOLFIRINOX.
The results revealed that FOLFIRINOX was associated with a significant PFS advantage relative to FOLFIRI (26.29 weeks versus 10.57 weeks; p = 0.0001). However, there was no significant difference in PFS between the FOLFIRINOX and FOLFOX-6 groups (26.29 weeks versus 23.07 weeks; p = 0.29). Furthermore, analysis of OS2 revealed that FOLFIRINOX provided longer OS2 than FOLFIRI (47.86 weeks versus 25.00 weeks; p = 0.038; HR: 0.48, 95% CI: 0.25–0.93). Nevertheless, there was no significant difference in OS2 between the FOLFIRINOX and FOLFOX-6 groups (47.86 versus 42.00 weeks; p = 0.37).
The results from our study were consistent with the historical data, although the present study revealed slightly better outcomes in terms of survival (PFS and OS2), DCR, ORR, and toxicity profile. For example, the main adverse events were grade 2 anemia, thrombocytopenia, neuropathy, vomiting, diarrhea, and asthenia, while neutropenia was the main grade 3 toxicity (FOLFIRINOX: 20%, FOLFOX-6: 6%, FOLFIRI: 0%). These results are likely related to the fact that the patients received very personalized dose/schedule selection and good supportive care for adverse events, as chemotherapy at a reduced dose (80%) was administered to most patients (FOLFIRINOX: 81%, FOLFOX-6: 91%, FOLFIRI: 80%). Moreover, none of the patients required further dose reductions, which suggests that careful treatment selection might have been related to the survival benefits and manageable toxicity profiles.
The efficacy and safety data for second-line FOLFIRINOX treatment is based on retrospective analyses,16,17 and a few recent phase II trials.18,19 The first phase I/II trial was conducted in 2017 to investigate second-line FOLFIRINOX treatment for metastatic pancreatic cancer after failure of first-line gemcitabine monotherapy. The FOLFIRINOX treatment was administered to 18 patients (median age: 63 years, ECOG PS: 0–1) and provided an ORR of 22.2% and a DCR of 61.1%, which is difficult to obtain in the second-line setting. Interestingly, the median OS2 (after starting FOLFIRINOX treatment) was 9.8 months and the median OS1 was 15.5 months, which suggests that the second-line treatment was responsible for most of the OS benefit.18 Those results subsequently led to FOLFIRINOX being evaluated as a first-line option.3
We compared the survival outcomes between the FOLFOX-6 and FOLFIRI groups, which revealed a significant PFS advantage for FOLFOX-6 relative to FOLFIRI (23.07 weeks versus 10.57 weeks; p = 0.0005, HR: 0.43, 95% CI: 0.25–0.75). However, the median OS2 was not significantly different between the two groups (42.00 weeks versus 25.00 weeks; p = 0.17), although the median advantage of 17 weeks would be considered clinically relevant. It is also worth noting that the OS2 analysis considered more events in the FOLFIRI group than in the FOLFOX-6 group (83% versus 65%, respectively). In addition, the FOLFOX-6 group had a significantly better DCR than the FOLFIRI group (59% versus 26%; p = 0.01). The only trial that has compared these chemotherapeutic schedules revealed favorable efficacy and toxicity profiles for both regimens, without any differences in survival outcomes.12 That phase II trial compared the FOLFIRI3 regimen with a modified-FOLFOX regimen (mFOLFOX) in 61 patients with advanced PDAC that progressed during first-line gemcitabine-based chemotherapy. There was no significant difference between the FOLFIRI3 and mFOLFOX groups in terms of the primary endpoint (6-month survival rate: 27% versus 30%) or in terms of the ORR (23% versus 17%), PFS (8.3 weeks versus 6 weeks), and OS (16.6 weeks versus 14.9 weeks). Nevertheless, the drug doses for both regimens did not reflect the current standard of care.12
The use of irinotecan for treating pancreatic cancer is supported by retrospective studies and prospective phase II trials.11,13–15 A nanoliposomal formulation of irinotecan was recently tested alone and in combination with 5FU in the randomized phase III NAPOLI-1 trial.20 That trial compared 5FU/LV alone versus Nal-IRI (nanoliposomal irinotecan) monotherapy or the combination of 5FU/LV plus Nal-IRI as second-line treatment for patients with metastatic PDAC. Patients in the 5FU/LV+ Nal-IRI group achieved longer OS than patients in the 5FU/LV group (6.1 months versus 4.2 months; p = 0.012, HR: 0.67), although no significant difference in OS was observed between the 5FU/LV and Nal-IRI monotherapy groups (4.2 months versus 4.9 months; p = 0.94, HR: 0.99).20 Data regarding the efficacy and safety of the 5FU/LV plus Nal-IRI regimen as second-line treatment for metastatic PDAC have gradually been accumulating. A recent comparison of 5FU plus Nal-IRI versus fluoropyrimidine-based chemotherapy plus oxaliplatin also revealed better survival outcomes and toxicity profiles for the 5FU plus Nal-IRI regimen.21 However, Nal-IRI still has not been approved by the Italian regulatory agency.
The present study also evaluated data regarding third-line treatments, which were administered to 60% of patients in the FOLFIRINOX group, 70% of patients in the FOLFIRI group, and 31% of patients in the FOLFOX-6 group. Nevertheless, survival outcomes remained poor despite the high proportion of patients who started third-line treatment. This may be related to the fact that few patients could tolerate third-line combination regimens, such as gemcitabine plus capecitabine, and few patients who received second-line FOLFOX-6 were able to start third-line FOLFIRI (and vice versa).
The present study failed to detect a significant global OS difference (i.e., in terms of OS1) between second-line FOLFIRINOX and FOLFOX-6 treatment of metastatic PDAC (103.7 weeks versus 76.29 weeks; p = 0.56) or between second-line FOLFIRINOX and FOLFIRI treatment (103.7 weeks versus 66.29 weeks; p = 0.13). Nevertheless, the FOLFIRINOX group had a clinically relevant survival advantage in terms of the median PFS and OS (>6 months), which may be related to the significantly better rates of DCR and ORR. Moreover, second-line FOLFIRINOX treatment appeared to have a manageable toxicity profile.
The present study has several limitations. First, the patients in the FOLFIRINOX group were significantly younger and had better ECOG PS at the beginning of the second-line treatment. Second, the small sample sizes in each group might explain the lack of a significant difference in the OS outcomes (FOLFIRINOX: 15 patients, FOLFIRI: 30 patients, FOLFOX-6: 32 patients). Third, we performed retrospective analysis of outcomes for second-line treatments that were selected based on physician and patient choices, rather than based on prospectively determined criteria. In addition, the second-line treatments would have been selected based on patient age, tolerability of the first-line treatment and its adverse events (e.g., neuropathy). These factors may explain why patients with a poor performance status (ECOG PS of 1–2) at the beginning of second-line treatment were more likely to receive the FOLFIRI or FOLFOX-6 regimens, as the proportion of patients with an ECOG PS of >0 were 86% for the FOLFIRI group, 73% for the FOLFOX-6 group, and 26% for the FOLFIRINOX group.
We are not aware of any trial that has compared second-line FOLFIRINOX with other chemotherapeutic schedules for metastatic PDAC. We are also not aware of any trials regarding second-line treatment options for patients with metastatic PDAC that progressed during first-line treatment using gemcitabine plus nab-paclitaxel. A recent retrospective study has compared fluoropyrimidine-oxaliplatin doublets (FOLFOX or XELOX) and fluoropyrimidine monotherapy (S-1) in this setting for Korean patients, although it failed to detect significant differences in PFS and OS.22 Therefore, to the best of our knowledge, ours is the first study to compare three second-line poly-chemotherapy regimens for advanced PDAC that progressed during first-line gemcitabine-based treatment. Thus, prospective trials are needed to investigate the role of second-line FOLFIRINOX treatment after failure of first-line gemcitabine-based treatment, and especially after failure of gemcitabine plus nab-paclitaxel, which is currently the standard of care in most countries.
Supplemental Material
Supplemental material, Supplementary_Materials_1 for FOLFIRINOX after first-line gemcitabine-based chemotherapy in advanced pancreatic cancer: a retrospective comparison with FOLFOX and FOLFIRI schedules by Francesca Foschini, Fabiana Napolitano, Alberto Servetto, Roberta Marciano, Eleonora Mozzillo, Anna Chiara Carratù, Antonio Santaniello, Pietro De Placido, Priscilla Cascetta, Giovanni Butturini, Isabella Frigerio, Paolo Regi, Nicola Silvestris, Sabina Delcuratolo, Enrico Vasile, Caterina Vivaldi, Cataldo Bianco, Sabino De Placido, Luigi Formisano and Roberto Bianco in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Author contributions: Conceptualization, Roberto Bianco, Luigi Formisano, Francesca Foschini, Fabiana Napolitano and Alberto Servetto; methodology, Roberto Bianco, Luigi Formisano; software and formal analysis, Luigi Formisano; validation, Roberto Bianco; investigation, Alberto Servetto, Roberta Marciano, Antonio Santaniello, Giovanni Butturini, Isabella Frigerio, Paolo Regi, Nicola Silvestris, Sabina Delcuratolo, Enrico Vasile, Caterina Vivaldi, Cataldo Bianco, Eleonora Mozzillo, Anna Chiara Carratù, Francesca Foschini, Fabiana Napolitano, Priscilla Cascetta, Pietro De Placido, Luigi Formisano, Roberto Bianco; resources, Roberto Bianco, Luigi Formisano, Sabino De Placido; data curation, Roberto Bianco, Luigi Formisano, Francesca Foschini; writing—original draft preparation, Francesca Foschini; writing—review and editing, Luigi Formisano, Roberto Bianco; supervision, Sabino De Placido; funding acquisition, Roberto Bianco, Luigi Formisano.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Luigi Formisano was supported by AIRC MFAG2018 grant number 21505. Roberto Bianco was supported by AIRC IG2018 grant number 21339. This study was supported by the Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO).
ORCID iD: Francesca Foschini  https://orcid.org/0000-0001-5389-7390
https://orcid.org/0000-0001-5389-7390
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Francesca Foschini, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Fabiana Napolitano, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Alberto Servetto, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Roberta Marciano, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Eleonora Mozzillo, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Anna Chiara Carratù, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Antonio Santaniello, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Pietro De Placido, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Priscilla Cascetta, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Giovanni Butturini, Pancreatic Surgery Unit, Pederzoli Hospital, Peschiera del Garda, Verona, Italy.
Isabella Frigerio, Pancreatic Surgery Unit, Pederzoli Hospital, Peschiera del Garda, Verona, Italy.
Paolo Regi, Pancreatic Surgery Unit, Pederzoli Hospital, Peschiera del Garda, Verona, Italy.
Nicola Silvestris, Medical Oncology Unit, IRCCS Istituto Tumori Giovanni Paolo II of Bari, Bari, Italy; Department of Biomedical Science and Human Oncology, University of Bari Aldo Moro, Bari, Italy.
Sabina Delcuratolo, Medical Oncology Unit, IRCCS Istituto Tumori Giovanni Paolo II of Bari, Bari, Italy.
Enrico Vasile, Unit of Oncology 2, University Hospital of Pisa, Italy.
Caterina Vivaldi, Unit of Oncology 2, University Hospital of Pisa, Italy.
Cataldo Bianco, Department of Experimental and Clinical Medicine, University of Catanzaro Magna Graecia, Catanzaro, Italy.
Sabino De Placido, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, 80131 Naples, Italy.
Luigi Formisano, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, Via Pansini 5, Naples, 80131, Italy.
Roberto Bianco, Department of Clinical Medicine and Surgery, Oncology Division, University of Naples Federico II, Via Pansini 5, Naples, 80131, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 4. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho IR, Kang H, Jo JH, et al. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: single-center cohort study. World J Gastrointest Oncol 2020; 12: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pusceddu S, Ghidini M, Torchio M, et al. Comparative effectiveness of gemcitabine plus nab-paclitaxel and FOLFIRINOX in the first-line setting of metastatic pancreatic cancer: a systematic review and meta-analysis. Cancers (Basel) 2019; 11: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 8. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011; 47: 1676–1681. [DOI] [PubMed] [Google Scholar]
- 9. Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 10. Gill S, Ko YJ, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 11. Taïeb J, Lecomte T, Aparicio T, et al. FOLFIRI.3, a new regimen combining 5-fluorouracil, folinic acid and irinotecan, for advanced pancreatic cancer: results of an Association des Gastro-Entérologues Oncologues (Gastroenterologist Oncologist Association) multicenter phase II study. Ann Oncol 2007; 18: 498–503. [DOI] [PubMed] [Google Scholar]
- 12. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009; 101: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuzillet C, Hentic O, Rousseau B, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol 2012; 18: 4533–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gebbia V, Maiello E, Giuliani F, et al. Irinotecan plus bolus/infusional 5-fluorouracil and leucovorin in patients with pretreated advanced pancreatic carcinoma: a multicenter experience of the Gruppo Oncologico Italia Meridionale. Am J Clin Oncol 2010; 33: 461–464. [DOI] [PubMed] [Google Scholar]
- 15. Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012; 69: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 16. Assaf E, Verlinde-Carvalho M, Delbaldo C, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011; 80: 301–306. [DOI] [PubMed] [Google Scholar]
- 17. Lee MG, Lee SH, Lee SJ, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. Epub ahead of print 18 January 2014. DOI: 10.1159/000356158. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi N, Shimamura T, Tokuhisa M, et al. Effect of FOLFIRINOX as second-line chemotherapy for metastatic pancreatic cancer after gemcitabine-based chemotherapy failure. Medicine (Baltimore) 2017; 96: e6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung MJ, Kang H, Kim HG, et al. Multicenter phase II trial of modified FOLFIRINOX in gemcitabine-refractory pancreatic cancer. World J Gastrointest Oncol 2018; 10: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 21. Kieler M, Unseld M, Bianconi D, et al. A real-world analysis of second-line treatment options in pancreatic cancer: liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther Adv Med Oncol 2019; 11: 1758835919853196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee K, Bang K, Yoo C, et al. Clinical outcomes of second-line chemotherapy after progression on nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic adenocarcinoma. Cancer Res Treat 2020; 52: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_1 for FOLFIRINOX after first-line gemcitabine-based chemotherapy in advanced pancreatic cancer: a retrospective comparison with FOLFOX and FOLFIRI schedules by Francesca Foschini, Fabiana Napolitano, Alberto Servetto, Roberta Marciano, Eleonora Mozzillo, Anna Chiara Carratù, Antonio Santaniello, Pietro De Placido, Priscilla Cascetta, Giovanni Butturini, Isabella Frigerio, Paolo Regi, Nicola Silvestris, Sabina Delcuratolo, Enrico Vasile, Caterina Vivaldi, Cataldo Bianco, Sabino De Placido, Luigi Formisano and Roberto Bianco in Therapeutic Advances in Medical Oncology