Abstract
Background
There are limited data on the seasonal variation in acute myocardial infarction (AMI) in the contemporary literature.
Hypothesis
There would be decrease in the seasonal variation in the management and outcomes of AMI.
Methods
Adult (>18 years) AMI admissions were identified using the National Inpatient Sample (2000‐2017). Seasons were classified as spring, summer, fall, and winter. Outcomes of interest included prevalence, in‐hospital mortality, use of coronary angiography, and percutaneous coronary intervention (PCI). Subgroup analyses for type of AMI and patient characteristics were performed.
Results
Of the 10 880 856 AMI admissions, 24.3%, 22.9%, 22.2%, and 24.2% were admitted in spring, summer, fall, and winter, respectively. The four cohorts had comparable age, sex, race, and comorbidities distribution. Rates of coronary angiography and PCI were slightly but significantly lower in winter (62.6% and 40.7%) in comparison to the other seasons (64‐65% and 42‐43%, respectively) (P < .001). Compared to spring, winter admissions had higher in‐hospital mortality (adjusted odds ratio [aOR]: 1.07; 95% confidence interval [CI]: 1.06‐1.08), whereas summer (aOR 0.97; 95% CI 0.96‐0.98) and fall (aOR 0.98; 95% CI 0.97‐0.99) had slightly lower in‐hospital mortality (P < .001). ST‐segment elevation (10.0% vs 9.1%; aOR 1.07; 95% CI 1.06‐1.08) and non‐ST‐segment elevation (4.7% vs 4.2%; aOR 1.07; 95% CI 1.06‐1.09) AMI admissions in winter had higher in‐hospital mortality compared to spring (P < .001). The primary results were consistent when stratified by age, sex, race, geographic region, and admission year.
Conclusions
Compared to other seasons, winter admission was associated with higher in‐hospital mortality in AMI in the United States.
Keywords: acute myocardial infarction, healthcare disparities, outcomes research, season, winter
Abbreviations
- AMI
acute myocardial infarction
- CI
confidence interval
- GWTG‐CAD
Get With The Guideline‐Coronary Artery Disease
- HCUP
Healthcare Cost and Utilization Project
- ICD‐10CM
International Classification of Diseases‐10 Clinical Modification
- ICD‐9CM
International Classification of Diseases‐9 Clinical Modification
- NIS
National/Nationwide Inpatient Sample
- NSTEMI
non‐ST‐segment elevation myocardial infarction
- OR
odds ratio
- PCI
percutaneous coronary intervention
- STEMI
ST‐segment elevation myocardial infarction
1. INTRODUCTION
Studies evaluating the chronobiology of acute myocardial infarction (AMI) have reported a circadian and seasonal periodicity for the incidence of AMI. 1 , 2 , 3 , 4 , 5 , 6 Reports from large national registries have shown seasonal variations in AMI‐related mortality with the majority reporting increased incidence and in‐hospital mortality during the winter months compared to other seasons. 4 , 5 , 6 Subsequent studies have evaluated the impact of daily weather and environmental conditions in well‐defined geographical areas further establishing the role of weather as a potential trigger for cardiovascular diseases. 7 , 8 However, only a selected few studies evaluated the seasonal association of AMI stratified by type of AMI (ST‐segment elevation myocardial infarction [STEMI] vs non‐ST‐segment elevation myocardial infarction [NSTEMI]). 9 , 10 Furthermore, several reports have shown that similarities exist between seasonal patterns of AMI and influenza infection. 11 , 12 Therefore through this study, we sought to assess the seasonal variations in clinical outcomes of AMI using an extensive national database over 18 years while comparing these differences in STEMI and NSTEMI populations. We hypothesized that with advances in healthcare deliveries there would be decrease in the seasonal variation in the management and outcomes of AMI.
2. MATERIAL AND METHODS
2.1. Study population, variables, and outcomes
The National (Nationwide) Inpatient Sample (NIS) is the largest all‐payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Quality and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality. 13 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP‐NIS does not capture individual patients but captures all information for a given admission. Institutional Review Board approval was not sought due to the publicly available nature of this de‐identified database. These data are available to other authors via the HCUP‐NIS database with the Agency for Healthcare Research and Quality.
Using the HCUP‐NIS data from 2000 to 2017, a retrospective cohort study of adult admissions (>18 years) with AMI in the primary diagnosis field (International Classification of Diseases 9.0 Clinical Modification [ICD‐9CM] 410.x and ICD‐10CM I21.x‐22.x) were identified. Similar to prior literature, we defined the seasons based on the meteorological classification of the Northern Hemisphere as—Spring (March‐May), Summer (June‐August), Fall (September‐November), and Winter (December‐February). 14 We excluded admissions that did not have information on admission month. The Deyo's modification of the Charlson Comorbidity Index was used to identify the burden of comorbid diseases (Table S1). 15 Demographic characteristics, hospital characteristics, acute organ failure, mechanical circulatory support, cardiac procedures, and noncardiac organ support use were identified for all admissions using previously used methodologies from our group. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 The four geographic regions included the Northeast, Midwest, South, and West as classified by the HCUP‐NIS. 35 Similar to prior literature, we defined early coronary angiography as that performed on the day of hospital admission (day 0). 31 , 42 , 43 We identified timing of coronary angiography and percutaneous coronary intervention (PCI) relative to the day of admission. 18 , 31 , 41 , 42
The primary outcome was the seasonal variation in the prevalence of AMI and the in‐hospital mortality in admissions with AMI. The secondary outcomes included receipt of coronary angiography, PCI and mechanical circulatory support, hospital length of stay, hospitalization costs, and discharge disposition. Stratified analyses were performed for type of AMI (STEMI vs NSTEMI) and patient characteristics (age, sex, race, tertile of study period, and geographic region).
2.2. Statistical analysis
As recommended by HCUP‐NIS, survey procedures using discharge weights provided with HCUP‐NIS database were used to generate national estimates. 48 Using the trend weights provided by the HCUP‐NIS, samples from 2000 to 2011 were reweighted to adjust for the 2012 HCUP‐NIS redesign. 48 Chi‐square and t tests were used to compare categorical and continuous variables, respectively. Multivariable logistic regression was used to analyze trends over time (referent year 2000). Univariable analysis for trends and outcomes was performed and was represented as odds ratio (OR) with 95% confidence interval (CI). Multivariable logistic regression analysis incorporating age, sex, race, primary payer status, weekend admission, socioeconomic stratum, hospital characteristics, comorbidities, organ failure, AMI‐type, cardiac procedures, and noncardiac procedures was performed for assessing temporal trends of prevalence and in‐hospital mortality. To confirm the results of the primary analysis, multiple subgroup analyses stratifying by age, sex, race, tertiles of study period, type of AMI, and geographic region were performed. For the multivariable modeling, regression analysis with purposeful selection of statistically (liberal threshold of P < .20 in univariate analysis) and clinically relevant variables was conducted. Two‐tailed P < .05 was considered statistically significant. All statistical analyses were performed using SPSS v25.0 (IBM Corp, Armonk, New York).
Best practices relating to the use of the HCUP‐NIS database, such as not assessing individual hospital‐level volumes (due to changes to sampling design detailed above), treating each entry as an “admission” as opposed to individual patients, restricting the study details to inpatient factors since the HCUP‐NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies, were adhered to during data analysis. 48
3. RESULTS
In the period from 1 January 2000 to 31 December 2017, there were 11 622 528 admissions for AMI, of which, 741 672 did not have data on the month of admission and were excluded. Among the final cohort of 10 880 856 (93.6%) admissions, 2 826 906 (24.3%), 2 660 729 (22.9%), 2 577 885 (22.2%), and 2 815 336 (24.2%), respectively were admitted in spring, summer, fall, and winter (Figure S1). The 18‐year temporal trends of AMI admissions showed a consistent increase in NSTEMI admissions with a concomitant decrease in STEMI rates during this study period without significant differences between the seasons (Figure 1A,B). The four cohorts had comparable distribution of STEMI vs NSTEMI, age, sex, race, insurance, socioeconomic status, and comorbidity (Table 1). NSTEMI admissions comprised 62% to 63% of all admissions across the four seasons during the study period. There were no clinically meaningful differences in the hospital characteristics (location, teaching status, bed‐size, and region) between the four cohorts (Table 1). Cardiac arrest, cardiogenic shock, and multiorgan failure were noted in about 5%, 5%, and 9%, respectively across the seasons and were comparable across the four cohorts.
FIGURE 1.
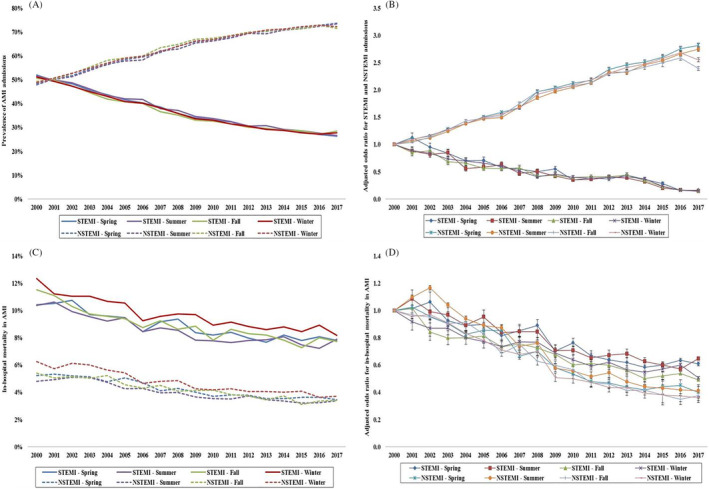
Trends in the prevalence and in‐hospital mortality in AMI admissions stratified by type of AMI. A, Unadjusted temporal trends of the proportion of AMI admissions stratified by type of AMI during spring, summer, fall, and winter (P < .001 for trend over time). B, Adjusted odds ratio for STEMI and NSTEMI weekend admissions by year (with 2000 as the referent); adjusted for age, sex, race, comorbidity, primary payer, socioeconomic status, STEMI location, hospital region, hospital location and teaching status, and hospital bed‐size (P < .001 for trend over time). C, Unadjusted in‐hospital mortality in AMI admissions stratified by type of AMI during spring, summer, fall, and winter (P < .001 for trend over time). D, Adjusted odds ratio for in‐hospital mortality by year (with 2000 as the referent) in AMI admissions stratified by type of AMI and weekend vs weekday admission; adjusted for age, sex, race, comorbidity, primary payer, hospital region, hospital location and teaching status, hospital bed‐size, weekend admission, multiorgan failure, cardiogenic shock, cardiac arrest, coronary angiography, PCI, pulmonary artery catheterization, mechanical circulatory support, invasive mechanical ventilation, and acute hemodialysis (P < .001 for trend over time). AMI, acute myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction
TABLE 1.
Baseline and clinical characteristics of AMI admissions stratified by seasons
| Characteristic | Spring (N = 2 826 906) | Summer (N = 2 660 729) | Fall (N = 2 577 885) | Winter (N = 2 815 336) | P |
|---|---|---|---|---|---|
| Type of AMI | <.001 | ||||
| STEMI | 37.1 | 37.4 | 37.1 | 37.1 | |
| NSTEMI | 62.9 | 62.6 | 62.9 | 62.9 | |
| Age (y) | 67.4 ± 14.2 | 67.0 ± 14.3 | 67.5 ± 14.2 | 67.9 ± 14.2 | <.001 |
| Female sex | 39.5 | 39.5 | 40.0 | 39.9 | <.001 |
| Race | <.00 | ||||
| White | 63.1 | 62.8 | 62.4 | 63.1 | |
| Black | 7.9 | 8.0 | 7.9 | 7.8 | 1 |
| Others a | 29.0 | 29.2 | 29.7 | 29.1 | |
| Primary payer | <.001 | ||||
| Medicare | 57.2 | 56.2 | 57.4 | 58.2 | |
| Medicaid | 6.3 | 6.4 | 6.2 | 6.1 | |
| Private | 28.3 | 28.8 | 28.1 | 27.6 | |
| Others b | 8.1 | 8.5 | 8.4 | 8.1 | |
| Quartile of median household income for zip code | <.001 | ||||
| 0‐25th | 24.6 | 24.5 | 24.1 | 24.4 | |
| 26th‐50th | 26.9 | 26.9 | 26.9 | 26.9 | |
| 51st‐75th | 24.2 | 24.3 | 24.3 | 24.3 | |
| 75th‐100th | 24.2 | 24.3 | 24.8 | 24.4 | |
| Charlson Comorbidity Index | <.001 | ||||
| 0–3 | 38.7 | 40.2 | 36.4 | 36.6 | |
| 4–6 | 43.8 | 43.1 | 44.8 | 44.9 | |
| ≥7 | 17.5 | 16.7 | 18.7 | 18.5 | |
| Prior coronary artery bypass grafting | 7.8 | 7.7 | 7.8 | 7.9 | <.001 |
| Hospital teaching status and location | <.001 | ||||
| Rural | 11.6 | 11.5 | 11.6 | 11.6 | |
| Urban nonteaching | 37.4 | 37.3 | 37.9 | 38.2 | |
| Urban teaching | 51.0 | 51.2 | 50.5 | 50.2 | |
| Hospital bed‐size | <.001 | ||||
| Small | 11.9 | 11.6 | 11.5 | 11.8 | |
| Medium | 26.1 | 25.9 | 25.8 | 26.1 | |
| Large | 62.0 | 62.4 | 62.7 | 62.1 | |
| Hospital region | <.001 | ||||
| Northeast | 20.8 | 20.9 | 21.0 | 21.0 | |
| Midwest | 24.6 | 24.7 | 24.5 | 23.9 | |
| South | 36.1 | 36.1 | 35.9 | 36.2 | |
| West | 18.4 | 18.3 | 18.5 | 18.9 | |
| Tertile of admission years | <.001 | ||||
| 2000‐2005 | 34.9 | 34.7 | 36.9 | 36.2 | |
| 2006‐2011 | 30.1 | 29.9 | 31.3 | 30.4 | |
| 2012‐2017 | 35.0 | 35.4 | 31.8 | 33.4 | |
| Weekend admission | 25.8 | 25.9 | 25.8 | 25.7 | <.001 |
| Fibrinolytic therapy (STEMI only) | 4.7 | 4.6 | 4.5 | 4.7 | <.001 |
| Coronary artery bypass grafting | 9.4 | 9.1 | 9.2 | 9.2 | <.001 |
| Cardiac arrest | 4.9 | 5.0 | 5.1 | 5.1 | <.001 |
| Cardiogenic shock | 4.9 | 4.8 | 4.8 | 4.9 | <.001 |
| Multiorgan failure | 9.4 | 9.2 | 9.3 | 9.8 | <.001 |
| Respiratory infections | |||||
| Influenza | 0.1 | 0.0 | 0.0 | 0.4 | <.001 |
| Pneumonia | 6.9 | 5.6 | 6.3 | 8.0 | <.001 |
| Fibrinolysis | 2.2 | 2.2 | 2.1 | 2.2 | <.001 |
| Pulmonary artery catheterization | 1.1 | 1.0 | 1.0 | 1.1 | <.001 |
| Invasive mechanical ventilation | 5.9 | 5.7 | 5.8 | 6.2 | <.001 |
| Acute hemodialysis | 0.5 | 0.5 | 0.6 | 0.6 | <.001 |
Note: Represented as percentage or mean ± SD.
Abbreviations: AMI, acute myocardial infarction; NSTEMI, non‐ST‐segment‐elevation myocardial infarction; STEMI, ST‐segment‐elevation myocardial infarction.
Hispanic, Asian or Pacific Islander, Native American, others.
Self‐pay, no charge, others.
Rates of coronary angiography and PCI were slightly but significantly lower in winter (62.6% and 40.7%) in comparison to the other three seasons (64%‐65% and 42%‐43%, respectively) (P < .001) (Table 2). During the 18‐year study period, the STEMI admissions underwent comparable rates of coronary angiography and PCI (Figure 2A,C), whereas the NSTEMI admissions in winter received consistently lower use of both procedures compared to other seasons (Figure 2B,D). The use of mechanical circulatory support was comparable during the seasons (Table 2). Hospital costs, length of hospital stay, and discharge dispositions were similar across seasons (Table 2).
TABLE 2.
Clinical outcomes of AMI admissions stratified by seasons
| Characteristic | Spring (N = 2 826 906) | Summer (N = 2 660 729) | Fall (N = 2 577 885) | Winter (N = 2 815 336) | P |
|---|---|---|---|---|---|
| Coronary angiography | 64.0 | 65.0 | 64.1 | 62.6 | <.001 |
| Percutaneous coronary intervention | 42.0 | 43.0 | 42.1 | 40.7 | <.001 |
| Mechanical circulatory support | 4.9 | 4.7 | 4.8 | 4.8 | <.001 |
| In‐hospital mortality | 6.0 | 5.8 | 6.1 | 6.7 | <.001 |
| Length of stay (days) | 5.1 ± 5.8 | 4.9 ± 5.6 | 5.0 ± 5.9 | 5.2 ± 6.0 | <.001 |
| Hospitalization costs (×1000 USD) | 60 ± 79 | 60 ± 77 | 60 ± 79 | 60 ± 81 | <.001 |
| Discharge disposition | <.001 | ||||
| Home | 62.9 | 64.0 | 63.2 | 62.1 | |
| Transfer | 12.4 | 12.2 | 12.4 | 12.4 | |
| Skilled nursing facility | 13.2 | 12.8 | 13.2 | 14.0 | |
| Home with HHC | 10.6 | 10.1 | 10.3 | 10.7 | |
| Against medical advice | 0.9 | 0.9 | 0.9 | 0.8 |
Note: Represented as percentage or mean ± SD.
Abbreviations: AMI, acute myocardial infarction; HHC, home healthcare; USD, United States Dollars.
FIGURE 2.
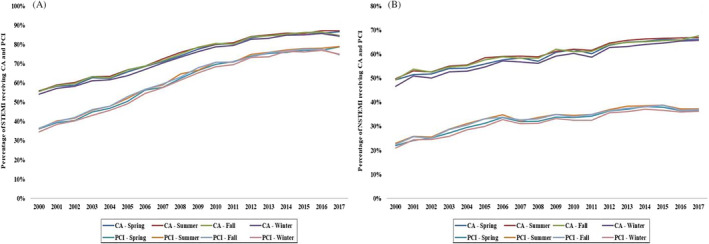
Temporal trends in the use of CA and PCI stratified by type of AMI. Eighteen‐year temporal trends in the use of CA and PCI in STEMI (A) and NSTEMI (B); all P < .001 for trend over time. AMI, acute myocardial infarction; CA, coronary angiography; NSTEMI, non‐ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction
The AMI admissions in winter had slightly higher in‐hospital mortality 6.7% compared to other seasons 5.8% to 6.1% (P < .001). During this 18‐year period, winter admissions had consistently higher in‐hospital mortality compared to other seasons in both STEMI and NSTEMI subgroups (Figure 1C,D). In a multivariable analysis, with spring as the referent category, AMI admissions in winter had slightly higher adjusted in‐hospital mortality (OR 1.07; 95% CI 1.06‐1.08), whereas those in summer (OR 0.97; 95% CI 0.97‐0.98) and fall (OR 0.98; 95% CI 0.98‐0.99) had slightly lower in‐hospital mortality (P < .001) (Table S2). These results were largely consistent in the subgroup analyses. Winter AMI admissions had higher in‐hospital mortality in both STEMI and NSTEMI admissions (Table 3). When stratifying by age, sex, and race, compared to spring, summer admissions had lower in‐hospital mortality whereas winter admissions had higher in‐hospital mortality (Table 3). When stratified by tertiles of study period, these differences became less pronounced over time (Table 3). The primary outcome did not differ between the four geographic regions (Table 3).
TABLE 3.
In‐hospital mortality in AMI admissions stratified by patient characteristics
| Patient characteristics a | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Type of AMI | ||||
| STEMI | ||||
| Spring | Reference category | |||
| Summer | 0.97 | 0.97 | 0.98 | <.001 |
| Fall | 0.98 | 0.98 | 0.99 | <.001 |
| Winter | 1.07 | 1.06 | 1.08 | <.001 |
| NSTEMI | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.97 | 0.99 | .003 |
| Fall | 1.00 | 0.99 | 1.01 | .64 |
| Winter | 1.07 | 1.06 | 1.09 | <.001 |
| Age group | ||||
| ≤75 years | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.96 | 0.99 | <.001 |
| Fall | 0.97 | 0.95 | 0.98 | <.001 |
| Winter | 1.07 | 1.06 | 1.08 | <.001 |
| >75 years | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.96 | 0.99 | <.001 |
| Fall | 1.00 | 0.99 | 1.01 | .88 |
| Winter | 1.07 | 1.06 | 1.08 | <.001 |
| Sex | ||||
| Male | ||||
| Spring | Reference category | |||
| Summer | 0.97 | 0.96 | 0.98 | <.001 |
| Fall | 0.99 | 0.98 | 1.01 | .18 |
| Winter | 1.08 | 1.06 | 1.09 | <.001 |
| Female | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.97 | 0.99 | .003 |
| Fall | 0.98 | 0.97 | 0.99 | <.001 |
| Winter | 1.06 | 1.05 | 1.08 | <.001 |
| Race | ||||
| White | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.97 | 0.99 | <.001 |
| Fall | 0.99 | 0.98 | 0.99 | .04 |
| Winter | 1.07 | 1.05 | 1.08 | <.001 |
| Non‐White b | ||||
| Spring | Reference category | |||
| Summer | 0.97 | 0.96 | 0.98 | <.001 |
| Fall | 0.98 | 0.96 | 0.99 | .003 |
| Winter | 1.08 | 1.06 | 1.09 | <.001 |
| Tertiles of study period | ||||
| 2000‐2005 | ||||
| Spring | Reference category | |||
| Summer | 0.99 | 0.99 | 1.01 | .80 |
| Fall | 1.02 | 1.00 | 1.03 | .01 |
| Winter | 1.10 | 1.08 | 1.11 | <.001 |
| 2006‐2011 | ||||
| Spring | Reference category | |||
| Summer | 0.95 | 0.94 | 0.97 | <.001 |
| Fall | 0.99 | 0.97 | 1.01 | .22 |
| Winter | 1.06 | 1.04 | 1.08 | <.001 |
| 2012‐2017 | ||||
| Spring | Reference category | |||
| Summer | 0.97 | 0.95 | 0.98 | <.001 |
| Fall | 0.94 | 0.92 | 0.96 | <.001 |
| Winter | 1.04 | 1.03 | 1.06 | <.001 |
| Geographic region | ||||
| Northeast | ||||
| Spring | Reference category | |||
| Summer | 0.97 | 0.95 | 0.98 | <.001 |
| Fall | 0.98 | 0.96 | 0.99 | .02 |
| Winter | 1.08 | 1.06 | 1.10 | <.001 |
| Midwest | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.96 | 0.99 | .007 |
| Fall | 0.98 | 0.97 | 1.00 | .05 |
| Winter | 1.08 | 1.06 | 1.09 | <.001 |
| South | ||||
| Spring | Reference category | |||
| Summer | 0.98 | 0.96 | 0.99 | .001 |
| Fall | 0.98 | 0.97 | 0.99 | .02 |
| Winter | 1.07 | 1.05 | 1.09 | <.001 |
| West | ||||
| Spring | Reference category | |||
| Summer | 0.99 | 0.97 | 1.01 | 0.16 |
| Fall | 1.00 | 0.98 | 1.02 | 0.71 |
| Winter | 1.05 | 1.03 | 1.07 | <.001 |
Abbreviations: AMIE, acute myocardial infarction; NSTEMI, non‐ST‐segment‐elevation myocardial infarction; STEMI, ST‐segment‐elevation myocardial infarction.
Each subgroup was adjusted for age, sex, race, insurance status, socioeconomic stratum, hospital characteristics, comorbidities, year of admission, weekend admission, cardiogenic shock, cardiac arrest, multiorgan failure, respiratory infections, coronary angiography, percutaneous coronary intervention, pulmonary artery catheterization, mechanical circulatory support, and invasive mechanical ventilation.
Black, Hispanic, Asian, Native American, others.
4. DISCUSSION
In the largest study evaluating seasonal effect on the management and outcomes of nearly 11 million AMI admissions, we noted winter admissions with AMI to have higher in‐hospital mortality which was more pronounced in the NSTEMI population. Despite comparable baseline characteristics and acuity of illness, the AMI admissions in winter had slightly, but statistically significant, lower rates of coronary angiography and PCI use. These disparities were persistent during the 18‐year study period, however, were less pronounced over time. These results were consistent in both STEMI and NSTEMI and across all patient demographics.
To date, in addition to several small cohort‐studies, 49 , 50 , 51 reports from large national databases of various countries have demonstrated a seasonal pattern to the incidence of AMI. 4 , 5 , 6 In the United States, two different studies from the National Registry of Myocardial Infarction have reported a higher number of AMI cases in winter and a significantly lower number in summers. 4 , 5 However, these data was obtained from about 15% of all acute medical/surgical hospitals in the United States, which limits the generalizability. 5 Subsequently, Patel et al evaluated seasonal variations in AMI incidence using the HCUP‐NIS database from 2000 to 2011 and identified a marked increase in AMI hospitalizations during winter among the elderly population but found no such association in those younger than 65 years. 52 More recently, data from Get With The Guideline‐Coronary Artery Disease (GWTG‐CAD) Program, which included a significant proportion of patients with AMI in the United States from 2003 to 2008, showed evidence of a seasonal variation in the incidence of AMI. However, the seasonal pattern was limited only to those with NSTEMI and was not significant in STEMI patients. Besides, they also reported that seasonal variation with winter predominance was identified only in the warmer states of the country. 9 These reports suggest that seasonal variation in AMI incidence may not be as uniform across the nation as previously believed. In comparison to these studies, our data did not show differences in the overall prevalence of AMI when evaluated by seasons. Due to the differences in the inclusion criteria (ie, AMI vs all acute coronary syndromes including unstable angina), the nationally representative nature of this study, the large sample size of our population and the evolution of medical therapy for AMI might explain some of these differences.
These differences may partly be explained by the inclusion criteria for these cohorts and patient selection. Our study represents the largest national cohort and spans over a significantly longer duration. Additionally, while we identified patients based on ICD codes, studies from NRMI and GWTG‐CAD used clinical findings in conjunction with ICD codes. Furthermore, it is important to consider the inclusion of all acute coronary syndrome patients (ie, including unstable angina) vs only those with a true AMI. Moreover, another population‐based study using clinical findings to identify AMI reported a lack of seasonal variation in incident AMI cases. 8 Discrepancies with results from other countries could potentially be explained by the fact that the United States has a diverse climate across the nation independent of seasons, unlike other countries which have reported a seasonal variation. In this regard, there appears to be no seasonal variation based upon geographic region. Another interesting trend identified in our study was the consistent increase in NSTEMI admissions over the 18 years. Contemporary evidence has shown a decline in the incidence of all types of AMI. 53 , 54 , 55 Improvements in evidence‐based management strategies, greater emphasis on primary prevention, and increased use of cardioprotective medications and prior coronary revascularization are the reasons for an overall decline in AMI incidence. 53 However, a simultaneous increase in the usage and sensitivity of cardiac biomarkers, specifically for NSTEMI, could potentially be one of the many reasons for the increasing trend in NSTEMI incidence. 54 , 55 Given the higher rate of NSTEMI in the elderly, an increase in the elderly population over the last decade could also explain this trend. 9 , 55 In order to ensure we are only capturing a type‐1 NSTEMI, this study only included admissions with a primary diagnosis of NSTEMI.
Despite the lack of seasonal association in the incidence of AMI, we did find small, but significant, variations in the in‐hospital mortality across various seasons. Both unadjusted and adjusted analyses showed increased in‐hospital mortality during winters and the lowest in summers in both STEMI and NSTEMI admissions, as well as the entire AMI cohort. Spencer et al reported higher in‐hospital case‐fatality rates for AMI during winter and the lowest in spring. 5 Another study from Canada identified a seasonal variation in mortality of nearly 10%, with the highest number of AMI deaths in winter and lowest in summer. 56 Similar findings were also reported from studies in German and Japanese populations. 6 , 50 In contrast, Nagarajan et al found no specific differences in in‐hospital mortality across seasons using the Get With The Guidelines registry from the United States. 9 However, when stratified into STEMI and NSTEMI, they found higher mortality among the STEMI admissions during the fall season. 9 Although inconclusive, these seasonal variations in AMI mortality have been attributed to factors such as intolerance to low temperatures among the elderly who constitute a significant part of the AMI population and others such as hemodynamic and physiologic changes associated with cooler temperatures. 5 , 50 , 52 The association between the lower rates of coronary angiography and PCI for winter admissions, as identified in our study, is of potential concern. It is possible that weather conditions might have impacted total ischemic time in the winter; however, our study did not show any differences across all four geographic regions. Lower use of angiography in winter may be postulated to be due to higher rates of NSTEMI or type‐2 AMI since these patients may typically be admitted with respiratory illnesses. Respiratory illnesses might be associated with higher in‐hospital mortality, as noted in this study, and may additionally serve as barriers to coronary angiography and PCI due to concerns for overall patient trajectory. 57 , 58
4.1. Limitations
This study has several limitations, despite the HCUP‐NIS database's attempts to mitigate potential errors by using internal and external quality control measures. The administrative codes for AMI have been previously validated that reduces the inherent errors in the study. Echocardiographic data, angiographic variables, and hemodynamic parameters were unavailable in this database which limits physiological assessments of disease severity. We are unable to assess further detailed metrics such as total ischemic time and door‐to‐balloon time. Important factors such as the delay in presentation from time of onset of AMI symptoms, timing of cardiogenic shock, cardiac arrest, and acute organ failure, reasons for not receiving aggressive medical care, and treatment‐limiting decisions of organ support could not be reliably identified in this database. It is possible that despite best attempts at controlling for confounders by a multivariate analysis, winter admission was a marker of greater illness severity due to residual confounding. Despite these limitations, this study addresses an important knowledge gap highlighting the national temporal evolution of the seasonal effect and the impact of concomitant influenza infection on AMI.
5. CONCLUSIONS
In this study of nearly 11 million AMI admissions, winter admission was associated with higher in‐hospital mortality during this 18‐year study period. Though concomitant respiratory infections may explain this mortality, further data on the seasonal differences in outcomes relating to weather and travel and delayed presentations are needed to help understand this phenomenon better.
AUTHOR CONTRIBUTIONS
SV, SHP, WC did study design, literature review, statistical analysis. SV, SHP, WC did data management, data analysis, drafting manuscript. SV, SHP, WC, DRH, BJG: Access to data. DRH, BJG: Manuscript revision, intellectual revisions, mentorship. SV, SHP, WC, DRH, BJG. Final approval.
DISCLOSURE OF INTERESTS
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supporting information
Figure S1. Temporal trends of total AMI, STEMI, and NSTEMI admissions during the study period. Unadjusted temporal trends of total AMI, STEMI, and NSTEMI admissions during the 18‐year study period (P < .001 for trend over time). AMI, acute myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction
Table S1. Administrative codes used for identification of diagnoses and procedures
Table S2. Multivariable regression for in‐hospital mortality
ACKNOWLEDGMENTS
Dr. Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Vallabhajosyula S, Patlolla SH, Cheungpasitporn W, Holmes DR Jr, Gersh BJ. Influence of seasons on the management and outcomes acute myocardial infarction: An 18‐year US study. Clin Cardiol. 2020;43:1175–1185. 10.1002/clc.23428
REFERENCES
- 1. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315‐1322. [DOI] [PubMed] [Google Scholar]
- 2. Master A, Dack S, Jaffe H. Factors and events associated with onset of coronary artery thrombosis. JAMA. 1937;109(8):546‐549. [Google Scholar]
- 3. Nicolau GY, Haus E, Popescu M, Sackett‐Lundeen L, Petrescu E. Orcadian, weekly, and seasonal variations in cardiac mortality, blood pressure, and catecholamine excretion. Chronobiol Int. 1991;8(2):149‐159. [DOI] [PubMed] [Google Scholar]
- 4. Ornato JP, Peberdy MA, Chandra NC, Bush DE. Seasonal pattern of acute myocardial infarction in the national registry of myocardial infarction. J Am Coll Cardiol. 1996;28(7):1684‐1688. [DOI] [PubMed] [Google Scholar]
- 5. Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonal distribution of acute myocardial infarction in the second National Registry of Myocardial Infarction. J Am Coll Cardiol. 1998;31(6):1226‐1233. [DOI] [PubMed] [Google Scholar]
- 6. Keller K, Hobohm L, Münzel T, Ostad MA. Sex‐specific differences regarding seasonal variations of incidence and mortality in patients with myocardial infarction in Germany. Int J Cardiol. 2019;287:132‐138. [DOI] [PubMed] [Google Scholar]
- 7. Mohammad MA, Koul S, Rylance R, et al. Association of weather with day‐to‐day incidence of myocardial infarction: a SWEDEHEART nationwide observational study. JAMA Cardiol. 2018;3(11):1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, 1979 to 2002. J Am Coll Cardiol. 2006;48(2):287‐292. [DOI] [PubMed] [Google Scholar]
- 9. Nagarajan V, Fonarow GC, Ju C, et al. Seasonal and circadian variations of acute myocardial infarction: findings from the Get With The Guidelines–Coronary Artery Disease (GWTG‐CAD) program. Am Heart J. 2017;189:85‐93. [DOI] [PubMed] [Google Scholar]
- 10. Leibowitz D, Planer D, Weiss T, Rott D. Seasonal variation in myocardial infarction is limited to patients with ST‐elevations on admission. Chronobiol Int. 2007;24(6):1241‐1247. [DOI] [PubMed] [Google Scholar]
- 11. Eickhoff TC, Sherman IL, Serfling RE. Observations on excess mortality associated with epidemic influenza. JAMA. 1961;176(9):776‐782. [DOI] [PubMed] [Google Scholar]
- 12. Warren‐Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systematic review. Lancet Infect Dis. 2009;9(10):601‐610. [DOI] [PubMed] [Google Scholar]
- 13.Introduction to the HCUP Nationwide Inpatient Sample. 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. Accessed January 18, 2015.
- 14. Akintoye E, Briasoulis A, Egbe A, et al. Seasonal variation in hospitalization outcomes in patients admitted for heart failure in the United States. Clin Cardiol. 2017;40(11):1105‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 16. Bathini T, Thongprayoon C, Chewcharat A, et al. Acute myocardial infarction among hospitalizations for heat stroke in the United States. J Clin Med. 2020;9(5):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Subramaniam AV, Barsness GW, Vallabhajosyula S, Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol Ther. 2019;8(2):211‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vallabhajosyula S, Arora S, Lahewala S, et al. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. 2018;7(22):e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vallabhajosyula S, Arora S, Sakhuja A, et al. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol. 2019;123(3):489‐497. [DOI] [PubMed] [Google Scholar]
- 20. Vallabhajosyula S, Barsness GW, Vallabhajosyula S. Multidisciplinary teams for cardiogenic shock. Aging (Albany NY). 2019;11(14):4774‐4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vallabhajosyula S, Bell MR, Sandhu GS, Jaffe AS, Holmes DR Jr, Barsness GW. Complications in patients with acute myocardial infarction supported with extracorporeal membrane oxygenation. J Clin Med. 2020;9(3):E839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako‐tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7(18):e009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr, Prasad A. Hospital‐level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124(4):491‐498. [DOI] [PubMed] [Google Scholar]
- 24. Vallabhajosyula S, Dunlay SM, Barsness GW, et al. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction‐related cardiogenic shock. PLoS One. 2019;14(9):e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vallabhajosyula S, Dunlay SM, Bell MR, et al. Epidemiological trends in the timing of in‐hospital death in acute myocardial infarction‐cardiogenic shock in the United States. J Clin Med. 2020;9(7):E2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vallabhajosyula S, Dunlay SM, Kashani K, et al. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol. 2019;285:6‐10. [DOI] [PubMed] [Google Scholar]
- 27. Vallabhajosyula S, Dunlay SM, Murphree DH Jr, et al. Cardiogenic shock in takotsubo cardiomyopathy versus acute myocardial infarction: an 8‐year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail. 2019;7(6):469‐476. [DOI] [PubMed] [Google Scholar]
- 28. Vallabhajosyula S, Dunlay SM, Prasad A, et al. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73(14):1781‐1791. [DOI] [PubMed] [Google Scholar]
- 29. Vallabhajosyula S, El Hajj SC, Bell MR, et al. Intravascular ultrasound, optical coherence tomography, and fractional flow reserve use in acute myocardial infarction. Catheter Cardiovasc Interv. 2019;96:E59‐E66. 10.1002/ccd.28543. [DOI] [PubMed] [Google Scholar]
- 30. Vallabhajosyula S, Jentzer JC, Zack CJ. Cardiac arrest definition using administrative codes and outcomes in acute myocardial infarction. Mayo Clin Proc. 2020;95(3):611‐613. [DOI] [PubMed] [Google Scholar]
- 31. Vallabhajosyula S, Kashani K, Dunlay SM, et al. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000‐2014. Ann Intensive Care. 2019;9(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vallabhajosyula S, Kumar V, Vallabhajosyula S, et al. Acute myocardial infarction‐cardiogenic shock in patients with prior coronary artery bypass grafting: a 16‐year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9‐15. [DOI] [PubMed] [Google Scholar]
- 33. Vallabhajosyula S, O'Horo JC, Antharam P, et al. Venoarterial extracorporeal membrane oxygenation with concomitant impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2020;66:497‐503. [DOI] [PubMed] [Google Scholar]
- 34. Vallabhajosyula S, O'Horo JC, Antharam P, et al. Concomitant intra‐aortic balloon pump use in cardiogenic shock requiring veno‐arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11(9):e006930. [DOI] [PubMed] [Google Scholar]
- 35. Vallabhajosyula S, Patlolla SH, Dunlay SM, et al. Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail. 2020;13(2):e006661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vallabhajosyula S, Patlolla SH, Miller PE, et al. Weekend effect in the management and outcomes of acute myocardial infarction in the United States, 2000‐2016. Mayo Clin Proc Innov Qual Outcomes. 2020. 10.1016/j.mayocpiqo.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vallabhajosyula S, Patlolla SH, Sandhyavenu H, et al. Periprocedural cardiopulmonary bypass or venoarterial extracorporeal membrane oxygenation during transcatheter aortic valve replacement: a systematic review. J Am Heart Assoc. 2018;7(14):e009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vallabhajosyula S, Patlolla SH, Verghese D, et al. Burden of arrhythmias in acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2020;125:1774‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vallabhajosyula S, Prasad A, Bell MR, et al. Extracorporeal membrane oxygenation use in acute myocardial infarction in the United States, 2000 to 2014. Circ Heart Fail. 2019;12(12):e005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vallabhajosyula S, Prasad A, Bell MR, et al. Outcomes of ST‐segment elevation myocardial infarction involving the left main coronary artery. Mayo Clin Proc Innov Qual Outcomes. 2020;4(3):345‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vallabhajosyula S, Prasad A, Dunlay SM, et al. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: a 15‐year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8(15):e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vallabhajosyula S, Vallabhajosyula S, Bell MR, et al. Early vs. delayed in‐hospital cardiac arrest complicating ST‐elevation myocardial infarction receiving primary percutaneous coronary intervention. Resuscitation. 2020;148:242‐250. [DOI] [PubMed] [Google Scholar]
- 43. Vallabhajosyula S, Prasad A, Sandhu GS, et al. Mechanical circulatory support‐assisted early percutaneous coronary intervention in acute myocardial infarction with cardiogenic shock: 10‐year national temporal trends, predictors and outcomes. EuroIntervention. 2019. 10.4244/EIJ-D-19-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vallabhajosyula S, Shankar A, Patlolla SH, et al. Pulmonary artery catheter use in acute myocardial infarction‐cardiogenic shock. ESC Heart Fail. 2020;7(3):1234‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vallabhajosyula S, Vallabhajosyula S, Burstein B, et al. Epidemiology of in‐hospital cardiac arrest complicating non‐ST‐segment elevation myocardial infarction receiving early coronary angiography. Am Heart J. 2020;223:59‐64. [DOI] [PubMed] [Google Scholar]
- 46. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, et al. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review. ASAIO J. 2020. 10.1097/MAT.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 47. Vallabhajosyula S, Ya'Qoub L, Dunlay SM, et al. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail. 2019;6(4):874‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318(20):2011‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hopstock LA, Wilsgaard T, Njølstad I, et al. Seasonal variation in incidence of acute myocardial infarction in a sub‐Arctic population: the Tromsø study 1974‐2004. Eur J Cardiovasc Prev Rehabil. 2011;18(2):320‐325. [DOI] [PubMed] [Google Scholar]
- 50. Rumana N, Kita Y, Turin TC, et al. Seasonal pattern of incidence and case fatality of acute myocardial infarction in a Japanese population (from the Takashima AMI registry, 1988 to 2003). Am J Cardiol. 2008;102(10):1307‐1311. [DOI] [PubMed] [Google Scholar]
- 51. Marchant B, Ranjadayalan K, Stevenson R, Wilkinson P, Timmis AD. Circadian and seasonal factors in the pathogenesis of acute myocardial infarction: the influence of environmental temperature. Br Heart J. 1993;69(5):385‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patel NJ, Pant S, Deshmukh AJ, et al. Seasonal variation of acute myocardial infarction related hospitalizations in the United States: perspective over the last decade. Int J Cardiol. 2014;172(3):e441‐e442. [DOI] [PubMed] [Google Scholar]
- 53. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155‐2165. [DOI] [PubMed] [Google Scholar]
- 54. Parikh NI, Gona P, Larson MG, et al. Long‐term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute's Framingham Heart Study. Circulation. 2009;119(9):1203‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smedegaard L, Charlot MG, Gislason GH, Hansen PR. Temporal trends in acute myocardial infarction presentation and association with use of cardioprotective drugs: a nationwide registry‐based study. Eur Heart J Cardiovasc Pharmcother. 2017;4(2):93‐101. [DOI] [PubMed] [Google Scholar]
- 56. Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol. 1999;33(7):1916‐1919. [DOI] [PubMed] [Google Scholar]
- 57. Meier CR, Jick SS, Derby LE, et al. Acute respiratory‐tract infections and risk of first‐time acute myocardial infarction. Lancet. 1998;351(9114):1467‐1471. [DOI] [PubMed] [Google Scholar]
- 58. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378(4):345‐353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Temporal trends of total AMI, STEMI, and NSTEMI admissions during the study period. Unadjusted temporal trends of total AMI, STEMI, and NSTEMI admissions during the 18‐year study period (P < .001 for trend over time). AMI, acute myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction
Table S1. Administrative codes used for identification of diagnoses and procedures
Table S2. Multivariable regression for in‐hospital mortality


