Abstract
Cysts are commonly seen on CT scans of the lungs, and diagnosis can be challenging. Clinical and radiographic features combined with a multidisciplinary approach may help differentiate among various disease entities, allowing correct diagnosis. It is important to distinguish cysts from cavities because they each have distinct etiologies and associated clinical disorders. Conditions such as emphysema, and cystic bronchiectasis may also mimic cystic disease. A simplified classification of cysts is proposed. Cysts can occur in greater profusion in the subpleural areas, when they typically represent paraseptal emphysema, bullae, or honeycombing. Cysts that are present in the lung parenchyma but away from subpleural areas may be present without any other abnormalities on high-resolution CT scans. These are further categorized into solitary or multifocal/diffuse cysts. Solitary cysts may be incidentally discovered and may be an age related phenomenon or may be a remnant of prior trauma or infection. Multifocal/diffuse cysts can occur with lymphoid interstitial pneumonia, Birt-Hogg-Dubé syndrome, tracheobronchial papillomatosis, or primary and metastatic cancers. Multifocal/diffuse cysts may be associated with nodules (lymphoid interstitial pneumonia, light-chain deposition disease, amyloidosis, and Langerhans cell histiocytosis) or with ground-glass opacities (Pneumocystis jirovecii pneumonia and desquamative interstitial pneumonia). Using the results of the high-resolution CT scans as a starting point, and incorporating the patient’s clinical history, physical examination, and laboratory findings, is likely to narrow the differential diagnosis of cystic lesions considerably.
Key Words: cystic lung disease, diffuse lung disease, focal lung luciencies, lung cysts, pulmonary cysts
Abbreviations: BHD, Birt Hogg Dubé; DIP, desquamative interstitial pneumonia; HRCT, high-resolution CT scan; LAM, lymphangioleiomyomatosis; LCDD, light-chain deposition disease; LIP, lymphoid interstitial pneumonia; PJP, Pneumocystis jirovecii pneumonia; PLCH, pulmonary Langerhans cell histiocytosis; TS, tuberous sclerosis
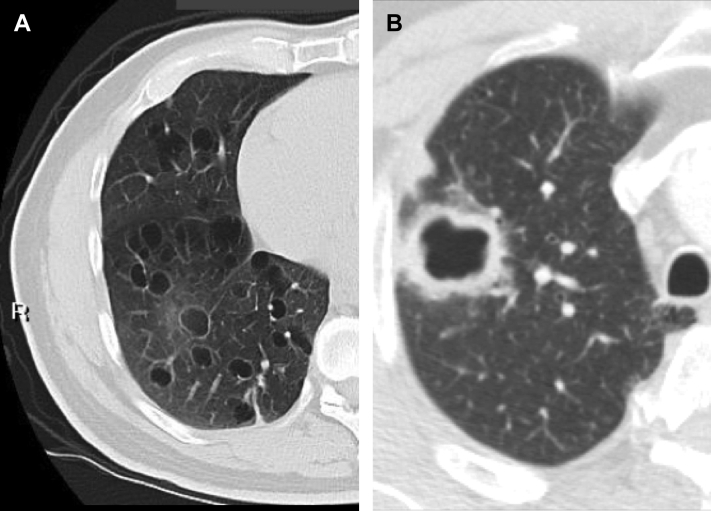
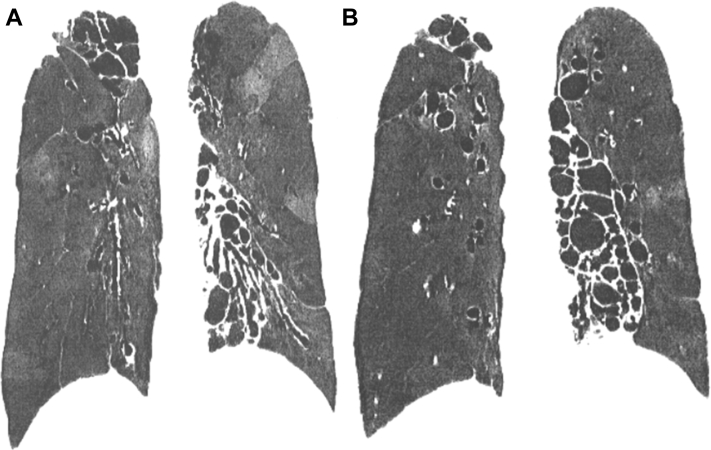
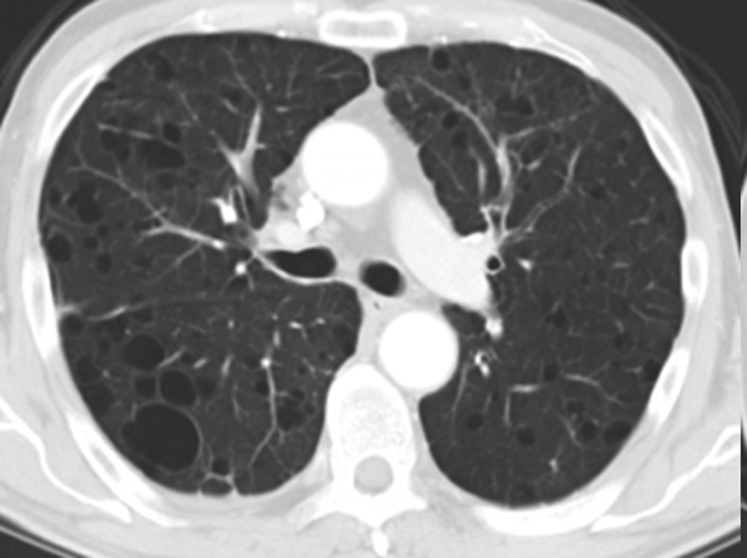
A pulmonary cyst is a round air space defined pathologically by an epithelial or fibrous outer wall and radiographically as a round parenchymal lucency or low attenuation area with a well-defined interface with normal lung.1 Cysts are common in otherwise normal individuals but may occur in association with other lung diseases. Cysts must be differentiated from pulmonary cavities. A cavity is a discrete air and/or fluid-containing space, identifiable as a focal lucency or low-attenuation area when air-filled, frequently identified as an isolated lesion or lesions or in association with pulmonary consolidation and/or mass. Most importantly, compared with cysts that, by definition, have thin walls (< 2 mm) (Fig 1A), cavities characteristically present with a wall thickness > 4 mm (Fig 1B).1 The thin wall of cysts may appear to be deceptively thicker due to compression of the adjacent lung parenchyma (Fig 2). Occasionally, a condition such as lung contusion may result in the development of cyst and an adjacent area of lung parenchymal density (Fig 3). The combination of these findings may also give the impression of a thick walled cyst or cavity.
Figure 1.
A, True cysts: Round parenchymal lucencies surrounded by distinct wall < 2 mm in thickness, exhibiting a well-defined interface with normal lung tissue. Cysts may be seen in otherwise normal individuals but may occur in association with other lung diseases. B, Cavity. This is a gas-filled space, seen as a lucency or low-attenuation area, with a wall thickness > 4 mm. It is seen within pulmonary consolidation, a mass, or nodule.
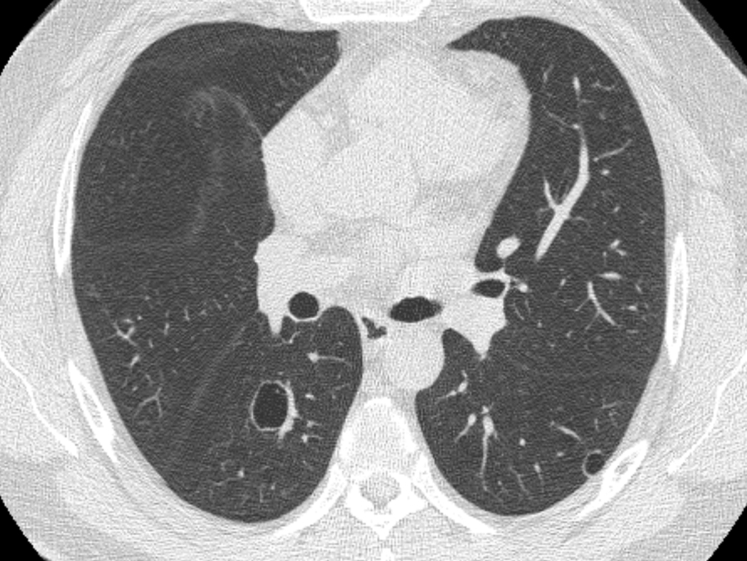
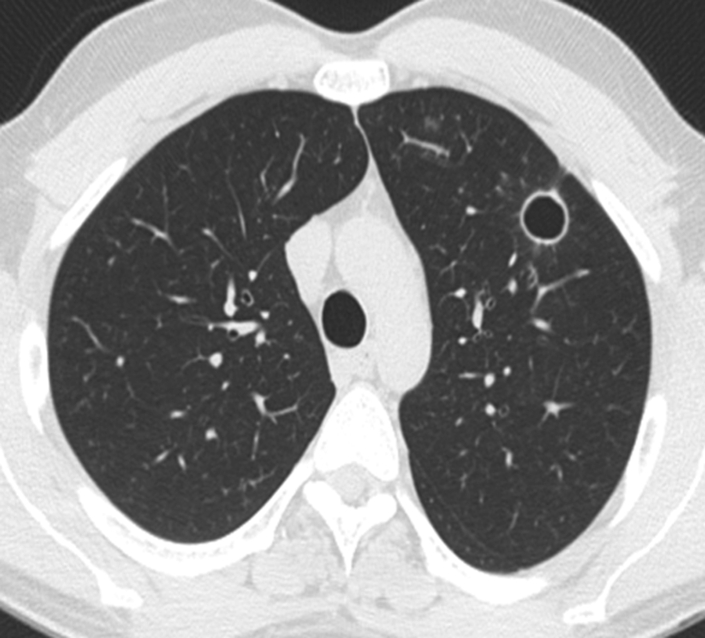
Figure 2.
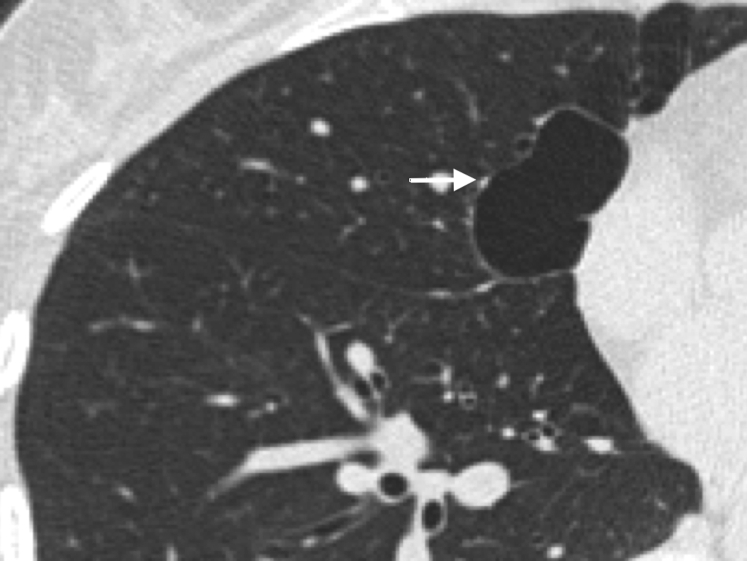
Tracheobronchial papillomatosis. Note that the thin-walled cysts may compress portions of the lung, giving the spurious impression of asymmetric wall thickening.
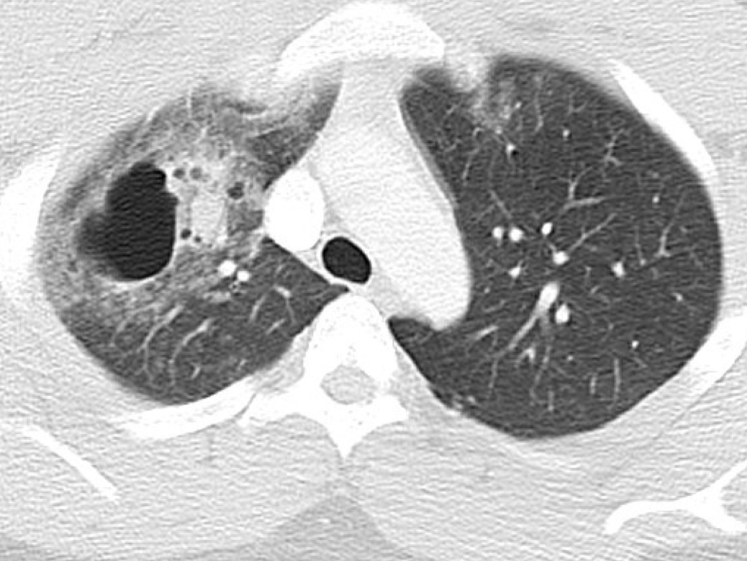
Figure 3.
Traumatic pneumatocele. Thin-walled, gas-filled space in the lungs, usually transient, that can be caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluids. In this illustration, there are adjacent areas of increased parenchymal density from lung contusion.
Pulmonary parenchymal cysts are commonly seen on CT scans, and their differential diagnosis may be challenging. Regardless of the cause of cysts, patients usually present with either no symptoms, with the cysts discovered on chest imaging for another reason, or with nonspecific symptoms such as cough and shortness of breath. Sometimes the shortness of breath is acute due to development of pneumothorax. Careful review and characterization of the radiographic abnormalities, coupled with assessment of clinical and laboratory features that may point to an underlying pulmonary or systemic disease, are helpful in distinguishing among numerous possibilities to arrive at the correct diagnosis. The current article presents a classification of cysts allowing for a simplified algorithmic approach to diagnosis.
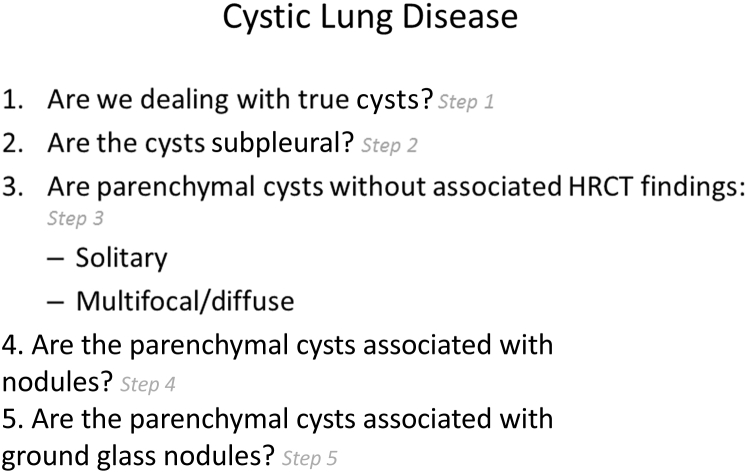
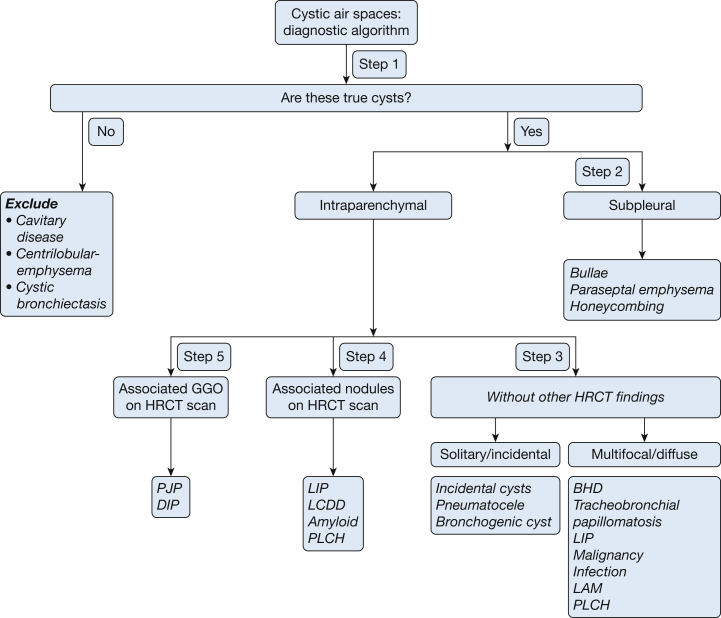
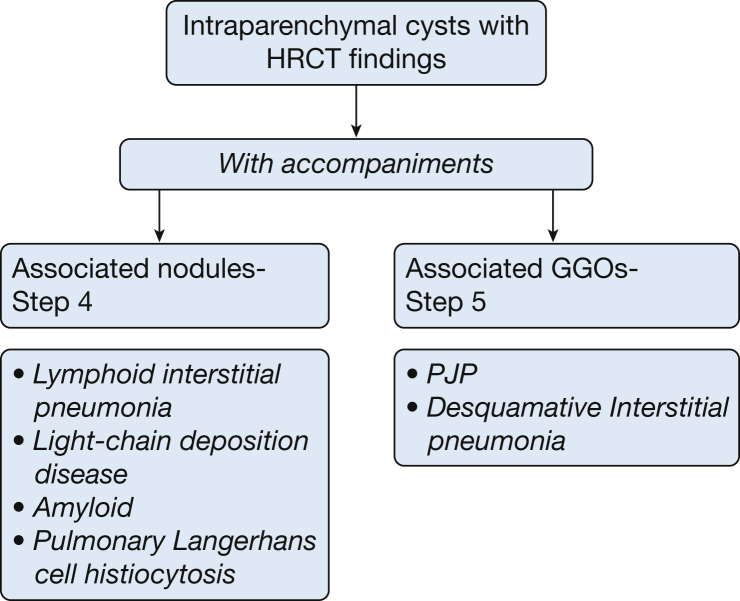
Lung cysts can be conveniently divided into five specific categories on the basis of their location, number, distribution, and associated CT findings (Fig 4). These categories include: (1) subpleural cysts, either solitary or multifocal; (2) parenchymal cysts without associated high-resolution CT scan (HRCT) findings, either solitary or multiple; (3) parenchymal cysts seen in association with nodules; and (4) parenchymal cysts seen in association with ground-glass attenuation. Each of these categories carries a limited number of diagnostic possibilities, and differentiating them may be relatively straightforward.
Figure 4.
A five-step approach to managing cystic lung disease.
Subpleural cysts are usually bullae, paraseptal emphysema or represent honeycomb changes. Parenchymal cysts can be solitary (frequently the sequelae of previous infections or trauma) or multifocal lesions including lymphangioleiomyomatosis (LAM)/tuberous sclerosis (TS) and Birt Hogg Dubé (BHD) syndrome. Cysts associated with discrete lung nodules can be seen with lymphoid interstitial pneumonia (LIP), amyloidosis, or (less frequently) in light-chain deposition disease (LCDD) and pulmonary Langerhans cell histiocytosis (PLCH). Finally, cysts may be seen in association with diffuse ground-glass attenuation, as occurs in patients with various infections (including Pneumocystis jirovecii pneumonia [PJP]) and less commonly in patients with diffuse lung diseases such as desquamative interstitial pneumonia (DIP). Table 1 highlights the radiographic and clinical features of the cystic lung diseases.
Table 1.
Definitions and Radiographic Features of Conditions Included Under Cystic Lung Disease
| Condition | Description | Wall Thickness | Other Features |
|---|---|---|---|
| Cyst (Fig 1A) | A round parenchymal lucency or low-attenuation area exhibiting a well-defined interface with normal lung and usually containing air | Thin-walled (< 2 mm) | Not associated with pulmonary emphysema. May rarely contain fluid or solid material |
| Bulla (Fig 12) | A rounded focal lucency or area of decreased attenuation measuring >1 cm (usually several centimeters) | Thin-walled, usually < 1 mm | Often accompanied by emphysema in the adjacent lung |
| Paraseptal emphysema (Fig 13) | Subpleural and peribronchovascular regions of low attenuation separated by intact interlobular septa, sometimes associated with bullae | No walls | Bounded by pleural surface and interlobular septa. Rest of lung architecture preserved. Centrilobular emphysema may be present |
| Honeycombing (Fig 10) | Closely approximated ring shadows 3-10 mm in diameter, usually subpleural with well-defined walls | 1-3 mm | Late stage of various lung diseases, with complete loss of acinar architecture |
The radiologic definitions have been taken from the Fleischner Society (Glossary of terms for thoracic imaging. Radiology. 2008).22
Sometimes a disorder can be included in > 1 category. For example, PLCH may present as a diffuse cystic disease lung with nodules or without any other radiographic abnormalities. In some cases, cysts and cavities may both be present. Although overlap is anticipated when using this classification, we emphasize a multimodality approach to diagnosis. Central to this algorithmic approach is the role of HRCT imaging as the starting point. HRCT produces images of great clarity and detail in different orientations and is critical in the diagnostic evaluation.2, 3 In this regard, volumetric image acquisition, obtaining contiguous 1- or 1.5-mm sections using an edge-enhancing reconstructive algorithm throughout the thorax in a single breath-hold is essential. This method will precisely define the walls, extent, and distribution of lucent lung lesions, and it will also generate high-quality coronal and sagittal image reconstructions. Images obtained by using this technique are especially valuable for illustrating the relationship between cysts and peripheral airways. In specific situations, additional imaging techniques, such as minimum intensity projection, may enhance visualization of low density structures such as cysts by eliminating interference from high density structures such as blood vessels (Figs 5A, 5B). Minimum intensity projections (MiniIP) is a technique that projects the lowest attenuation or density voxels on each view, projected as a 2-dimensional image. This technique allows endobronchial air and air in the lung parenchyma to be differentiated.4 The MiniIP effectively eliminates high density structures and enhances the distinction between cysts and bronchiectasis. Images in exhalation may also be valuable to identify focal or diffuse air trapping or morphologic changes in the central airways and parenchymal cysts. A critical review of HRCT features can reveal patterns that are diagnostic in a large proportion of patients with cystic lung disease. In prior analyses, expert radiologists were able to correctly assign the diagnosis of cystic lung diseases in 80% of cases based on review of HRCT features alone.4, 5, 6
Figure 5.
A-B, Minimum intensity projection (MINiP) imaging. Sequential coronal images through the posterior aspect of the lungs show extensive cystic bronchiectasis within the right apex and the entire left lower lobe. With MINiP imaging, only those voxels with the lowest density in any row are projected in two dimensions, effectively eliminating all high-density structures, including most pulmonary vessels. Note that there is also evidence of mosaic lung attenuation identifiable as alternating geographical areas of low-density and higher lung density, indicative of extensive air-trapping in this case due to associated obstructive small airways disease. These changes are seen to especially good advantage using the MINiP technique.
Although the current article encompasses the majority of cysts encountered in clinical practice, it is not intended to be an exhaustive review of all cystic lung disease. For the sake of completion, we have tabulated rare causes of cystic lung disease that are not covered in this algorithm (Table 2).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 We have included uncommon disorders where cystic abnormalities are common (eg, BHD syndrome), and excluded more common diseases where cystic abnormalities are uncommon (eg, 10% cases of hypersensitivity pneumonitis).11 We have also excluded congenital pulmonary airway malformation,21 a rare condition in which cysts communicate with small airways or bronchioles in only one of its four classes. We do not include cystic bronchiectasis in this classification because the air spaces do not have true walls. Regardless, some patients with otherwise typical radiographic and clinical features may still require a biopsy to establish a diagnosis.
Table 2.
Description of HRCT Features of Rare Cystic Lung Diseases
| Condition | HRCT Features |
|---|---|
| HP | Centrilobular ground glass nodules with mosaic attenuation and airways involvement.6, 7 In < 10% of cases of HP, cysts are seen. These are usually few in number and random in distribution |
| MALToma | Diffuse multiple thin-walled cysts and small ill-defined nodules exhibiting a perilymphatic pattern in bilateral lung fields7 |
| Follicular bronchiolitis | Small centrilobular nodules with occasional peribronchial nodules and areas of ground-glass opacity. It is included in the same category of lymphoproliferative diseases as lymphoid interstitial pneumonia. In this disease, a major distinguishing feature from lymphoid interstitial pneumonia is that the findings are confined primarily to the peribronchiolar region and that ground-glass opacities are uncommon8, 9 |
| Lymphomatoid granulomatosis | Small thin-walled cysts, small lung nodules distributed in peribronchovascular (core) areas, coarse irregular opacities, and mediastinal nodal enlargement10 |
| Ankylosing spondylitis | Upper lobe predominant with apical fibrosis, associated interstitial lung disease and bronchiectasis. Seen in 10% of patients with ankylosing spondylitis11 |
| Neurofibromatosis | Numerous upper lobe predominant cysts seen bilaterally. Cysts more commonly seen in smokers12, 13 |
| Proteus syndrome | Rare syndrome characterized by vascular malformations and asymmetric postnatal overgrowth of connective tissues. Thin- and thick-walled cystic changes, as well as emphysematous enlargement of air spaces can be seen in approximately 10% of patients with Proteus syndrome14, 15 |
| Ehlers-Danlos Syndrome | Rare genetic disorder of connective tissues characterized by skin hyperextensibility, joint hypermobility, and tissue fragility. Multiple parenchymal cysts and cavities are rarely seen in patients with Ehlers-Danlos syndrome16 |
| Fire-eater’s lung | Chemical pneumonitis resulting from accidental aspiration of petroleum products. Cystic changes in lung parenchyma represent pneumatoceles, and they typically follow a benign course leading to resolution in a few weeks17, 18 |
| Hyper-IgE syndrome | Primary immunodeficiency condition characterized by multiple skin and sino-pulmonary infections, as well as an elevated IgE level. Lung cysts likely represent pneumatoceles secondary to staphylococcal infections19, 20 |
| CPAM | Presents as cystic or solid lung masses. The types of CPAM, based mainly on the size of the cysts, are as follows21, 36: Type 1 (70%): most common type, with large cysts (2-10 mm) Type 2 (20%): small uniform cysts, 0.5-2 cm in diameter, associated with air-filled multicystic masses or focal areas of consolidation Type 3 (10%): poor prognosis; lesions are visualized as multiple cysts < 2 mm in size that usually involve entire lobe of the lung Type 4: These are very large cysts, sometimes ≥10 cm in size usually affecting one lobe |
These are either rare or uncommon manifestations of cystic lung diseases that are not discussed in the current article. CPAM = congenital pulmonary airway malformation; HP = hypersensitivity pneumonitis; HRCT = high-resolution CT scan; MALToma = mucosa-associated lymphoid tissue lymphoma.
Systematic Approach to Cystic Lung Disease
A summary of the systematic approach to cystic lung disease is given in Figure 6.
Figure 6.
Detailed algorithmic approach to cystic lung disease. It is important to exclude mimics of cystic lung disease before proceeding with the actual algorithm. BHD = Birt Hogg Dube syndrome; DIP = desquamative interstitial pneumonia; HRCT = high-resolution CT scan; LAM = lymphangioleiomyomatosis; LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; PJP = pneumocystis jirovecii pneumonia; PLCH = pulmonary Langerhans cell histiocytosis.
Step 1: Are There True Cystic Lesion(s)?
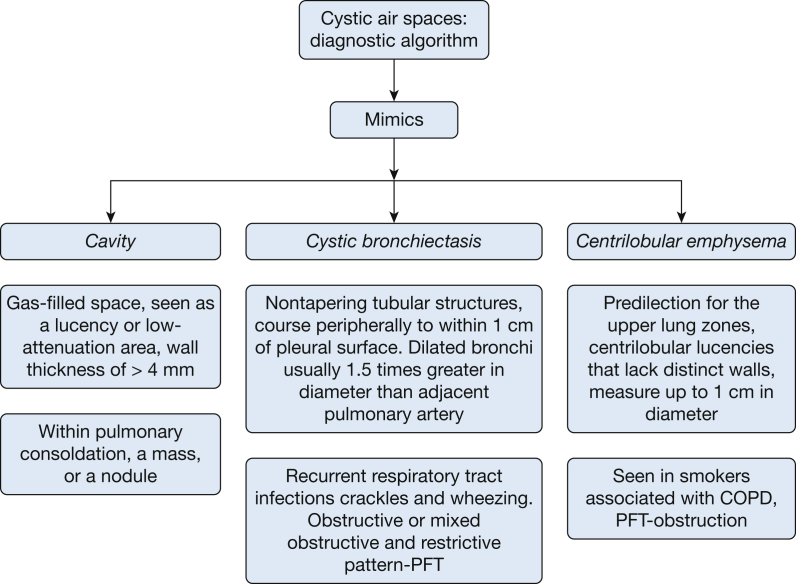
Evaluation of cystic lung disease begins with ascertaining that the lesions on HRCT scan are true cysts. It is imperative that mimics of cysts, including cavities, centrilobular emphysema and cystic bronchiectasis be excluded first (Fig 7). These conditions are characterized as follows:
-
•
A cavity is a gas-filled space, seen as a lucency or low-attenuation area with a wall thickness of > 4 mm noted within pulmonary consolidation, a mass, or a nodule22, 23 (Fig 1B).
-
•
Centrilobular emphysema is visualized on HRCT imaging as centrilobular lucencies that lack distinct walls, measure up to 1 cm in diameter, and have a predilection for the upper lung zones (Fig 8). Many of these luciencies exhibit a central dot that is a branch of the pulmonary artery within the secondary lobule.22, 24, 25
-
•
Cystic bronchiectasis is typically associated with bronchial dilation adjacent to the accompanying pulmonary artery (“signet-ring sign”), absence of tapering of bronchi, and identification of bronchi within 1 cm of the pleural surfaces. There may be accompanying bronchial wall thickening, tree-in-bud opacities, and a mosaic pattern (Fig 9). Coronal images or sequential axial images help distinguish cystic bronchiectasis from true cysts.22, 26
-
•
Honeycombing results from advanced and irreversible fibrotic lung disease that may lead to cysts that are typically 3 to 10 mm in diameter, clustered (the walls of the cyst abut each other), with thick walls (1-3 mm or larger), and with architectural distortion and reticulation of intervening lung (Fig 10).27
Figure 7.
The mimics of cystic lung disease along with their radiographic and clinical features. PFT = pulmonary function test.
Figure 8.
Centrilobular emphysema. These lucencies demonstrate a predilection for the upper lung zones. Centrilobular lucencies are seen that lack distinct walls and measure up to 1 cm in diameter. The lucencies may have a dot in the center, representing a branch of pulmonary artery.
Figure 9.
Cystic bronchiectasis. Nontapering tubular structures that course peripherally to within 1 cm of pleural surface. The contiguity of these dilated bronchi can be appreciated on adjacent transverse sections of airways and on coronal planes, distinguishing them from true individual cysts.
Figure 10.
Honeycombing. Lower lobe predominance, with cysts of similar diameter, demonstrating thicker walls. Cysts are present in one or more layers. There is architectural distortion of the lung parenchyma with traction bronchiectasis, representing end-stage lung disease.
Step 2: Are the Cysts Located in the Subpleural Areas?
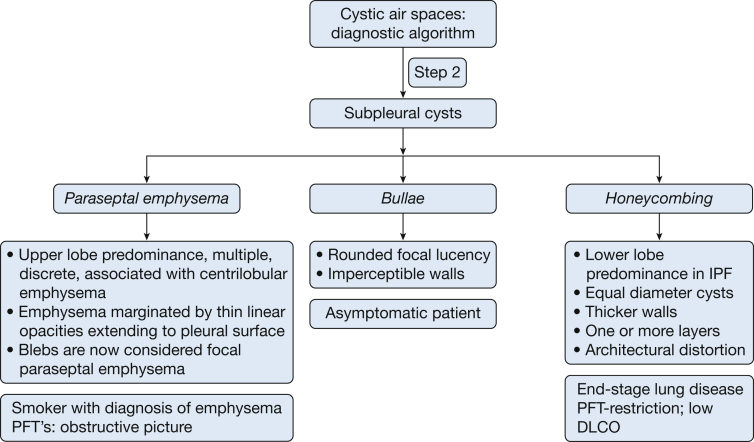
The next step is to determine if the cysts are located primarily in the subpleural areas (Fig 11). If so, the differential diagnosis includes bullae (mostly ≥ 1 cm in diameter or paraseptal emphysema and honeycombing (usually < 1 cm diameter). It should be pointed out that BHD syndrome is associated with cysts that may show predisposition for basal and paraseptal areas.
Figure 11.
The major etiologies of cysts that are present predominantly in the subpleural areas. Of note, Birt-Hogg-Dubé (BHD) syndrome may also give rise to cysts in the subpleural area. See Figure 7 legend for expansion of abbreviation.
Bullae
A bulla is a rounded focal lucency or area of decreased attenuation ≥ 1 cm in diameter, bounded by a thin, almost imperceptible wall. It may be formed by the coalescence of cysts of paraseptal emphysema (Fig 12).22 Bullae can enlarge over time and compress normal lung, resulting in restrictive physiology.28, 29 They can present with spontaneous pneumothorax, hemoptysis, or infection. Primary bullous disease may be seen in patients with Ehlers-Danlos,30, 31 Marfan syndrome, and other conditions.27
Figure 12.
Bulla. Large (> 1 cm), air-containing structures with very thin walls (≤ 1 mm). Walls of bulla are formed by lung tissues, septa, or pleura. Bullae are usually associated with emphysema.
Paraseptal Emphysema
This subtype of emphysema involves distal alveoli, including alveolar ducts and sacs (Fig 13). The cysts are discrete, thin-walled, and multiple. The cyst walls do not abut each other but are characteristically bound by pleural surfaces and interlobar septa. They are seen as radiolucent abnormalities, ranging from a few millimeters to 1 cm in diameter in subpleural and peribronchovascular distribution with predominance in the dorsal surfaces of lung.22, 24, 25 As opposed to honeycombing, the lung architecture is well preserved. The coalescence of cysts in paraseptal emphysema leads to formation of bullae. A bleb is a small gas-containing space within the visceral pleura or in the subpleural lung, no larger than 1 cm in diameter, usually not seen on chest radiographs.22 The Fleischner Society recommends that blebs be considered as focal paraseptal emphysema.
Figure 13.
Paraseptal emphysema. Subpleural lucencies marginated by interlobular septa and pleura. They exhibit upper lobe predominance and are usually associated with centrilobular emphysema.
It may be argued that paraseptal emphysema is not a true cyst because pathologically, a true cyst should have its walls lined by epithelium or fibrous tissue. However, we have chosen to include paraseptal emphysema within the classsification of cystic lung disease since on the HRCT scan, the former projects as a hyper lucency bounded by distinct thin walls–albeit formed by pleural surface and/or interlobular septae.23
Honeycombing
This results from advanced fibrotic lung disease and may lead to cysts that are typically uniform in size (3-10 mm diameter), clustered (the walls of the cysts abut each other) with thick walls (1-3 mm or larger).
Step 3: Are Cysts Detected in Lung Parenchyma Without Other Radiographic Abnormalities?
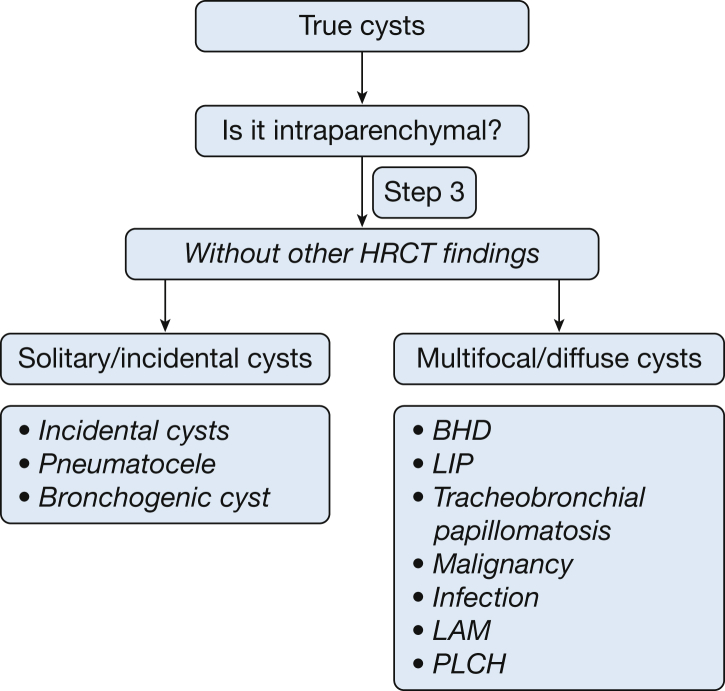
If cysts can be detected in lung parenchyma without other radiographic abnormalities, these lung parenchymal cysts can be classified into two groups: (1) solitary or incidental; and (2) focal, multifocal, or diffuse (Fig 14).
Figure 14.
Intraparenchymal cysts. These are either solitary or multiple. See Figure 6 legend for expansion of abbreviations.
Solitary (single) cysts are usually randomly distributed. In many instances, they are discovered in images obtained for other reasons. Focal disease is defined as > 1 cyst in one lobe of the lung, whereas multifocal disease involves > 1 lobe but does not involve all the lobes. Diffuse is defined as involving all five lobes of the lung. Within the category of diffuse involvement, there may be a greater profusion of cysts in one zone of the lung. For example, PLCH is considered a diffuse disease, even though it has a predilection for the upper and middle lobes.
Are the Lung Parenchymal Cysts Solitary?
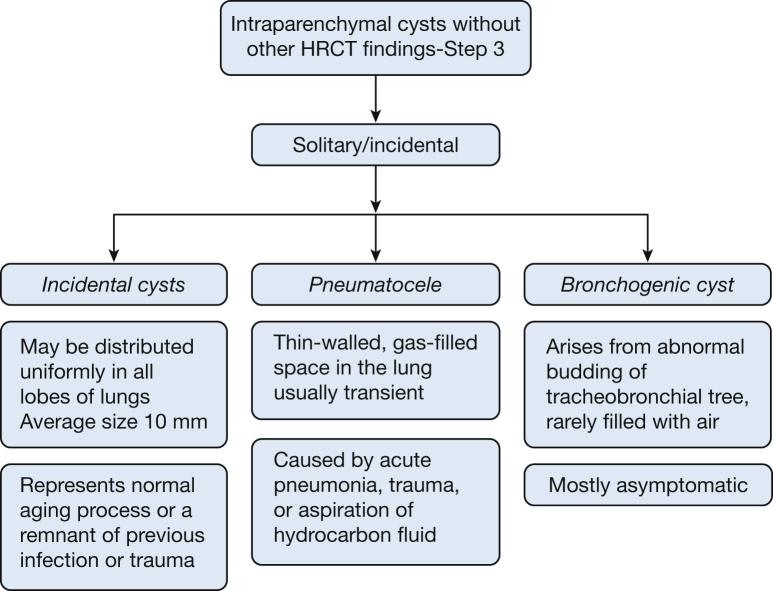
Examples of solitary cysts (Fig 15) include incidental cysts (Fig 16), pneumatocele (Fig 3), and bronchogenic cysts (Fig 17).
Figure 15.
Algorithm for solitary intraparenchymal cysts.
Figure 16.
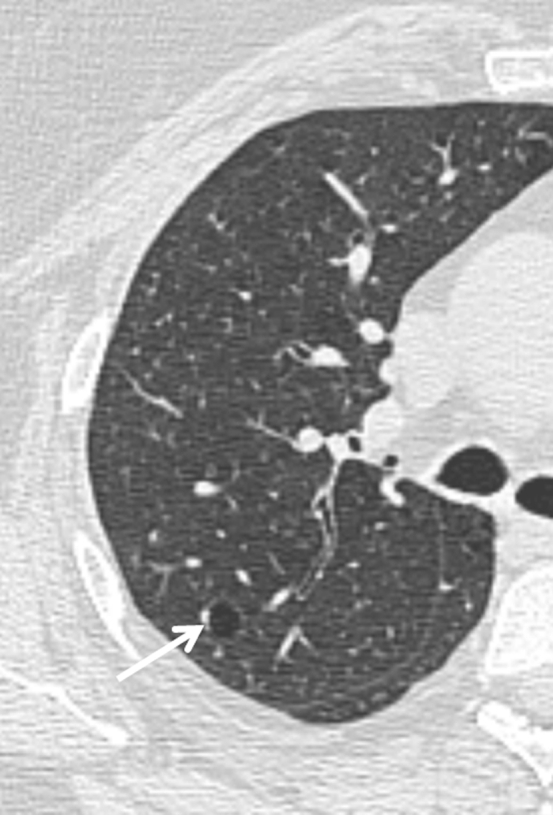
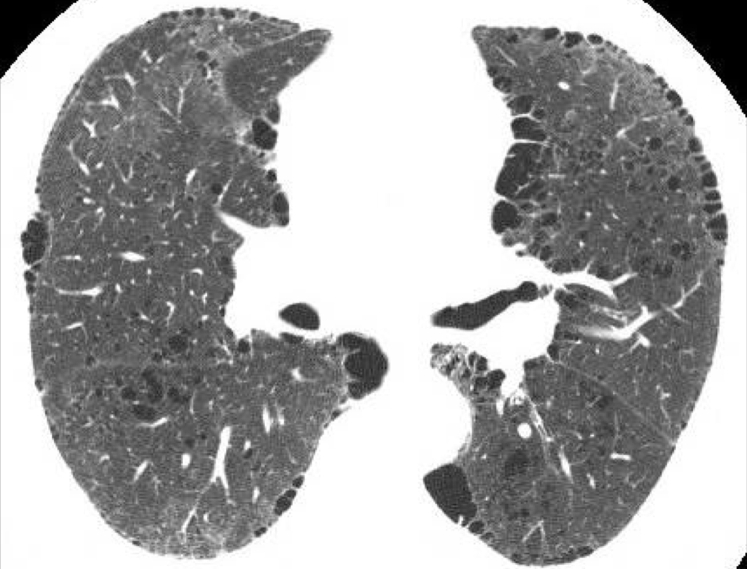
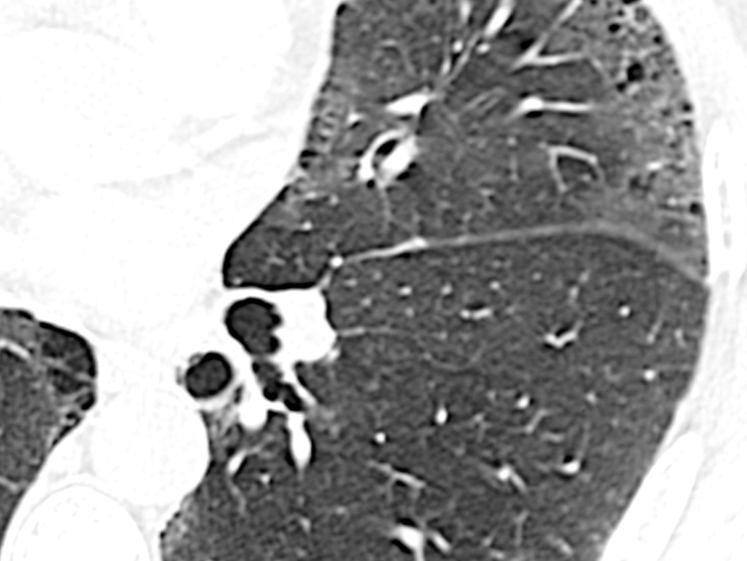
Incidental cyst. These cysts can occur in any lobe of the lung. The average size is 10 mm, and they may represent the normal aging process or persist as a remnant of previous infection or trauma.
Figure 17.
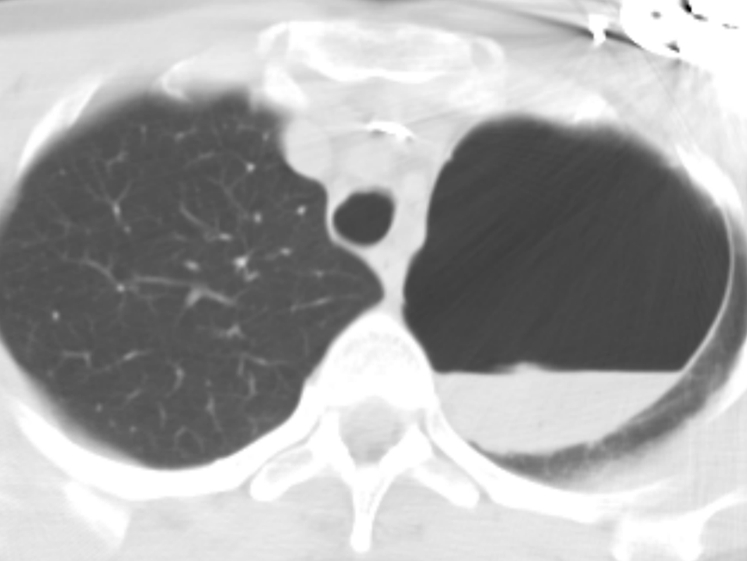
Bronchogenic cyst. Usually asymptomatic, these cysts arise from abnormal budding of the tracheobronchial tree. Rarely, they may communicate with the tracheobronchial tree and become infected. An air-fluid level is seen in this large bronchogenic cyst.
Incidental Cysts
In a study of asymptomatic individuals evaluated by using CT scans of the chest,32 cysts were discovered in approximately 25% of patients aged > 75 years, and they almost never caused symptoms. These cysts were equally prevalent among smokers and nonsmokers, distributed uniformly in all lobes of the lungs, and were, on average, 10 mm in size, raising the possibility that they may represent part of the normal aging process. Of note, none of the patients aged < 55 years demonstrated cysts on their HRCT imaging.32 A single cyst may also be the remnant of a previous infection or trauma.
Pneumatocele
A pneumatocele is a transient, thin-walled, gas-filled space in the lung. It is most frequently caused by acute pneumonia, trauma, or aspiration of hydrocarbon fluid and is usually transient. The mechanism is believed to be a combination of parenchymal necrosis and check-valve airway obstruction.22 It can be distinguished from other cysts by its transient nature and relevant history such as pneumonia or trauma (Fig 3). Occasionally, it can persist.
Bronchogenic Cysts
Bronchogenic cysts arise from abnormal budding of the tracheobronchial tree during lung development.21 Although they commonly present as middle mediastinal masses,33 they also present as lower lobe intraparenchymal masses in about one third of cases. These are most commonly filled with fluid. Air-filled intraparenchymal cysts are rare. On a CT scan, it presents as a cyst with well-defined boundaries and attenuation of water or soft tissue.21
Are There More Than One (or Several, or Many) Lung Parenchymal Cysts? (Fig 18)
Figure 18.
Algorithmic approach to intraparenchymal cysts–multiple. See Figure 6 and 7 legends for expansion of abbreviations.
Birt Hogg Dubé syndrome
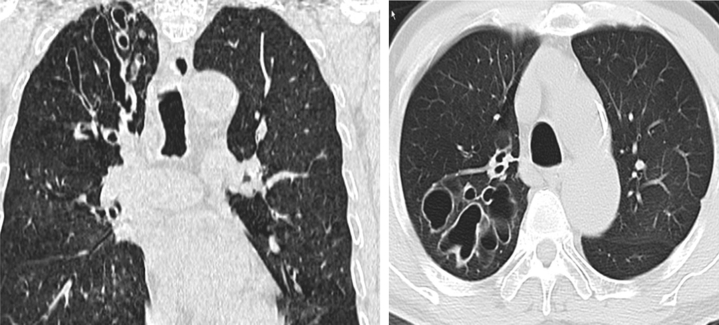
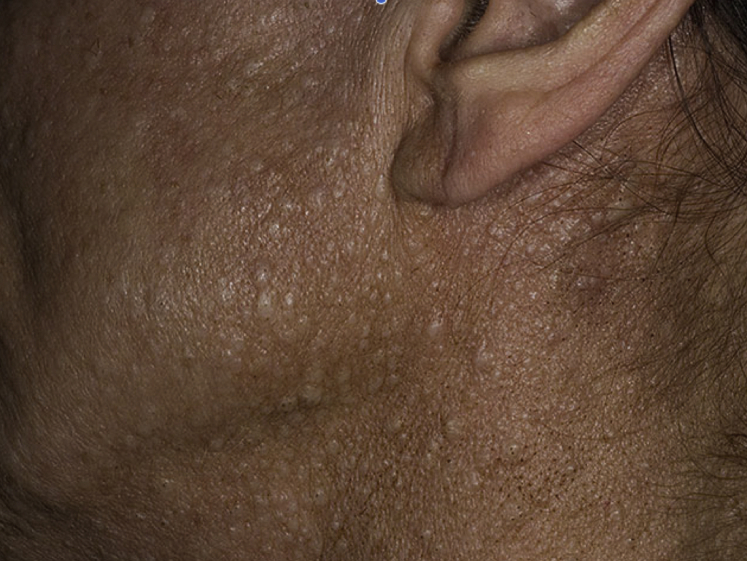
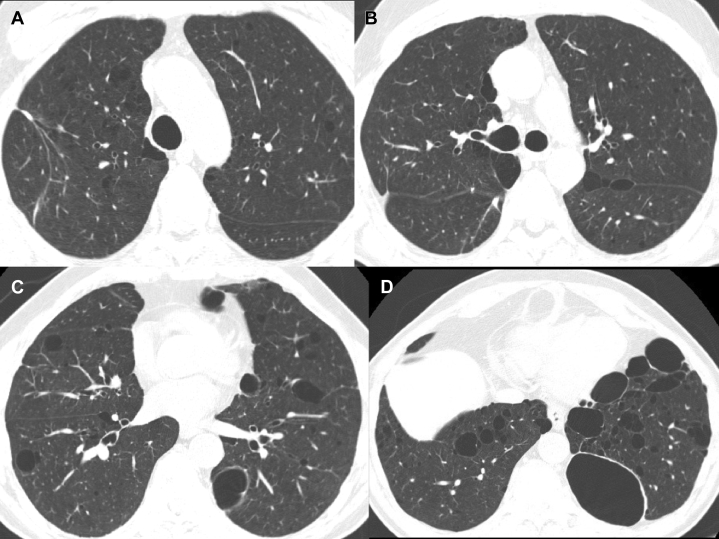
BHD syndrome is an autosomal dominant syndrome characterized by cutaneous fibrofolliculomas (Fig 19), multiple lung cysts, spontaneous pneumothorax, and renal cancer.34 HRCT findings of the lung include multiple, thin-walled cysts in > 80% patients, predominantly seen in peripheral lung zones at lung bases and along the mediastinum.4 These cysts are unique in that they either abut or encase the proximal portion of the lower pulmonary veins. The cysts are less profuse compared with LAM, and intervening lung parenchyma is normal (Fig 20 A-D).30 Spontaneous pneumothorax occurs in approximately 24% of patients with BHD syndrome, albeit with a very high likelihood (almost 75%) of recurrence.35 These lesions usually appear after the age of 20 years. Renal cancer occurs in approximately 25% of patients with this disorder.34
Figure 19.
Fibrofolliculomas (dome shaped, whitish papules) of the face, neck and upper trunk are seen in Birt-Hogg-Dubé (BHD) syndrome.
Figure 20.
A-D, Axial CT chest images of a patient with Birt-Hogg-Dubé (BHD) syndrome. Multiple, thin-walled cysts are seen in approximately 80% of patients. These cysts are distributed predominantly in the peripheral lung zones, at lung bases, and along the mediastinum. These are less in number generally, compared with those seen in lymphangioleiomyomatosis (LAM).
Lymphoid Interstitial Pneumonia
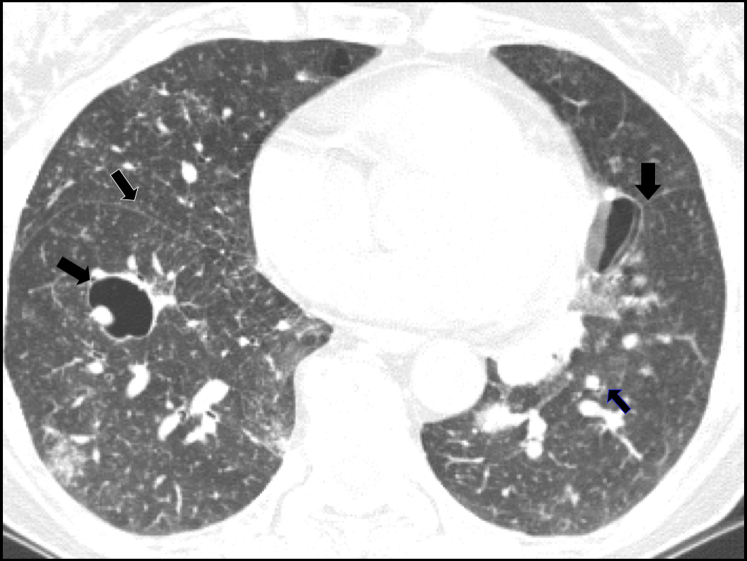
This disease may present with randomly distributed, thin-walled cystic air spaces (68%), some of which may be up to 30 mm, involving and usually affecting < 10% of the lung (Fig 21). Of note, LIP may also present with diffuse ground-glass opacities, poorly defined centrilobular nodules (100%), and perilymphatic nodules mimicking lymphangitic spread of carcinoma (86%).36 A more detailed description of LIP is included later in the article (in the section entitled “Step 4: Are the Lung Parenchymal Cysts Associated With Nodules?”).
Figure 21.
Lymphoid interstitial pneumonia. Cysts are thin-walled, random in distribution, and typically involve < 10% of lung parenchyma. Note the scattered foci of increased lung density in close proximity to the medial aspect of a cyst in the right lower lobe (solid black arrow). There are multiple small nodules. The thin black arrow on the right side shows these nodules in the fissure.
Tracheobronchial Papillomatosis
HRCT findings reveal lesions in the larynx and trachea. Lung manifestations include nodules that are solid or cystic with thin or thick walls. The homogeneous small nodules progress to cystic lesions, which are distributed randomly in central, peripheral, and occasionally in subpleural locations (Fig 2).37 Juvenile papillomas commonly occur in the larynx but can involve the lung by direct extension from the larynx. Lung involvement occurs about 10 to 12 years after diagnosis of laryngeal papillomas.37, 38 The most common initial presenting symptoms are hoarseness of voice and cough. Most patients have a history of undergoing multiple endoscopies and for resections of papillomas and tracheostomies. Complications include respiratory infection, sepsis, and death.
Malignancy (Metastatic) Cancer
If cystic lung lesions are seen in patients with a known malignancy, metastatic disease should be suspected, and tissue sampling may be needed to establish the diagnosis. In metastatic adenocarcinoma, cavities rarely develop. The walls of cavities in some cases become thin from inflation by a ball valve mechanism, transforming them into true cystic lesions (Fig 22).23 Cysts in squamous carcinoma may result from cornification of squamous epithelium in the center of the lesion with subsequent liquefaction and evacuation into small airways; these lesions appear most frequently in the upper lobes.38, 39, 40, 41, 42
Figure 22.
Metastatic colon cancer with thin-walled cysts. The images are from a 62-year-old male patient who was an ex-smoker with metastatic colon cancer. This CT scan was obtained 10 months after resection of the colon cancer.
Cystic metastasis should be considered when there is a history of squamous cell cancer and especially with head and neck primary tumors.38 Thin-walled cystic lesions are seen occasionally within cavitary metastases from seminoma, Ewing sarcoma, myxosarcoma, Wilms’ tumor, osteogenic sarcoma, angiosarcoma, transitional cell carcinoma, teratocarcinoma, and sarcoma of unknown type.38, 43
Infections
Cystic lesions can be seen in many pulmonary infections. Chest CT scans in patients with PJP often disclose multiple pulmonary cysts, predominantly in the upper lobes. Commonly, the cysts are associated with ground-glass opacities. A more detailed description of PJP is included later this article (in the section entitled “Step 5: Are lung parenchymal cysts associated with ground glass opacities?”) These cysts occasionally rupture and lead to pneumothorax.44, 45 Although tuberculosis causes cavities that usually resolve with treatment, they can persist as cystic lesions. Atypical mycobacterial infections can also cause thin-walled cystic lesions. Lung cysts can be seen in paragonimiasis, echinococcosis, and coccidioidomycosis (Fig 23).38
Figure 23.
The cyst in a patient with coccidioidomycosis is well-delineated with little reaction in the surrounding lung parenchyma.
Lymphangioleiomyomatosis
LAM may be associated with tuberous sclerosis (TSC-LAM), or occurs sporadically (Table 3). TSC-LAM occurs almost in a third of patients with tuberous sclerosis but is usually less severe than sporadic LAM. Sporadic LAM is seen almost exclusively in women of childbearing age. Occasionally, it may be seen in postmenopausal women. Most common symptoms are slowly progressive dyspnea, spontaneous pneumothorax, hemoptysis, chyloxthorax, or chylous ascites. Renal angiomyolipomas may also be seen.46, 47
Table 3.
Radiographic, Clinical, and Pathologic Characteristics of TS-LAM and Sporadic LAM
| Characteristic | TS-LAM | Sporadic LAM |
|---|---|---|
| Radiology | HRCT may show diffuse nodular lesions along with thin-walled cysts, especially when MMPH coexists45 | Thin-walled cysts surrounded by normal parenchyma49 |
| Severity (clinical signs/symptoms) | Less severe | More severe |
| Genetic predisposition | Familial | Occurs sporadically |
| Associated findings | CNS (hamartomas, developmental delays, seizure disorder) Skin (hypomelanotic macules, ash-leaf spots, shagreen patches on the lower back or nape of neck, subungual fibromas, skin tags, and café au lait spots) Eye (retinal phakomas) Hepatic and renal angiomyolipomas |
Renal angiomyolipomas46, 47 |
LAM = lymphangioleiomyomatosis; MMPH = multifocal micronodular pneumocyte hyperplasia; TS-LAM = tuberous sclerosis–associated lymphangioleiomyomatosis. See Table 2 legend for expansion of other abbreviation.
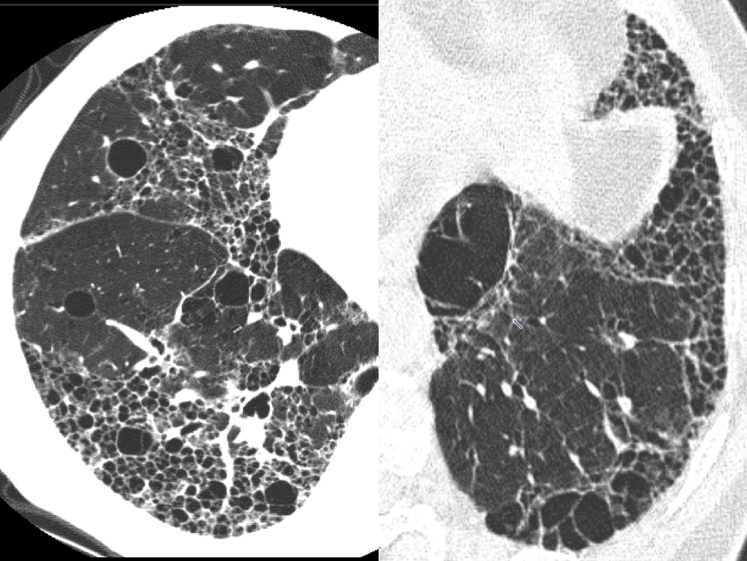
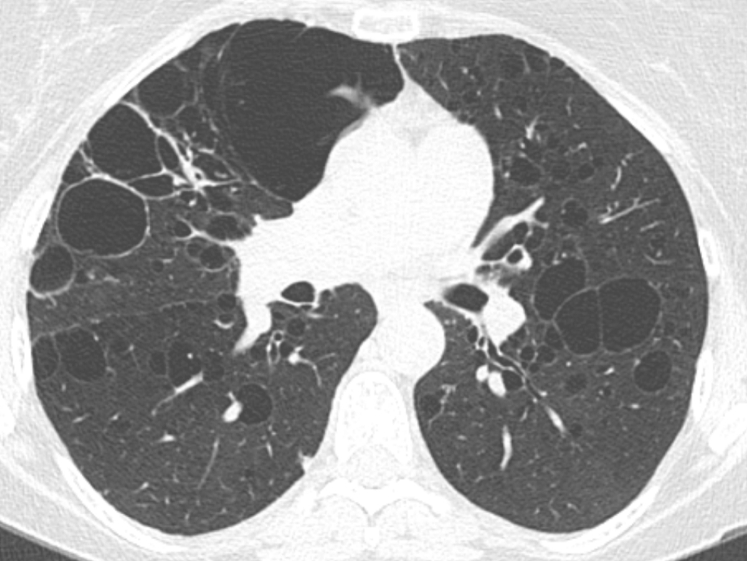
HRCT shows numerous thin-walled (from imperceptible up to <4 mm) cysts surrounded by normal-appearing lung parenchyma. Unlike PLCH, cysts caused by LAM are distributed throughout the lung (Fig 24A). Other findings include spontaneous pneumothorax (69%) and chylothorax (23%).48 In TSC-LAM, the HRCT may show diffuse nodular changes along with thin walled cysts. Pathology shows the airways, lymphatics and alveolar spaces infiltrated with smooth muscle cells. LAM cells have cosinophilic cytoplasm, appear spindle-shaped, and stain with HMB -45 (Fig 24B).
Figure 24.
A, Lymphangioleiomyomatosis (LAM). Thin-walled round (2-5 mm) cysts usually involve juxtaphrenic recesses and spare the extreme apices. The intervening lung between cysts is normal. B, LAM pathologic findings. (a) Low power histology shows multiple cysts surrounded by bundles of smooth muscle. (b) Surrounding cystic spaces are nodules of haphazardly arranged smooth muscle bundles. (c) HMB-45 immunohistochemistry shows positive cytoplasmic staining of the LAM cells.
Pulmonary Langerhans Cell Histiocytosis
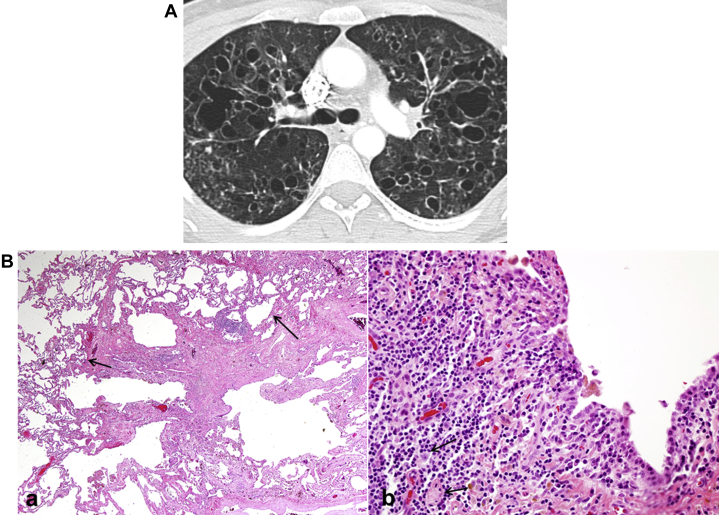
PLCH is a rare disease of lung and other organs characterized by accumulation of Langerhans cells and other inflammatory cells in small airways resulting in formation of inflammatory nodules (Fig 25A).49
Figure 25.
A, Pulmonary Langerhans cell histiocytosis. Generally seen in young smokers (most commonly between 20 and 40 years of age). Cysts are seen predominantly in upper and middle lobes, are variable in size, are thick- or thin-walled, and have bizarre shapes. The costophrenic angles are spared. The intervening lung parenchyma exhibits architectural distortion. B, Langerhans cell histiocytosis pathologic findings. (a) Low-power histology shows a stellate nodule composed of fibrosis with some cellular infiltrates. At the edges of the fibrotic scar is paracicatricial emphysema, giving some cystic appearance. (b) High-power shows a cellular proliferation of Langerhans cells.
PLCH is seen commonly in young adult smokers between the ages of 20 and 40 years with no gender predilection.50, 51 Most patients with PLCH are asymptomatic, but cough and dyspnea are common and pneumothorax may occur in about 15% of patients. Systematic symptoms like weight loss and fatigue may be seen in a few patients.52 Diabetes insipidus (polydipsia and polyuria), pain due to skeletal involvement and skin rashes are extra pulmonary manifestations of the disease.49, 50
HRCT findings vary according to the stage of disease. Nodules are seen in early stages and cysts are seen in later stages. Cysts are usually thin walled ranging in size from 1 to 20 mm (Fig 25A). These can progress to form thick, irregular walled, bizzare-shaped cysts. There is relative sparing of the lung bases and costophrenic angles.49, 50
Pathological examination in early stages shows destruction of the distal bronchioles with poorly formed granulomas comprised of predominantly Langerhans cells. Langerhans cells stain positive with S-100, although this stain is not specific to them. In late stages, there are nodules of stellate fibrosis alternating with cystic spaces (Fig 25B).
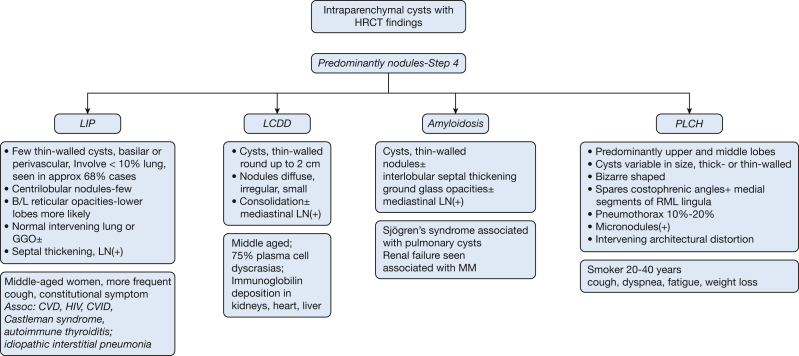
Step 4: Are the Lung Parenchymal Cysts Associated With Nodules?
If lung parenchymal cysts (Fig 26) are associated with nodules, the differential diagnosis includes LIP, LCDD, amyloidosis, and PLCH (Fig 27). Some conditions may be classified in more than one category. LIP and PLCH are associated with cysts and nodules in some cases and predominantly cysts in others. PLCH, depending on the stage of evolution of disease, may be concentrated in the upper and middle lobes and can be associated with nodules without cysts.
Figure 26.
Multiple intraparenchymal cysts with associated findings on high-resolution CT scan (HRCT) imaging. See Figure 6 legend for expansion of abbreviation.
Figure 27.
Algorithm for multiple intraparenchymal cysts with associated nodules on high-resolution CT scan (HRCT) imaging and HRCT scan. CVD = collagen vascular disease; CVID = common variable immunodeficiency; LCDD = light-chain deposition disease. See Figure 6 legend for expansion of other abbreviation.
Lymphoid Interstitial Pneumonia
LIP is a benign lymphoproliferative disorder limited to the lung, characterized pathologically by lymphocytic and plasma cell infiltration of alveolar and interlobular septa. LIP is more common in women than in men (ratio: 2.5-3:1) and is most commonly seen in 4th to 6th decades of life.53 Idiopathic LIP is rare. Most commonly LIP is associated with connective tissue diseases (Sjögren syndrome, rheumatoid arthritis, systemic lupus erythematosus) immune deficiency states, such as HIV infection and common variable immunodeficiency, infections, drug exposure, and allogeneic hematopoietic stem cell transplants.54
CT chest findings in LIP include alveolar opacities, ground glass attenuation (mostly diffuse), poorly defined centrilobular nodules, small subplerual nodules, thickening of bronchovascular bundles in a patchy distribution, interlobular septal thickening and mediastinal and hilar lymphadenopathy.43, 55 Cysts in LIP are random in distribution, although they may have a basilar or perivascular distribution. They are thin walled and range in size from 1-30 mm and are seen in 68% of cases (Fig 21).36
Light-Chain Deposition Disease
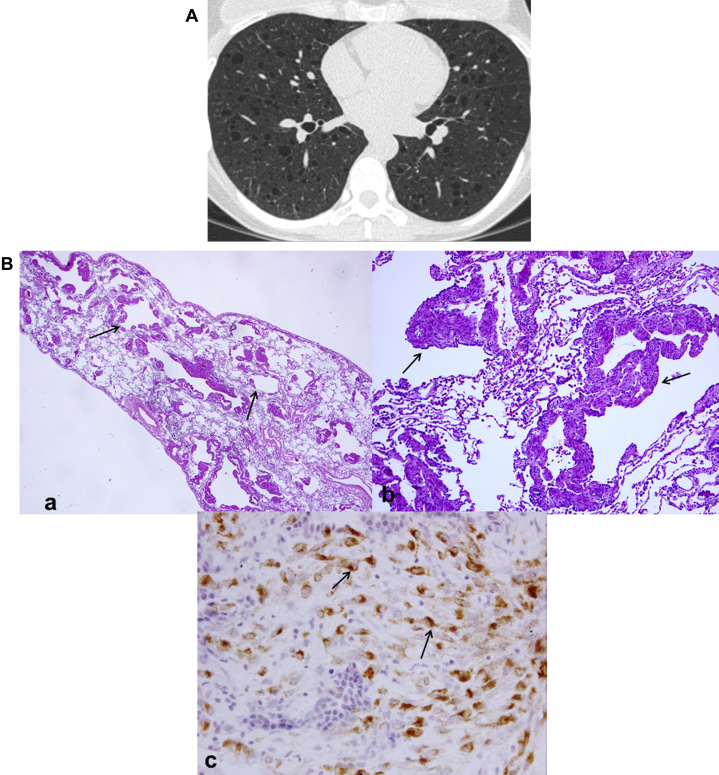
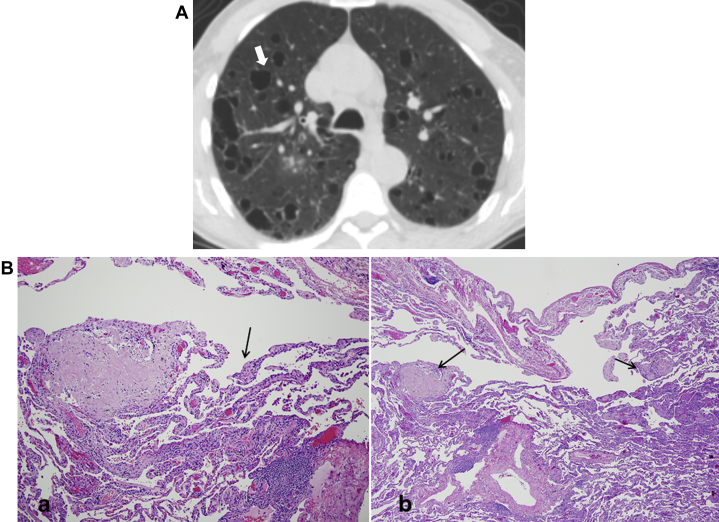
LCDD is a rare disorder of middle-aged patients (mean age, 67 years) characterized by deposition of monoclonal immunoglobulin light chains in various organs, including kidneys, lungs, skin, joints, or blood vessels.43 Seventy-five percent of LCDD cases occur in association with multiple myeloma or macroglobulinemia.56 Although lung involvement is rare, LCDD can result in respiratory failure and may require lung transplantation.57 HRCT manifestations commonly include nodules, lymphadenopathy, and cysts.42, 58 The cysts are thin-walled, round, and measure up to 2 cm in size (Fig 28A). Cyst formation is believed to correspond to dilation of the small airways. The associated nodules are irregular, multiple, and can be bilateral or unilateral. Patchy areas of consolidation can also be seen.56 Pathology shows characteristic focii of amorphous eosinophilic material (Fig 28B).
Figure 28.
A, Light-chain deposition disease (LCDD). Cysts are thin-walled, round, and may reach up to 2 cm in diameter (white arrow). Associated nodules are diffuse, irregular, and small. Consolidation of lung parenchyma may be present, as well as associated mediastinal lymphadenopathy. B, LCDD pathologic findings. (a) Low power shows a subpleural cystic area. (b) Focally in the wall of one of the cysts is a nodular focus of amorphous eosinophilic material characteristic of LCDD (black arrow).
Amyloidosis
Amyloidosis of the lung tends to occur in the 6th decade of life, commonly in association with Sjögren's syndrome (with or without LIP), lymphoproliferative disease or mucosa-associated lymphoid tissue (MALT) lymphoma.59 Depending on the type of amyloidosis, clinical manifestations may include: (1) tracheobronchial form causing diffuse or focal tracheal narrowing, (2) nodular form with lung parenchymal nodules that may be rather large, and (3) diffuse form with resultant miliary nodules, reticulonodular opacities, and honeycombing.60 When symptomatic, dyspnea on exertion is the most common symptom followed by cough, wheezing, hemoptysis, and recurrent pneumonia. Amyloid deposition in the lungs can present with large thin walled cysts with internal septae abutting bronchiocentric nodules. The cysts may have a peripheral predilection (Fig 29). The nodules in nodular pulmonary amyloidosis tend to be localized to lower lobes and subpleural areas. The nodules have sharp, smooth, lobulated contours, varying in size from 0.5 to 15 cm, with slow growth and often central cavitation or calcification.60
Figure 29.
Amyloidosis. Cysts are thin-walled with or without nodules. Interlobular septal thickening may be prominent. Ground-glass opacities can be seen, along with associated mediastinal lymphadenopathy.
Pulmonary Langerhans Cell Histiocytosis
Irregular, generally small nodules may be visible initially in PLCH. With disease progression, the nodules cavitate, giving rise to thick- and thin-walled, sometimes bizarre-shaped cysts and cavities (Fig 25A). (A more detailed description of PLCH was given earlier in the article.) Table 4 summarizes the HRCT features of cystic lung diseases associated with nodules.
Table 4.
Salient Features of Cystic Lung Diseases Associated With Nodules: Characteristics of Nodules Seen in Cystic Diseases
| LIP | Centrilobular, ill-defined ground glass nodules, more profuse in subpleural regions, demonstrating a patchy distribution |
| LCDD | Nodules are irregular, multiple, and can be bilateral or unilateral |
| Amyloid | The nodules tend to be localized to lower lobes and subpleural areas. They have sharp, smooth lobulated contours, varying in size from 0.5 to 15 cm, with slow growth and often central cavitation or calcification |
| PLCH | Irregular mainly in upper and middle lobes, sparing the costophrenic angles |
LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; PLCH = pulmonary Langerhans cell histiocytosis.
Step 5: Are Cysts Associated With Ground-Glass Opacities?
The differential diagnosis includes PJP and DIP (Fig 30).
Figure 30.
Algorithm for multiple intraparenchymal cysts with ground-glass opacities. D(A-a)O2 = alveolar arterial oxygen gradient; LDH = lactate dehydrogenase; PaO2 = partial pressure of oxygen in arterial blood. See Figure 6 legend for expansion of other abbreviations.
Pneumocystis Jirovecii Pneumonia
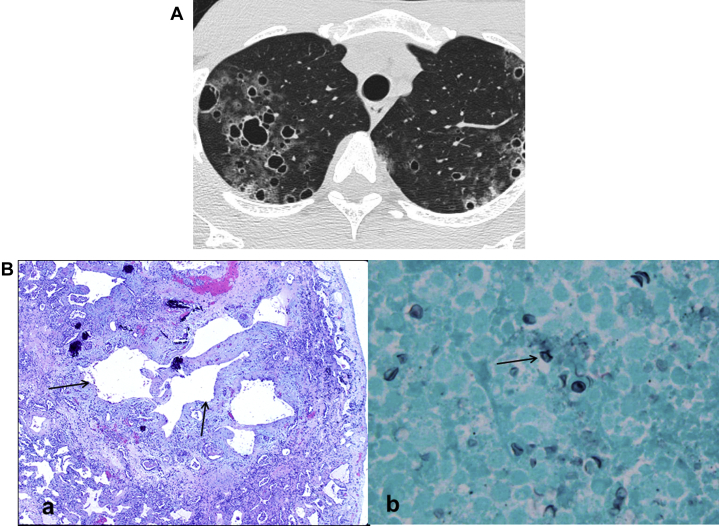
Despite effective anti-retroviral therapy, PJP is still the most common opportunistic pulmonary infection in patients with HIV infection. CT features of PJP include extensive ground-glass opacities or dense air-space disease in the central and perihilar distribution, along with septal wall thickening. Cystic lesions of the lung are relatively common, occurring in HRCT in up to 34% cases.61 Cysts are multiple, with upper lobe predominance and variations in size, shape, and wall thickness (Fig 25A). Cysts can rupture and cause pneumothorax, which is often difficult to treat. Disease in secondary pulmonary lobules alternating with normal lobules appears in, and presents a striking contrast on, HRCT; this pattern is referred to as “crazy paving”.62 Pathological exam of lung tissue demonstrates alveolar spaces filled with pinkish, amorphous material. P jirovecii may be seen as boat-shaped organisms. On high power field, Gomori methenamine silver stain, cysts of P jirovecii are visible with darkly stained foci (Fig 25B).
Desquamative Interstitial Pneumonia
DIP usually occurs in patients 40 to 60 years of age, presenting with nonspecific symptoms such as shortness of breath and cough. It occurs in men more commonly than in women (2:1), and 90% of cases occur in smokers. However, it can be associated with other conditions, including inhalation of inorganic particles, connective tissue disease, rheumatoid arthritis, sirolimus treatment (rare), and the heavy use of marijuana.63
The characteristic CT findings of DIP are ground-glass attenuation seen most prominently in lower lung zones and subpleural regions. Unlike the honeycomb appearance of advanced fibrotic lung disease, cysts in DIP have imperceptible walls; they are mostly discrete but occasionally they can be clustered and are surrounded by ground-glass opacities (Fig 31).4 The lung architecture is well preserved, and traction bronchiectasis is rare.64, 65 Cystic lesions seen in DIP may represent dilation of bronchioles (Fig 32), and they can disappear spontaneously or with treatment.65
Figure 31.
Desquamative interstitial pneumonia (DIP). Ninety percent of cases of DIP are seen in smokers. Small cysts are seen in the milieu of universal ground-glass opacities and scanty ground-glass nodules.
Figure 32.
A, Pneumocystis jirovecii: Diffuse ground glass opacities with septal thickening and occasional cysts (long-standing). B, P jirovecii pathologic findings. (a) Low- power histology from a case of long-standing P jirovecii pneumonia shows cystic spaces surrounded by thickened fibrotic walls containing some calcification. (b) High power of GMS (Gomori methenamine silver) stain shows characteristic cysts of P jirovecii with darkly stained foci.
The presence of small cysts within areas of ground-glass opacity is a unique feature of DIP. This feature is reported in approximately one-third of patients with DIP.63
Additional Testing (Other than HRCT Scan)
While HRCT scan is extremely useful in diagnosing cystic lung disease, further testing and confirmation may be required to establish a definite diagnosis and to initiate specific treatment. Diseases where confirmation of diagnosis (in the presence of compatible cystic change on HRCT) is recommended prior to initiation of treatment include:
-
1)
LAM: Confirmatory features may include the presence of angiomyolipomas, chylous effusions, lymphangioleiomyoma, elevated serum vascular endothelial growth factor-D (VEGF-D) levels, and lung biopsy showing changes consistent with LAM.
-
2)
BHD: Confirmatory features include skin biopsy revealing fibrofolliculomas and genetic testing showing the presence of pathogenic FLCN gene mutations.
-
3)
PLCH: While a diagnosis of PLCH can be established in typical cases by HRCT findings alone, tissue confirmation is needed in atypical cases, and to aid in detection of underlying genetic abnormalities (eg, BRAF mutation).
-
4)
Diseases such as amyloidosis and LCDD typically require tissue confirmation for the diagnosis.
-
5)
Sjögren's syndrome with characteristic cystic changes does not generally require a biopsy for confirmation. However, a biopsy in these cases is recommended if there is a concern for lymphoma.63, 64
Conclusions
In this review, we provide a systemic approach to aid clinicians in the diagnosis of cystic lung disease. Rather than classify each diagnosis in a single category, we have taken the most common presentations of each entity and placed them in one or more categories. Characteristics of cysts on HRCT imaging, considered together with clinical features, are helpful in guiding diagnostic and management strategies. First, we differentiate cysts from cavities, panlobular and centrilobular emphysema, and cystic bronchiectasis. The next step is to classify true cystic lesions by location into subpleural and parenchymal cysts. Subpleural cysts are commonly caused by paraseptal emphysema, bullae, blebs, and honeycombing. Parenchymal cysts are further classified into three groups based on whether they are the only HRCT abnormality or whether they are associated with nodules or ground-glass opacities.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: D. L. is a consultant to Parexel, Inc, Boehringer Ingelheim, Inc, Genentech, Inc, Gilead, Inc, and Veracyte, Inc. He has received support from Parexel, Inc, Siemens, Inc, and the National Heart, Lung, and Blood Institute. D. N. is a consultant to Siemens Medical, Inc for their development of a computer-assisted detection device. None declared: (Suhail Raoof, P. B., R. V., J. R., N. G., Sabiha Raoof, J. G., M. J. R., W. T., S. M., R. L.).
Other contributions: We gratefully acknowledge the secretarial assistance provided by Ms. Patrice Balistreri.
References
- 1.Ryu J.H., Swensen S.J. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744–752. doi: 10.4065/78.6.744. [DOI] [PubMed] [Google Scholar]
- 2.Echeveste J., Fernández-Velilla M., Torres M.I., Pardo M., Berrocal T., Martín-Hervás C. Cystic diseases of the lung: high-resolution computed tomography findings. Arch Bronconeumol. 2005 Jan;41(1):42–49. doi: 10.1016/s1579-2129(06)60393-5. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 3.Devakonda A., Raoof S., Sung A., Travis W.D., Naidich D. Bronchiolar disorders: a clinical-radiological diagnostic algorithm. Chest. 2010;137(4):938–951. doi: 10.1378/chest.09-0800. [DOI] [PubMed] [Google Scholar]
- 4.Koyama M., Johkoh T., Honda O. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol. 2003;180(3):827–835. doi: 10.2214/ajr.180.3.1800827. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N., Meraj R., Tanase D. Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases. Eur Respir J. 2015;46(4):1196–1199. doi: 10.1183/13993003.00570-2015. [DOI] [PubMed] [Google Scholar]
- 6.Lynch D.A., Newell J.D., Logan P.M., King T.E., Jr., Müller N.L. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? AJR Am J Roentgenol. 1995;165(4):807–811. doi: 10.2214/ajr.165.4.7676971. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi S., Yatera K., Kido T. Pulmonary mucosa-associated lymphoid tissue (MALT) lymphoma with multiple thin-walled pulmonary cysts: a case report and review of the literature. Intern Med. 2013;52(20):2325–2329. doi: 10.2169/internalmedicine.52.0377. [DOI] [PubMed] [Google Scholar]
- 8.Howling S.J., Hansell D.M., Wells A.U. Follicular bronchiolitis: thin-section CT and histologic findings. Radiology. 1999;212(3):637–642. doi: 10.1148/radiology.212.3.r99se04637. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.S., Tuder R., Lynch D.A. Lymphomatoid granulomatosis: radiologic features and pathologic correlations. AJR Am J Roentgenol. 2000;175(5):1335–1339. doi: 10.2214/ajr.175.5.1751335. [DOI] [PubMed] [Google Scholar]
- 10.Casserly I.P., Fenlon H.M., Breatnach E., Sant S.M. Lung findings on high-resolution computed tomography in idiopathic ankylosing spondylitis –correlation with clinical findings, pulmonary function testing and plain radiography. Br J Rheumatol. 1997;36(6):677–682. doi: 10.1093/rheumatology/36.6.677. [DOI] [PubMed] [Google Scholar]
- 11.Franquet T., Hansell D.M., Senbanjo T., Remy-Jardin M., Müller N.L. Lung cysts in subacute hypersensitivity pneumonitis. J Comput Assist Tomogr. 2003;27(4):475–478. doi: 10.1097/00004728-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Zamora A.C., Collard H.R., Wolters P.J., Webb W.R., King T.E. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J. 2007;29(1):210–214. doi: 10.1183/09031936.06.00044006. [DOI] [PubMed] [Google Scholar]
- 13.Ueda K., Honda O., Satoh Y. Computed tomography (CT) findings in 88 neurofibromatosis 1 (NF1) patients: Prevalence rates and correlations of thoracic findings. Eur J Radiol. 2015;84(6):1191–1195. doi: 10.1016/j.ejrad.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Newman B., Urbach A.H., Orenstein D., Dickman P.S. Proteus syndrome: emphasis on the pulmonary manifestations. Pediatr Radiol. 1994;24(3):189–193. doi: 10.1007/BF02012188. [DOI] [PubMed] [Google Scholar]
- 15.Irion K.L., Hocchegger B., Marchiori E. Proteus syndrome: high-resolution CT and CT pulmonary densitovolumetry findings. J Thorac Imaging. 2009;24(1):45–48. doi: 10.1097/RTI.0b013e31818b20cd. [DOI] [PubMed] [Google Scholar]
- 16.Dowton S.B., Pincott S., Demmer L. Respiratory complications of Ehlers-Danlos syndrome type IV. Clin Genet. 1996;50(6):510–514. doi: 10.1111/j.1399-0004.1996.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 17.Franquet T., Gómez-Santos D., Giménez A., Torrubia S., Monill J.M. Fire eater's pneumonia: radiographic and CT findings. J Comput Assist Tomogr. 2000;24(3):448–450. doi: 10.1097/00004728-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Bankier A.A., Brunner C., Lomoschitz F., Mallek R. Pyrofluid inhalation in “fire-eaters”: sequential findings on CT. J Thorac Imaging. 1999;14(4):303–306. doi: 10.1097/00005382-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Nowicka U., Wiatr E., Kupis W. [Pneumatocele during long-lasting observation of hyper IgE patient] Pneumonol Alergol Pol. 2007;75(2):200–207. [PubMed] [Google Scholar]
- 20.Shamberger R.C., Wohl M.E., Perez-Atayde A., Hendren W.H. Pneumatocele complicating hyperimmunoglobulin E syndrome (Job's Syndrome) Ann Thorac Surg. 1992;54(6):1206–1208. doi: 10.1016/0003-4975(92)90100-i. [DOI] [PubMed] [Google Scholar]
- 21.Jain A., Anand K., Singla S., Kumar A. Congenital cystic lung diseases. J Clin Imaging Sci. 2013 Jan 30;3:5. doi: 10.4103/2156-7514.106620. 13 Franquet T, Hansell DM, Senbanjo T, Remy-Jardin M, Müller NL. Lung cysts in subacute hypersensitivity pneumonitis. J Comput Assist Tomogr. 2003;27(4):475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 23.Gadkowski L.B., Stout J.E. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305–333. doi: 10.1128/CMR.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi M., Fukuoka J., Nitta N. Imaging of pulmonary emphysema: a pictorial review. Int J Chron Obstruct Pulmon Dis. 2008;3(2):193–204. doi: 10.2147/copd.s2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern E.J., Frank M.S. CT of the lung in patients with pulmonary emphysema: diagnosis, quantification, and correlation with pathologic and physiologic findings. AJR Am J Roentgenol. 1994;162(4):791–798. doi: 10.2214/ajr.162.4.8140992. [DOI] [PubMed] [Google Scholar]
- 26.Lee K.H., Lee J.S., Lynch D.A., Song K.S., Lim T.H. The radiologic differential diagnosis of diffuse lung diseases characterized by multiple cysts or cavities. J Comput Assist Tomogr. 2002;26(1):5–12. doi: 10.1097/00004728-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ogilvie C., Catterall M. Patterns of disturbed lung function in patients with emphysematous bullae. Thorax. 1959;14:216–224. doi: 10.1136/thx.14.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Bael K., La Meir M., Vanoverbeke H. Video-assisted thoracoscopic resection of a giant bulla in vanishing lung syndrome: case report and a short literature review. J Cardiothorac Surg. 2014;9:4. doi: 10.1186/1749-8090-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mura M., Zompatori M., Mussoni A. Bullous emphysema versus diffuse emphysema: a functional and radiologic comparison. Respir Med. 2005;99(2):171–178. doi: 10.1016/j.rmed.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Dowton S.B., Pincott S., Demmer L. Respiratory complications of Ehlers-Danlos syndrome type IV. Clin Genet. 1996;50(6):510–514. doi: 10.1111/j.1399-0004.1996.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 31.Murray R.A., Poulton T.B., Saltarelli M.G. Rare pulmonary manifestation of Ehlers-Danlos syndrome. J Thorac Imaging. 1995;10(2):138–141. doi: 10.1097/00005382-199521000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Copley S.J., Wells A.U., Hawtin K.E. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251(2):566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 33.Staatz G, Honnef D, Piroth W, et al. Pediatric Imaging. George Thieme Verlag; 2007.
- 34.Menko F.H., van Steensel M.A., Giraud S., European BHD Consortium Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10(12):1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 35.Toro J.R., Pautler S.E., Stewart L. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175(10):1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johkoh T., Müller N.L., Pickford H.A. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999;212(2):567–572. doi: 10.1148/radiology.212.2.r99au05567. [DOI] [PubMed] [Google Scholar]
- 37.Kramer S.S., Wehunt W.D., Stocker J.T., Kashima H. Pulmonary manifestations of juvenile laryngotracheal papillomatosis. AJR Am J Roentgenol. 1985;144(4):687–694. doi: 10.2214/ajr.144.4.687. [DOI] [PubMed] [Google Scholar]
- 38.Godwin J.D., Webb W.R., Savoca C.J., Gamsu G., Goodman P.C. Multiple, thin-walled cystic lesions of the lung. AJR Am J Roentgenol. 1980;135(3):593–604. doi: 10.2214/ajr.135.3.593. [DOI] [PubMed] [Google Scholar]
- 39.Woodring J.H., Fried A.M., Chuang V.P. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269–1271. doi: 10.2214/ajr.135.6.1269. [DOI] [PubMed] [Google Scholar]
- 40.Singh N., Bal A. Lung cyst caused by centrally located bronchogenic carcinoma. Arch Bronconeumol. 2012;48(3):99–101. doi: 10.1016/j.arbres.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Farooqi A.O., Cham M., Zhang L., International Early Lung Cancer Action Program Investigators Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781–786. doi: 10.2214/AJR.11.7812. [DOI] [PubMed] [Google Scholar]
- 42.Ye M.X., Zhao Y.L., Zhang J. Lung cysts as radiological manifestations of benign and malignant diseases: pitfalls in the diagnosis. Arch Bronconeumol. 2012;48(4):138. doi: 10.1016/j.arbres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Seaman D.M., Meyer C.A., Gilman M.D., McCormack F.X. Diffuse cystic lung disease at high-resolution CT. AJR Am J Roentgenol. 2011;196(6):1305–1311. doi: 10.2214/AJR.10.4420. [DOI] [PubMed] [Google Scholar]
- 44.Sandhu J.S., Goodman P.C. Pulmonary cysts associated with Pneumocystis carinii pneumonia in patients with AIDS. Radiology. 1989;173(1):33–35. doi: 10.1148/radiology.173.1.2789413. [DOI] [PubMed] [Google Scholar]
- 45.Allen C.M., Al-Jahdali H.H., Irion K.L., Al Ghanem S., Gouda A., Khan A.N. Imaging lung manifestations of HIV/AIDS. Ann Thorac Med. 2010;5(4):201–216. doi: 10.4103/1817-1737.69106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor J.R., Ryu J., Colby T.V., Raffin T.A. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990;323(18):1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 47.Johnson S. Lymphangioleiomyomatosis: Clinical features, management and basic mechanisms. Thorax. 1999;54:254–264. doi: 10.1136/thx.54.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juvet S.C., Hwang D., Downey G.P. Rare lung diseases I–Lymphangioleiomyomatosis. Can Respir J. 2006;13(7):375–380. doi: 10.1155/2006/696573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suri H.S., Yi E.S., Nowakowski G.S., Vassallo R. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis. 2012;7:16. doi: 10.1186/1750-1172-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartman T.E., Tazelaar H.D., Swensen S.J., Müller N.L. Cigarette smoking: CT and pathologic findings of associated pulmonary diseases. Radiographics. 1997;17(2):377–390. doi: 10.1148/radiographics.17.2.9084079. [DOI] [PubMed] [Google Scholar]
- 51.Vassallo R., Ryu J.H., Schroeder D.R., Decker P.A., Limper A.H. Clinical outcomes of pulmonary Langerhans'-cell histiocytosis in adults. N Engl J Med. 2002;346(7):484–490. doi: 10.1056/NEJMoa012087. [DOI] [PubMed] [Google Scholar]
- 52.Gupta N., Vassallo R., Wikenheiser-Brokamp K.A., McCormack F.X. Diffuse Cystic Lung Disease: Part I. Am J Respir Crit Care Med. 2015;191(12):1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cha S.I., Fessler M.B., Cool C.D., Schwarz M.I., Brown K.K. Lymphoid interstitial pneumonia: clinical features, associations and prognosis. Eur Respir J. 2006;28(2):364–369. doi: 10.1183/09031936.06.00076705. [DOI] [PubMed] [Google Scholar]
- 54.Koss M.N., Hochholzer L., Langloss J.M., Wehunt W.D., Lazarus A.A. Lymphoid interstitial pneumonia: clinicopathological and immunopathological findings in 18 cases. Pathology. 1987;19(2):178–185. doi: 10.3109/00313028709077131. [DOI] [PubMed] [Google Scholar]
- 55.Travis W.D., Galvin J.R. Non-neoplastic pulmonary lymphoid lesions. Thorax. 2001;56(12):964–971. doi: 10.1136/thorax.56.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombat M., Stern M., Groussard O. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med. 2006;173(7):777–780. doi: 10.1164/rccm.200510-1620CR. [DOI] [PubMed] [Google Scholar]
- 57.Gupta N., Vassallo R., Wikenheiser-Brokamp K.A., McCormack F.X. Diffuse Cystic Lung Disease: Part II. Am J Respir Crit Care Med. 2015;192(1):17–29. doi: 10.1164/rccm.201411-2096CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luraine R., Sohier L., Kerjouan M., Desrues B., Delaval P., Jouneau S. An unusual cause of cystic lung disease: light chain deposition disease. Rev Mal Respir. 2013;30(7):567–571. doi: 10.1016/j.rmr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Lantuejoul S., Moulai N., Quetant S. Unusual cystic presentation of pulmonary nodular amyloidosis associated with MALT-type lymphoma. Eur Respir J. 2007;30(3):589–592. doi: 10.1183/09031936.00136605. [DOI] [PubMed] [Google Scholar]
- 60.Chew K.M., Clarke M.J., Dubey N., Seet J.E. Nodular pulmonary amyloidosis with unusual, widespread lung cysts. Singapore Med J. 2013;54(5):e97–e99. doi: 10.11622/smedj.2013062. [DOI] [PubMed] [Google Scholar]
- 61.Boiselle P.M., Crans C.A., Jr., Kaplan M.A. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172(5):1301–1309. doi: 10.2214/ajr.172.5.10227507. [DOI] [PubMed] [Google Scholar]
- 62.Murayama S., Murakami J., Yabuuchi H., Soeda H., Masuda K. “Crazy paving appearance” on high resolution CT in various diseases. J Comput Assist Tomogr. 1999;23(5):749–752. doi: 10.1097/00004728-199909000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Godbert B., Wissler M.P., Vignaud J.M. Desquamative interstitial pneumonia: an analytic review with an emphasis on aetiology. Eur Respir Rev. 2013;22(128):117–123. doi: 10.1183/09059180.00005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akira M., Yamamoto S., Hara H., Sakatani M., Ueda E. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax. 1997;52(4):333–337. doi: 10.1136/thx.52.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuo K., Tada S., Kataoka M. Spontaneous remission of desquamative interstitial pneumonia. Intern Med. 1997;36(10):728–731. doi: 10.2169/internalmedicine.36.728. [DOI] [PubMed] [Google Scholar]