Abstract
Background
The capability of bronchoscopy in the diagnosis of peripheral pulmonary nodules (PPNs) remains limited. Despite decades of effort, evidence suggests that the diagnostic accuracy for electromagnetic navigational bronchoscopy (EMN) and radial endobronchial ultrasound (EBUS) approach only 50%. New developments in robotic bronchoscopy (RB) may offer improvements in the assessment of PPNs.
Methods
A prospective single-blinded randomized controlled comparative study to assess success in localization and puncture of PPNs, using an ultrathin bronchoscope with radial EBUS (UTB-rEBUS) vs EMN vs RB in a human cadaver model of PPNs < 2 cm, was performed. The primary end point was the ability to successfully localize and puncture the target nodule, verified by cone-beam CT comparing RB and EMN. Secondary end points included needle to target position “miss” distance, and UTB-rEBUS comparisons.
Results
Sixty procedures were performed to target 20 PPNs over the study period. Implanted PPNs were distributed across all lobes, with 80% located within the lung periphery. The target PPN mean diameter was 16.5 ± 1.5 mm, with 50% noted to have a CT bronchus sign. The rate of successful PPN localization and puncture was superior when using RB, compared with EMN (80% vs 45%; P = .02). Among unsuccessful needle passes, the median needle to target “miss” distance was significantly different when comparing UTB-rEBUS, EMN, and RB (P = .0014).
Conclusions
In a cadaver model, use of RB significantly increased the ability to localize and successfully puncture small PPNs when compared with existing technologies. This study demonstrates the potential of RB to precisely reach, localize, and puncture small nodules in the periphery of the lung.
Key Words: interventional bronchoscopy, lung cancer, lung nodule, navigational bronchoscopy, robotic bronchoscopy
Abbreviations: EMN, electromagnetic navigation; FB, flexible bronchoscopy; PPN, peripheral pulmonary nodule; RB, robotic bronchoscopy; rEBUS, radial endobronchial ultrasound; UTB-rEBUS, ultrathin bronchoscopy with radial endobronchial ultrasound
Over 50 years ago, the introduction of the flexible bronchoscope (FB) launched a novel, minimally invasive endobronchial approach to diagnose peripheral pulmonary nodules (PPNs).1 The original design used fiber-optic bundles and an external light source to provide users with an image to guide their progression through the airways. The first prototypes incorporated an outer diameter of 5 mm with the ability to provide 180 and 120 degrees of flexion and extension, respectively. Since the initial unveiling of the FB, revisions have been made to both bronchoscope design and maneuverability for PPN evaluation; however, aside from small improvements to outer diameter size and adoption of digital imaging, little substantive change has been made.2
The initial reported diagnostic yield of FB paired with fluoroscopic guidance when sampling pulmonary nodules measuring less than 2 cm was reported to be up to 34%.3 Over the past 20 years, new technologies have been introduced to assist with improving diagnostic performance of bronchoscopy for pulmonary nodules. Electromagnetic navigation (EMN), virtual bronchoscopy, radial endobronchial ultrasound (rEBUS), guide sheaths, and advances in peripheral sampling tools have emerged in hopes of optimizing yield with a reported diagnostic yield of 70% regardless of which modality was used. These initial reported yields resulted in the most recent American College of Chest Physicians guidelines on nodule treatment recommending the use of guided bronchoscopy with modalities such as EMN or rEBUS.3 However, more recent prospective and randomized trial data have shown that the diagnostic accuracies of EMN and rEBUS approach only 50%, and an analysis of a large quality improvement registry reported a diagnostic yield of guided bronchoscopy, using EMN, of only 38.5%, which is nearly identical to the reported yields of decades ago, using only conventional bronchoscopy and fluoroscopy alone.3,4
As new technologies have been introduced there have been limited comparative data between the different modalities. A recent randomized controlled trial comparing the diagnostic yield of a thin bronchoscope and rEBUS with standard bronchoscopy and fluoroscopy in PPN lesions (mean diameter, 31.2 mm) showed the diagnostic yield of rEBUS to be 49% and that of fluoroscopy to be 34%.5 In addition, a prospective observational trial assessing the performance of a bronchial genomic classifier reported diagnostic yields of peripheral bronchoscopy with all modalities at 57%.6 These studies have highlighted the critical importance of randomized and comparative data to evaluate new technologies in order to approximately assess clinical usefulness.
Newer developments in robotic assisted technologies have led to the introduction of robotic bronchoscopy (RB).7 These novel devices may allow bronchoscopists to more precisely maneuver into the periphery of the lungs with greater stability and accuracy; this is done by advancing further into the lung periphery by generation count and insertion depth, compared with conventional bronchoscopy, despite having scope outer diameters of similar sizes.8
Limited data currently exist regarding the efficacy of RB, and no comparative data exist comparing RB with either EMN or rEBUS.9,10 We report here the results of a randomized controlled trial of RB vs EMN vs rEBUS with an ultrathin bronchoscope (UTB-rEBUS) in a cadaveric model to evaluate pulmonary nodules measuring less than 2 cm.
Methods
Study Design
A prospective single-blinded randomized controlled comparative trial to assess successful localization and puncture of implanted PPNs was performed. This preclinical trial compared three guided peripheral bronchoscopic navigation systems—UTB-rEBUS, EMN, and RB—in a human cadaveric model of PPNs measuring less than 2 cm.
Preclinical Study Validation
Before study implementation a series of cadaveric experiments were performed to validate the use of UTB-rEBUS and EMN in this study. First, validation of the rEBUS system was needed to ensure that the nodule implants used in this study would produce reliable ultrasound images consistent with standard rEBUS imaging. Second, EMN room mapping is suggested by the manufacturer to minimize electromagnetic interference to ensure that there is no discernible electromagnetic interference to offset the system’s ability to localize lesions. Internal study validation was needed and performed because industry methods could not be used during this study.
During the validation phase, two cadavers were prepared and a total of 10 nodules were placed in an endobronchial location to allow for visual confirmation during the validation phase of EMN and radial EBUS in this study. The placement of the validation nodules was performed by an independent proceduralist who was not involved in the navigation validation procedures discussed below.
The procedure room was then configured under the same map that was used on the days the index study procedures were performed. Cadaver positioning, navigation equipment, bronchoscopy equipment, and fluoroscope positioning were all marked with colored tape on the floor to allow for precise repositioning during all procedures during the validation phase in the same room and layout that the study was ultimately performed in. After the cadaver was prepared, CT imaging was performed followed by EMN planning. Two bronchoscopists blinded to the initial placement of the nodules were asked to navigate with the EMN system to all 10 nodules. All nodules were localized by EMN, and these locations were all confirmed by visual bronchoscopy. This portion of the experiment provided robust validation that the room map used in the study did not produce any discernible electromagnetic interference to limit the ability of the EMN system to localize lesions.
To validate the rEBUS portion, after localization and visual confirmation of all lesions by EMN, the radial probe was passed through the working channel of the bronchoscope. All lesions were identified by standard concentric rEBUS imaging (Fig 1).
Figure 1.
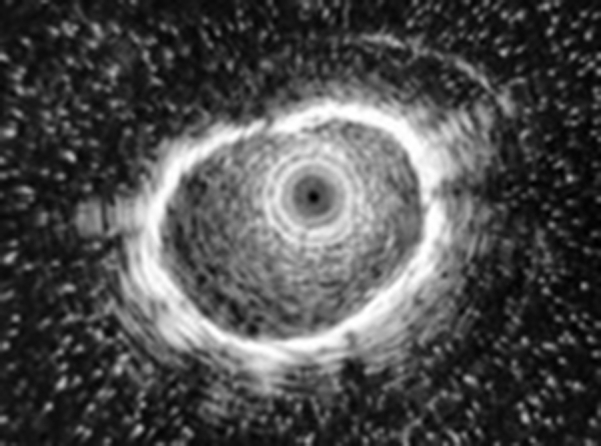
Concentric radial endobronchial ultrasound view of a peripheral target nodule placed during the validation phase of this study.
The initial phase of this study confirmed that EMN appropriately localized all lesions and that radial EBUS images were all consistent with concentric imaging, validating the use and accuracy of both technologies in this setting.
Peripheral Navigation Systems
UTB-rEBUS was performed with an ultrathin conventional flexible bronchoscope with an outer diameter of 3.0 mm and a 1.7-mm working channel (BF-MP190F; Olympus) in conjunction with an rEBUS probe (UM-S20-17S; Olympus) and a 21G (NA-403D-2021; Olympus) peripheral needle for sampling. The EMN system was composed of a superDimension EMN system (superDimension version 7.1; Medtronic) in conjunction with a 90-degree extended working channel (SDK3900-FT; Medtronic), a 21G (AKI00101-01; Medtronic) peripheral needle, and a flexible bronchoscope with a 2.8-mm working channel (BF-1TH190; Olympus) to allow passage of the extended working channel. The RB system consisted of the Ion endoluminal system with a fully articulating catheter with an outer diameter of 3.5 mm and 2.0-mm working channel (Ion IF-1000 version 1.1; Intuitive Surgical) in conjunction with a 21G (Flexision needle; Intuitive Surgical) peripheral needle. This system incorporates shape-sensing technology, consisting of a fiber optic sensor that measures the shape of the catheter multiple times per second and provides real-time location and scope information.11
Peripheral Nodule Model
Five human cadaveric torsos were intubated, using an 8.5-mm endotracheal tube. Intubation was followed by bilateral placement of a combination of large-bore surgical and 14F pigtail chest tubes and lung inflation. To emulate PPNs, pseudotumors were prepared with animal protein and iodinated contrast visible by rEBUS and chest CT imaging. After preparation, each cadaver underwent CT imaging to allow planning of peripheral target placements of the pseudotumors, using a floor-mounted system with DynaCT and guidance software (Artis zeego SW VD11C; Siemens Inc.). The nonintervention team selected target PPN placement locations to achieve a distribution of nodules in either the peripheral or mid-lung zones across all lobes. Four to six nodules were placed percutaneously in separate, distinct lobes, using a transthoracic needle under fluoroscopic guidance. Eighty percent of the nodules were placed in the periphery, defined as the outer one-third of the lung, with the goal of PPN target lesions measuring approximately 15 mm in each cadaver. After PPN target deployment, a second CT scan was performed (1-mm slice thickness with 5-mm overlap) to confirm nodule size and location and to select four PPNs for targeting. The same scan was then used for procedural planning of EMN and RB navigation as well as for guidance review before and during UTB-rEBUS to ensure image consistency across all three platforms.
Procedures
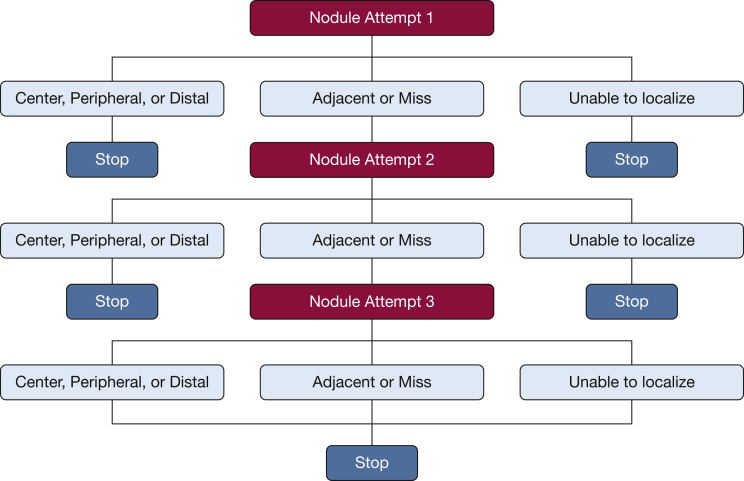
All procedures occurred over a period of five consecutive days and were performed by one of six experts in advanced diagnostic bronchoscopy (interventional pulmonologists or thoracic surgeons), all of whom had extensive prior experience with rEBUS and EMN. At the start of each day, the study team prepared a single cadaver as delineated in the section “Peripheral Nodule Model.” The nonintervention team selected four nodules (from a total of four to six per cadaver) before procedure initiation. Proceduralists attempted to reach and puncture the same four nodules with each bronchoscopic modality (Fig 2).
Figure 2.
Trial schematic illustrating nodule localization and puncture attempts.
On each study day, the three guided bronchoscopic procedures were performed on the mechanically ventilated cadaver model by a single proceduralist, with the exception of the third study day, during which two proceduralists alternated in performing the procedures. To limit any virtual mapping navigation bias, all proceduralists began with UTB-rEBUS and were prohibited from using computer-generated virtual navigation mapping preoperatively. Block randomization was used to determine which of the other two modalities, EMN and RB, would be performed second and third. Proceduralists were prohibited from using rEBUS for navigational assistance during EMN or RB procedures.
Using each bronchoscopic modality, proceduralists could perform up to three separate and consecutive attempts to reach and puncture each nodule via needle advancement. Fluoroscopic guidance was used with all three modalities to assist with target localization before needle puncture. After each independent navigation and needle advancement, the scope was stabilized and cone-beam CT images were acquired with the proceduralist blinded to the image findings.
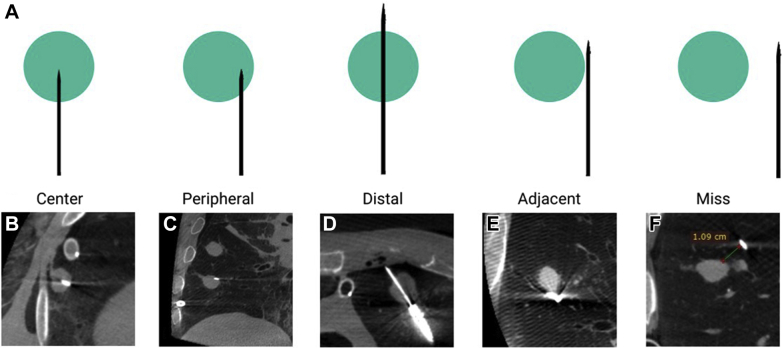
Localization of the nodule was defined as successful if the proceduralist had sufficient confidence to attempt a needle puncture pass after nodule visibility through rEBUS in the UTB arm or the virtual target was reached in either the EMN or RB arm. If no needle pass was attempted because of inability to sufficiently localize the lesion, the attempt was considered a navigation failure. After each needle pass, a nonproceduralist assessor (blinded to the device used and the proceduralist) evaluated the needle-target relationship for all attempted needle passes on cone-beam CT in a limited view magnified for nodule and needle placement. The following assessment scheme was used: center, peripheral, distal, adjacent, miss, and no localization (Fig 3A).
Figure 3.
A, Graphic depiction of needle-to-nodule puncture scenarios seen on cone-beam CT imaging. Cone-beam CT images are shown, illustrating needle-to-nodule puncture relationships. B, Central needle tip position. C, Peripheral needle position. D, Distal needle tip position. E, Adjacent needle-to-nodule position. F, “Miss,” unsuccessful localization or puncture (needle-to-nodule distance noted in centimeters).
Nodule puncture attempts received an assessment of “center” when the needle was located within the inner two-thirds of the nodule; an assessment of “peripheral” was assigned when the needle was located within the outer one-third of the nodule; an assessment of “distal” was used when the needle entered and went through the distal edge of the nodule; “adjacent” was defined as needle positioning in direct contact with the peripheral edge of the nodule; and “miss” was defined as a needle positioning without any contact or puncture of the nodule (Figs 3B-3F). Regardless of modality, nodule attempts were terminated after either three consecutive passes to reach the nodule or successful puncture of the nodule.
Study End Points
The study’s primary end point was successful PPN localization and puncture, defined as an assessment of needle-to-nodule localization (center, peripheral, or distal) achieved by the third attempt with a single bronchoscopic modality as defined above. The main secondary end point included a broader definition of puncture success—defined by an assessment that also included “adjacent” needle position achieved by the third attempt for a PPN with a single bronchoscopic modality. Additional secondary end points assessed included distance between needle and target in attempts where the PPN was not successfully localized and punctured (ie, passes assessed as “miss”).
Statistical Analysis
Descriptive statistics including means (± SD), medians (IQR25-75), proportions, and raw numbers were used as appropriate when reporting PPN pseudotumor size, parenchymal distribution, and procedural success rates. Each nodule attempt was characterized as successful or unsuccessful, based on the definitions provided above for the primary and secondary end points. Study end points were reported as rates, calculated as the proportion of PPNs successfully localized and punctured divided by the total number of PPNs attempted. Proportions are reported by study arm and separately for the primary and secondary outcome definitions. Successful puncture and localization between the two randomized arms—EMN and RB—was compared by χ2 analysis employing both the primary and secondary end-point definitions of successful puncture. A comparison of puncture and localization rates between RB and UTB-rEBUS and between EMN and UTB-rEBUS was done by χ2 analysis. Finally, the distance between needle and target in unsuccessful PPN attempts by study arm was assessed by Kruskal-Wallis test. All analyses were performed with Stata version 15 (StataCorp) and GraphPad Prism 8 (GraphPad Software, Inc.).
Results
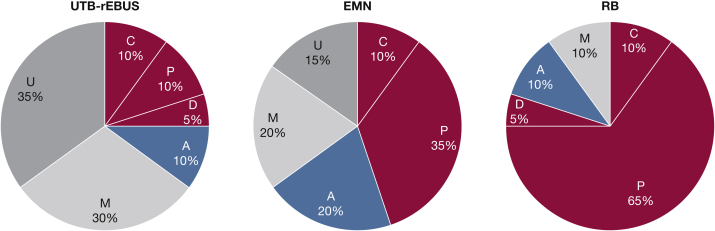
Sixty procedures were performed to target 20 PPN pseudotumors, which were implanted and assessed over the entire study period. Pseudotumors were distributed across all lobes and had a mean nodule size of 16.5 ± 1.5 mm. Eighty percent were located in the outer one-third of the lung, and 50% had a CT bronchus sign. Twenty procedures were successfully completed with each of the three bronchoscopic modalities, with 10 randomized to EMN as the second procedure followed by RB and 10 nodules randomized to RB as the second procedure followed by EMN. The distribution of the procedural outcomes for all three modalities is shown in Figure 4.
Figure 4.
Percent distribution of the procedural outcomes for all three modalities. Successful central (C), peripheral (P), or distal (D) localization and puncture is delineated by the color green. Adjacent (A) localization and puncture is shown in yellow. Localization but puncture “miss” (M) is shown in light gray. Inability to localize (U) is shown in dark gray. EMN = electromagnetic navigation; RB = robotic bronchoscopy; UTB-rEBUS = ultrathin bronchoscopy with radial endobronchial ultrasound.
Primary End Point: Center, Peripheral, Distal Needle Puncture
Nodule localization resulting in at least one needle pass attempt for a single PPN was achieved in 65% of UTB-rEBUS, 85% of EMN, and 100% of RB cases (Table 1). Use of RB resulted in a significantly higher rate of successful localization and needle puncture compared with EMN (80%, 16/20 vs 45%, 9/20; P = .022). Successful localization and puncture by UTB-rEBUS was considerably lower when compared with RB (25%, 5/20 vs 80%, 16/20; P < .001) (Fig 4). There was no significant difference between UTB-rEBUS and EMN (P = .19).
Table 1.
Localization, Puncture, and Successful Navigation Study Outcomes
| Study Arm | No. | Study Outcomes |
||
|---|---|---|---|---|
| Localization and Puncture (Primary End Point)a |
Localization and Puncture (Secondary End Point)b |
Successful Navigation |
||
| % (No.) | % (No.) | % (No.) | ||
| UTB-rEBUS | 20 | 25 (5) | 35 (7) | 65 (13) |
| EMN | 20 | 45 (9) | 65 (13) | 85 (17) |
| RB | 20 | 80 (16) | 90 (18) | 100 (20) |
EMN = electromagnetic navigation; PPN = peripheral pulmonary nodule; RB = robotic bronchoscopy; UTB-rEBUS = ultrathin bronchoscopy with radial endobronchial ultrasound.
Successful PPN localization and puncture defined by an assessment of needle-to-nodule localization (grade of center, peripheral, or distal).
Successful PPN localization and puncture defined by an assessment of needle-to-nodule localization (grade of center, peripheral, distal, or adjacent).
Secondary End Points: Center, Peripheral, Distal, Adjacent Needle Position
For our secondary analyses, a broader definition of procedural success was applied, namely that successful localization and puncture included all procedures that achieved an assessed needle position of center, peripheral, distal, or adjacent. Use of this definition resulted in higher rates of successful localization and needle puncture across all groups (Fig 4). Although the rate of puncture success was 25% higher for RB compared with EMN, this did not reach statistical significance (90%, 18/20 vs 65%, 13/20; P = .058).
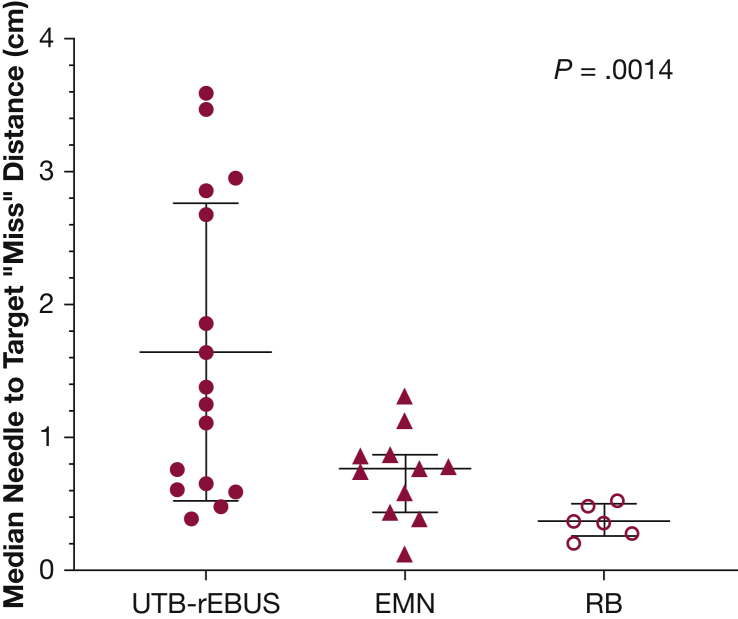
Needle-to-Target “Miss” Distance
This analysis calculated the difference in median distance between needle and target when the best outcome for an individual PPN was “miss.” This resulted in a total of six measurements (from two targets) for RB, 11 measurements (from four targets) for EMN, and 16 measurements (from six targets) for UTB-rEBUS for analysis. The median (IQR25-75) distance between the needle and target nodule was 1.3 cm (0.6-2.8 cm), 0.7 cm (0.4-0.9 cm), and 0.4 cm (0.3-0.5 cm) in the UTB-rEBUS, EMN, and RB arms, respectively (Fig 5) (P = .0014).
Figure 5.
Comparison of nodule-to-needle distances by technology; medians with interquartile range are shown. See Figure 4 legend for expansion of abbreviations.
Discussion
This trial is the first single-blinded prospective randomized trial comparing three peripheral navigation platforms in their ability to aid the bronchoscopist in localization and puncture of small (< 2 cm) PPN targets. In a human cadaveric model of PPNs, the use of RB with shape-sensing technology significantly increased the ability to localize and precisely puncture PPNs when compared with EMN. In addition to differences in measures of precision, assessment of the secondary end points allowed for novel measures of accuracy when comparing modalities by factoring in the needle-to-target “miss” difference, which was lower (more accurate) in the RB procedures. This suggests that additional factors such as catheter size, sampling tools, and navigational software likely play important additional roles in procedural success, which must be further investigated.
Although there have been important and significant efforts aimed at improving the diagnostic yield of PPN biopsy via FB, there remains an insufficient ability for the bronchoscopist to reliably and reproducibly achieve clinically meaningful results when attempting to biopsy PPNs. Despite single-center retrospective and registry studies reporting high variability in diagnostic yields,12, 13, 14 mounting pooled and prospective evidence evaluating adjunct technologies such as EMN and rEBUS have failed to show consistent significant improvements in diagnostic yield.4,5,15 The factors associated with the wide variation in diagnostic yields when using adjunct PPN sampling modalities have been reported to include center procedural volumes,16, 17, 18 lesion size, location, and the presence or absence of a bronchus sign.19,20 In addition, retrospective trials are associated with inherent biases that act as confounders when extrapolating consistent or expected diagnostic outcomes.21 Additional factors such as CT-to-body divergence, the effect of atelectasis, bleeding, and/or nodule motion may also result in diminished precision seen in “real-world” and prospective trials of peripheral FB.22,23
These data suggest that RB may provide the proceduralist with a reproducible improvement in diagnostic yield in the biopsy of PPNs. Although these findings are both promising and compelling, they also raise questions regarding which of the known and/or unknown factors associated with PPN diagnostic success are at play.
There are limitations of our study related to the cadaveric model employed, which, while anatomically intact, may not be fully generalizable (CT-to-body divergence, the effect of atelectasis, bleeding, and/or nodule motion, etc.) to in vivo procedural interventions. In addition, although the pseudotumors implanted proved to be excellent radiographic targets, they may not be representative of in vivo tumor tissue found in lung nodules. The lack of concurrent use of rEBUS with the navigation platforms may have resulted in lower yields; however, the goal of the study was to independently assess each modality. As discussed in the preclinical testing design, the EMN used in this study suggests mapping of the room to minimize interference. Although industry mapping could not be performed during this study, a robust validation model was performed to show that the mapping in this setting did not impact system accuracy, validating this approach. Another potential limitation in study design was that each proceduralist was allowed a maximum of only three attempts to localize and puncture the target, which may have resulted in lower success rates for all modalities tested, but the direct comparator design of this study equilibrated this factor across all arms.
The PRECISION-1 study represents the first comparative evaluation of a novel PPN biopsy platform (RB with shape-sensing technology) with existing technology. The results from this study demonstrate that RB may hold unique properties that allow for improved localization and puncture of small PPNs. Although these results are compelling, further studies with prospective multicenter comparative trials are needed to better understand the true diagnostic usefulness of this novel platform and to delineate what obstacles RB may be able to overcome to improve PPN diagnostic yields. Future studies evaluating new technology should consider incorporating combined modality approaches.
Acknowledgments
Author contributions: L. Y. takes responsibility for the content of the manuscript, including the data and analysis. L. Y. and A. V. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. L. Y., J. A., M. W., A. C., J. P. S., S. L. S., D. Y., F. M., J. C.-G., D. M., H. L., and A. V. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. Y. has received research, educational grants, and consulting fees from Olympus, superDimension, Veran Medical, Inspire Medical Consulting, Boston Scientific. M. W. has received research, educational grants, and consulting fees from Olympus, superDimension. H. L. has received research, educational grants, and consulting fees from Veran Medical, Olympus, Inspire Medical Consulting, Veracyte, and superDimension. A. C. has received research, educational grants, and consulting fees from Olympus, Auris, Boston Scientific, and Johnson & Johnson. D. M.has received research, educational grants, and consulting fees from Intuitive Surgical. J. A. has received research, educational grants, and consulting fees from Intuitive, Veran Medical, superDimension, and Boston Scientific. A. V. has received research, educational grants, and consulting fees from Veracyte and Johnson & Johnson. None declared (J. P. S., S. L. S., D. Y.)
Interventional Pulmonary Outcomes Group (IPOG) Collaborators: Johns Hopkins University, University of North Carolina, Duke University, Washington University in St Louis, University of Pennsylvania, Stanford University, University of Michigan, Sloan-Kettering Memorial Cancer Center and Vanderbilt University.
Other contributions: The IPOG investigators of the PRECISION-1 trial wish to acknowledge the Association of Interventional Pulmonary Program Directors for providing research funding to support this project; Intuitive Surgical for providing the Ion system and cadaveric facility; and Ben Cohn, Ruchi Bhatt, Hiba Lejmi, Sundeep Master, Oliver Wagner, MD, and the Intuitive Clinical Development Engineering team and the Johns Hopkins Interventional Pulmonary Research Core for providing equipment and support.
Footnotes
FUNDING/SUPPORT: This study was funded by the Association of Interventional Pulmonary Program Directors.
References
- 1.Ikeda S., Yanai N., Ishikawa S. Flexible bronchofiberscope. Keio J Med. 1968;17(1):1–16. doi: 10.2302/kjm.17.1. [DOI] [PubMed] [Google Scholar]
- 2.Oki M., Saka H., Ando M. Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions: a randomized trial. Am J Respir Crit Care Med. 2015;192(4):468–476. doi: 10.1164/rccm.201502-0205OC. [DOI] [PubMed] [Google Scholar]
- 3.Rivera M.P., Mehta A.C., Wahidi M.M. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e142S–e165. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 4.Ost D.E., Ernst A., Lei X. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193(1):68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanner N.T., Yarmus L., Chen A. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective, randomized trial. Chest. 2018;154(5):1035–1043. doi: 10.1016/j.chest.2018.08.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvestri G.A., Vachani A., Whitney D. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373(3):243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert H.B., Neimat J., Webster R.J., III Concentric tube robots as steerable needles: achieving follow-the-leader deployment. IEEE Trans Robot. 2015;31(2):246–258. doi: 10.1109/TRO.2015.2394331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen A.C., Gillespie C.T. Robotic endoscopic airway challenge: REACH assessment. Ann Thorac Surg. 2018;106(1):293–297. doi: 10.1016/j.athoracsur.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 9.Rojas-Solano J.R., Ugalde-Gamboa L., Machuzak M. Robotic bronchoscopy for diagnosis of suspected lung cancer: a feasibility study. J Bronchology Interv Pulmonol. 2018;25(3):168–175. doi: 10.1097/LBR.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding D.I.K., Bashirzadeh F., Son J.H. First human use of a new robotic-assisted fiber optic sensing navigation system for small peripheral pulmonary nodules. Respiration. 2019;98(2):142–150. doi: 10.1159/000498951. [DOI] [PubMed] [Google Scholar]
- 11.Galloway K.C., Chen Y., Templeton E., Rife B., Godage I.S., Barth E.J. Fiber optic shape sensing for soft robotics. Soft Robot. 2019;6(5):671–684. doi: 10.1089/soro.2018.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folch E.E., Pritchett M.A., Nead M.A. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE Study. J Thorac Oncol. 2019;14(3):445–458. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Gildea T.R., Mazzone P.J., Karnak D., Meziane M., Mehta A.C. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006;174(9):982–989. doi: 10.1164/rccm.200603-344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W., Chen S., Dong X., Lei P. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis. 2015;7(5):799–809. doi: 10.3978/j.issn.2072-1439.2015.04.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Memoli J.S., Nietert P.J., Silvestri G.A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birkmeyer J.D., Warshaw A.L., Finlayson S.R., Grove M.R., Tosteson A.N. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126(2):178–183. [PubMed] [Google Scholar]
- 17.Bach P.B., Cramer L.D., Schrag D., Downey R.J., Gelfand S.E., Begg C.B. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 18.Smith C.B., Wolf A., Mhango G., Wisnivesky J.P. Impact of surgeon volume on outcomes of older stage I lung cancer patients treated via video-assisted thoracoscopic surgery. Semin Thorac Cardiovasc Surg. 2017;29(2):223–230. doi: 10.1053/j.semtcvs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Gex G., Pralong J.A., Combescure C., Seijo L., Rochat T., Soccal P.M. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87(2):165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 20.Ali M.S., Sethi J., Taneja A., Musani A., Maldonado F. Computed tomography bronchus sign and the diagnostic yield of guided bronchoscopy for peripheral pulmonary lesions: a systematic review and meta-analysis. Ann Am Thorac Soc. 2018;15(8):978–987. doi: 10.1513/AnnalsATS.201711-856OC. [DOI] [PubMed] [Google Scholar]
- 21.Geneletti S., Richardson S., Best N. Adjusting for selection bias in retrospective, case-control studies. Biostatistics. 2009;10(1):17–31. doi: 10.1093/biostatistics/kxn010. [DOI] [PubMed] [Google Scholar]
- 22.Chen A., Pastis N., Furukawa B., Silvestri G.A. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147(5):1275–1281. doi: 10.1378/chest.14-1425. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett M.A., Schampaert S. Tipping point: cone beam CT with augmented fluoroscopy for the biopsy and treatment of peripheral nodules. J Bronchology Interv Pulmonol. 2019;26(1):e13–e15. doi: 10.1097/LBR.0000000000000561. [DOI] [PubMed] [Google Scholar]