Abstract
Planctomycetes of the family Gemmataceae are characterized by large genome sizes and cosmopolitan distribution in freshwater and terrestrial environments but their ecological functions remain poorly understood. In this study, we characterized a novel representative of this family, strain PL17T, which was isolated from a littoral tundra wetland and was capable of growth on xylan and cellulose. Cells of this isolate were represented by pink-pigmented spheres that multiplied by budding and occurred singly or in short chains and aggregates. Strain PL17T was obligately aerobic, mildly acidophilic chemoorganotrophic bacterium, which displayed good tolerance of low temperatures. The major fatty acids were C18:0, C16:1ω5, and βOH-C16:1; the major polar lipid was trimethylornithine. The genome of strain PL17T consisted of a 9.83 Mb chromosome and a 24.69 kb plasmid. The G + C contents of the chromosomal and plasmid DNA were 67.4 and 62.3 mol%, respectively. Over 8900 potential protein-coding genes were identified in the genome including a putative cellulase that contains a domain from the GH5 family of glycoside hydrolases. The genome of strain PL17T contained one linked and one unlinked rRNA operons with 16S rRNA gene sequences displaying 94.5% similarity to that in Gemmata obscuriglobus UQM2246T. Based on the results of comparative phenotypic, chemotaxonomic and phylogenomic analyses, we propose to classify strain PL17T (= CECT 9407T = VKM B-3467T) as representing a novel genus and species of the family Gemmataceae, Frigoriglobus tundricola gen. nov., sp. nov.
Keywords: Planctomycetes; Frigoriglobus tundricola gen. nov., sp. nov.; Psychrotolerance; Cellulose utilization; Large genome; Unlinked rRNA operon
Introduction
Planctomycetes of the family Gemmataceae inhabit a wide variety of freshwater and terrestrial environments including lakes [39], rivers [7], wetlands [12], [18], and soils [8], [46]. The first taxonomically characterized member of this family is Gemmata obscuriglobus, which was isolated from water collected from the surface waters of Maroon Dam, Queensland, Australia [14]. Representatives of three other described genera, i.e. Zavarzinella [21], Telmatocola [24], and Fimbriiglobus [22] were isolated from Sphagnum-dominated boreal wetlands. Finally, the genera Tuwongella [42] and Limnoglobus [23] were described based on characterization of strains obtained from freshwater lakes in Australia and Russia, respectively. The family Gemmataceae belongs to the order Gemmatales [13] and accommodates strictly aerobic, chemo-organotrophic planctomycetes with spherical or ellipsoidal cells, which occur singly, in pairs, or are assembled in large rosette-like clusters and dendriform-like structures [22]. Notably, all members of this family are characterized by large genome sizes, which vary between 9.0 and 9.2 Mb in Gemmata species [1] and 12.3 Mb in Fimbriiglobus ruber SP5T [40]. The analysis of genome-encoded capabilities of these planctomycetes reveals wide repertoires of carbohydrate-active enzymes, including many unclassified putative glycoside hydrolases. This suggests the presence of high glycolytic potential in these bacteria [23].
Until recently, the ecological functions of these microorganisms remained poorly understood. The ability of Gemmataceae planctomycetes to utilize some polysaccharides, such as xylan, laminarin, lichenin and starch, has been reported in several taxonomic descriptions of these bacteria [21], [22], [23], [24]. The presence of cellulolytic capabilities has only been demonstrated for Telmatocola sphagniphila [24], while the ability to degrade chitin and utilize it as a source of nitrogen has only been reported for Fimbriiglobus ruber [40]. Additional evidence for the presence of hydrolytic capabilities in members of the Gemmataceae was obtained in the metatranscriptome-based study of planctomycetes involved in biopolymer degradation in acidic peatlands [17]. Gemmata-related planctomycetes were one of the groups with increased 16S rRNA transcript pools in chitin-, xylan- and cellulose-amended peat samples, thus suggesting occurrence of as yet undescribed Gemmataceae planctomycetes with pronounced hydrolytic capabilities.
This study was undertaken in order to characterize a novel representative of the family Gemmataceae, strain PL17T, which was isolated from a shallow littoral tundra wetland. Apart from the standard tests used in taxonomic studies and the genome analysis, we also examined the ability of strain PL17T to utilize a number of key biopolymers, such as cellulose, chitin, xylan and pectin. We demonstrate the presence of cellulose- and xylan-degrading capabilities in this planctomycete and determine the genome-encoded basis for these capabilities.
Materials and methods
Sampling site and isolation procedure
Strain PL17T was isolated from water collected from a shallow littoral wetland in a forested tundra, Nadym region, Yamalo-Nenets Autonomous Okrug, Russia (N65°35′03″, E73°04′20″). The isolate was obtained on medium M31 (modification of medium 31 described by Staley et al., 1992 [45]), solidified with 10 g phytagel (Sigma–Aldrich), and containing (per L distilled water): 0.1 g KH2PO4, 20 ml Hutner's basal salts, 1.0 g N-acetylglucosamine, 0.1 g peptone, 0.1 g yeast extract, pH 5.8. After sterilization, the medium was complemented with 5 ml of 5% (w/v) glucose solution, 1 ml Staley's vitamin solution [45], 0.2 g ampicillin (sodium salt). Successive re-streaking on solid medium M31 was used to purify strain PL17T, which was then routinely maintained on this medium without ampicillin and was sub-cultured at 2 month intervals.
Microscopic studies
Morphological observations and cell size measurements were made with a Zeiss Axioplan 2 microscope and Axiovision 4.2 software (Zeiss, Germany).
Physiological tests
Physiological tests were performed in liquid medium M31. Growth of strain PL17T was monitored by nephelometry at 600 nm in an Eppendorf BioPhotometer for 7–14 days under a variety of conditions, with temperatures ranging from 4 to 37 °C, pH from 3.8 to 8.0 and NaCl concentrations from 0% to 3.0% (w/v). Variations in the pH were achieved by using MES (pH 4.0–6.5) and MOPS (pH 6.5–7.9) buffer systems. The pH range of 3–4 was achieved by adjusting the medium pH with 0.5 M H2SO4. Organic carbon utilization was determined using mineral medium M31 (N-acetylglucosamine and peptone were omitted from the medium and the concentration of yeast extract was reduced to 0.005%). The range of potential growth substrates of strain PL17T was examined by replacing glucose in medium M31 with various other organic carbon sources in a concentration 0.05% (w/v); the pH being adjusted to pH 5.8. The strain was cultivated in 100 ml flasks containing 10 ml medium and was incubated at 22 °C for 2–3 weeks on a shaker. Control incubations were run in parallel under the same conditions but without the organic carbon substrate.
Cellulolytic capabilities of strain PL17T were determined using fibrous cellulose (0.05%, w/v) as a carbon source. Fibrous cellulose was prepared from Whatman filter paper as described earlier [36]. Strain PL17T was grown under static conditions at 22 °C in 160 ml serum bottles containing 20 ml of liquid mineral medium M31 with fibrous cellulose. Control incubations without cellulose were run in parallel under the same conditions. All incubations were performed in triplicate. After 30 days of incubation, the culture suspensions were fixed with 4% (w/v) freshly prepared para-formaldehyde solution as described by Dedysh et al., 2001 [11]. A combination of two Planctomycetes-specific Cy3-labeled oligonucleotide probes PLA46 (5′-GACTTGCATGCCTAATCC-3′) and PLA886 (5′-GCCTTGCGACCATACTCCC-3′) [34] was applied for specific detection of cells on micro-fibrils of cellulose. The oligonucleotide probes were purchased from Syntol (Moscow, Russia). Hybridization was done on gelatin-coated (0.1%, w/v) and dried Teflon-laminated slides (MAGV, Germany) with eight wells. The fixed samples were applied to these wells, hybridized to the corresponding fluorescent probes, stained with the universal DNA stain 4′,6-diamidino-2-phenylindole (DAPI, 1 μM) and examined with a Zeiss Axioplan 2 microscope (Zeiss, Jena, Germany) equipped with the Zeiss Filters No 20 and 02 for Cy3-labeled probes and DAPI staining, respectively. Cell counting was performed on 100 randomly chosen fields of view (FOV) for each test sample. The number of target cells per ml of culture suspension was determined from the area of the sample spot, the FOV area, and the volume of the fixed aliquot used for hybridization.
Strain PL17T was tested for growth under anaerobic conditions in anaerobic jars by using AnaeroGen anaerobic system envelopes (Oxoid). Analyzes of enzymatic profiles, oxidase test, gelatin and urease hydrolysis were made with API ZYM and API 20NE kits (bioMérieux). Catalase test was carried out using the standard method [15]. Susceptibility to antibiotics was determined on solid M31 medium using discs (Oxoid) containing the following antibiotics: ampicillin (10 μg), gentamycin (10 μg), kanamycin (30 μg), neomycin (10 μg), novobiocin (30 μg), streptomycin (10 μg), chloramphenicol (30 μg), rifampicin (10 μg), tetracycline (10 μg) and lincomycin (10 μg). Growth of PL17T and occurrence of growth inhibition zones around these discs were assessed after 4 weeks of incubation at 22 °C. Only the inhibition zones exceeding 2 mm were taken into account.
Lipid analyses
For lipid analysis, cells of PL17T and Gemmata obscuriglobus DSM 5831T were grown in parallel in liquid M31 medium and harvested in the late exponential growth phase. Fatty acids were analyzed after acid hydrolysis of whole cells, following procedure described elsewhere [44]. The main intact polar lipids (IPLs) in PL17T were analyzed using procedures reported previously [29].
Genome sequencing, annotation and analysis
For genome analysis, cells of strain PL17T were grown in liquid M31 medium with 0.5% glucose and harvested after two weeks of incubation at 22 °C. Genomic DNA was isolated from strain PL17T using the PowerSoil DNA isolation kit (Mo Bio Laboratories Inc., Carlsbad, CA). The sequencing library for Illumina sequencing was prepared using the TruSeq nano DNA library prep kit (Illumina, USA) following the manufacturer's instructions. The sequencing of this library on the Illumina MiSeq platform using Miseq Reagent Kit v3 (600 cycles) sequencing reagents generated 1,502,025 paired-end reads with an average length of 289 nt (0.869 Gb in total). Primer sequences were removed from the Illumina reads using Cutadapt v.1.17 [28] with the default settings, and low quality read ends were trimmed using Sickle v.1.33 (option q = 30) (https://github.com/najoshi/sickle). For Nanopore sequencing the library was prepared using the 1D ligation sequencing kit (SQK-LSK108, Oxford Nanopore, UK). Sequencing of this library in an R9.4 flow cell (FLO-MIN106) using MinION device yielded 580,793 reads with a total length of 3.82 Gb. Hybrid assembly of Illumina and Nanopore reads was performed using Unicycler v. 0.4.8[47]. Two circular contigs of 9,832,708 bp and 24,691 bp were obtained.
Gene search and annotation were performed using the RAST server [35], followed by manual correction. Subsequent inspection was done in PROKKA package [43], RNAMMER [25], BLAST+ [9], and ARAGORN [26]. Annotation with PROKKA was performed against the UNIPROT database [2]. The analysis of strain PL17T genome sequence using the Kyoto Encyclopedia of Genes and Genomes (KEGG) was performed applying GhostKOALA tool [19]. The DNA–DNA hybridization values between the genome of strain PL17T and the genomes of phylogenetically related planctomycetes were estimated using formula 2 of the Genome-to-Genome-Distance-Calculator [4], [5]. The average nucleotide identity (ANI) values were determined using ANI calculator (http://enve-omics.ce.gatech.edu/ani/). The average amino acid identity (AAI) between the selected genomes was calculated using the aai.rb script from the enveomics collection [41]. Signal peptides were predicted using Signal P v. 5.0 for Gram-negative bacteria (http://www.cbs.dtu.dk/services/SignalP/).
The annotated chromosome and plasmid sequences of strain PL17T have been deposited in GenBank under the accession numbers CP053452 and CP053453, respectively.
Search for cellulase-encoding genes and subsequent phylogenetic analysis
Two full-length representatives from each of the fifteen (GH5, GH6, GH7, GH8, GH9, GH10, GH12, GH26, GH44, GH45, GH48, GH51, GH74, GH124 and GH148) cellulase-containing CAZy families [27], as well as the unclassified cellulase from Ruminococcus flavefaciens (GenPept, AAA26468.1), were used as queries for the BLAST search. Planctomycetal or experimentally-characterized bacterial proteins were preferred if available. At the next stage the obtained PL17T proteins were used as queries for the BLAST search, all revealed proteins were analyzed by PSI Protein Classifier [31] in order to detect proteins, which are deposited in the CAZy database. The most similar sequences were selected based on the BLAST search results. Closely related proteins of strains from the same species and species from the same genus except of those from planctomycetes were not used. Multiple sequence alignment was prepared manually using the program BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) on the basis of BLAST pairwise alignments [30]. After removing of the most variable regions, the multiple sequence alignment was used to implement classical phylogenetic inference programs, using either maximum parsimony (MP) or distance (NJ) methods. Programs PROTPARS and NEIGHBOR from the PHYLIP package (http://evolution.gs.washington.edu/phylip.html) were used. Moreover, programs SEQBOOT, PROTPARS, and CONSENSE and programs SEQBOOT, PROTDIST, NEIGHBOR, and CONSENSE were successively used to derive confidence limits, estimated by 1000 bootstrap replicates, for each node in the maximum parsimony and distance tree, respectively. The program TreeView Win32 (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html) was used for drawing the trees.
Phylogenomic analysis
The Genome Taxonomy Database toolkit (GTDB-Tk) [37], release 04-RS89 (https://github.com/Ecogenomics/GTDB-Tk) was used to identify the 120 single-copy, phylogenetically informative bacterial marker genes used in the GTDB classification system in the genome of strain PL17T. These were used to construct a multiple alignment of concatenated single-copy gene sequences, comprising those from PL17T and phylogenetically related planctomycetes. The multiple alignment built in GTDB-Tk was used to construct a phylogenetic tree in PhyML v. 3.3 [16] with default parameters.
Results and discussion
Cell morphology and physiology
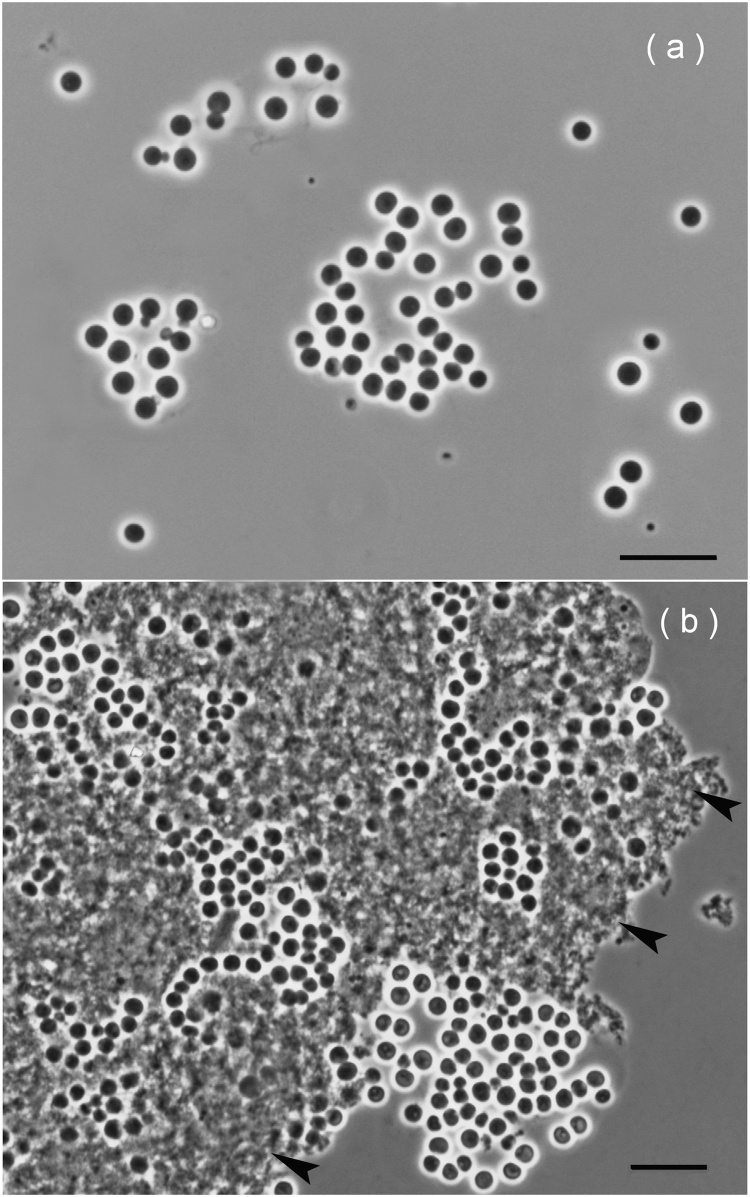
Mature cells of strain PL17T were spherical and varied in size from 1.8 to 2.6 μm. Cells occurred singly, in pairs, in short chains or in shapeless aggregates and reproduced by budding (Fig. 1a). The buds separated from the mother cells were highly motile. This cell morphology is characteristic for planctomycetes of the family Gemmataceae [22]. On phytagel-solidified M31 medium, strain PL17T formed small (1–3 mm in diameter), light-pink-pigmented, round colonies. Liquid cultures displayed light-pink turbidity.
Fig. 1.
(a) Phase-contrast image of cells of strain PL17T grown for 14 days on solid medium M31with glucose (a) and on liquid medium with xylan (b). Black arrows point to xylan micro-particles. Bars, 10 μm.
Strain PL17T grew in the temperature range of 4–28 °C, with an optimum at 15–22 °C. The specific growth rate displayed by strain PL17T at 10 °C (μ = 0.02 h−1; Td = 35 h) did not differ substantially from that observed at 22 °C (μ = 0.03 h−1; Td = 21 h). Slower but consistent growth was also detected at 4 °C (μ = 0.009 h−1; Td = 76 h). In contrast to all previously described Gemmataceae planctomycetes, strain PL17T did not grow at >28 °C. This planctomycete, therefore, is a psychrotolerant bacterium capable of growth at low temperatures (4–10 °C), characteristic of tundra ecosystems during the summer season. The pH range for growth was pH 4.2–6.8, with an optimum at pH 5.0–5.5. Growth was completely inhibited at NaCl concentrations above 0.5% (w/v).
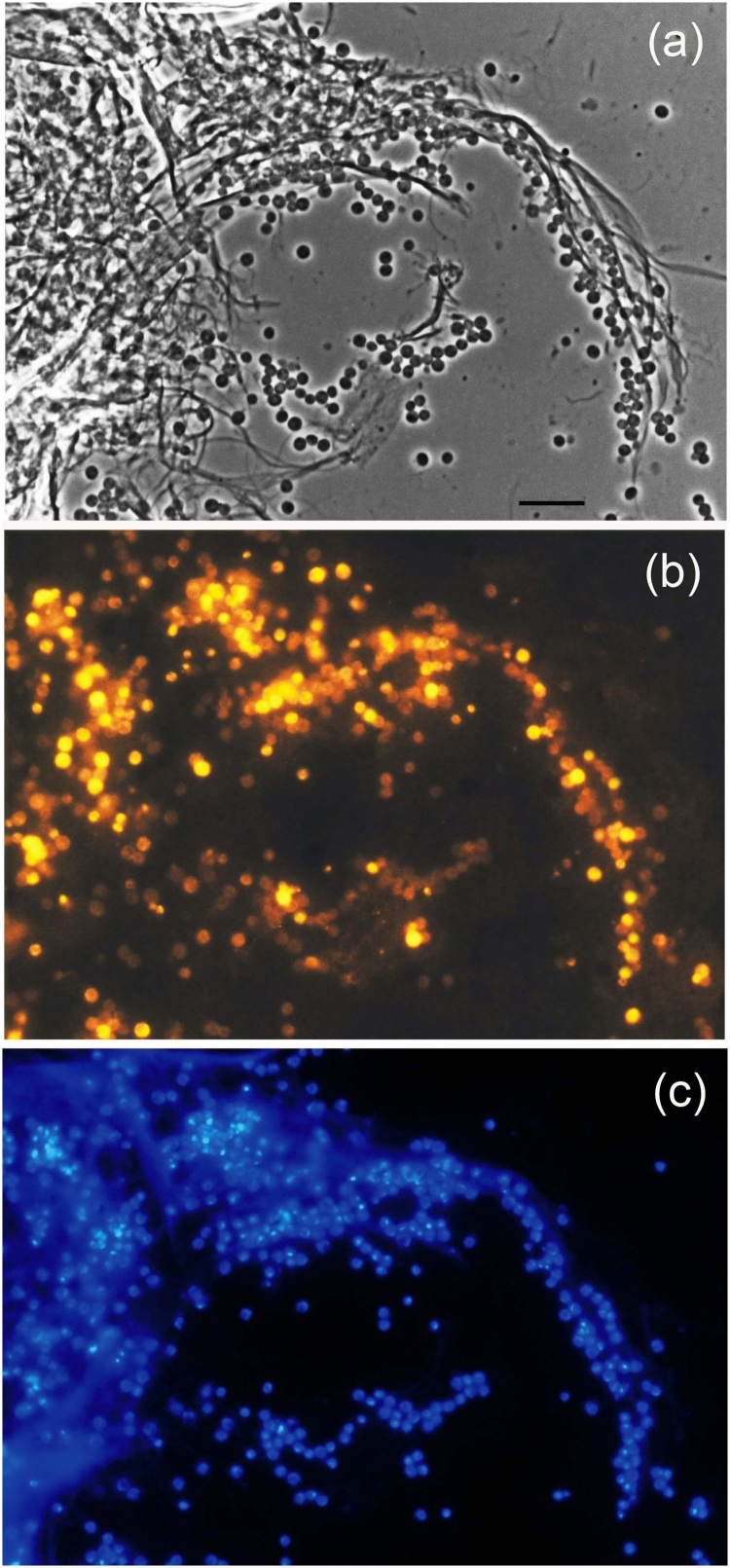
Strain PL17T is an obligately aerobic chemoorganotroph. It was not capable of growth under anoxic conditions. The preferred growth substrates were various sugars, including N-acetylglucosamine, and some polymeric substances, such as arabinogalactan, dextrin, chondroitin sulfate, laminarin, locust bean gum, gelatin, lichenin, pullulan, starch, and xanthan (see the species description). Good growth was observed on xylan (Fig. 1b). Organic acids and alcohols were not utilized. Casein, chitosan, chitin and pectin were not hydrolyzed. Similar to another representative of the family Gemmataceae, Telmatocola sphagniphila SP2T [24], strain PL17T displayed cellulolytic potential. Microscopic examination of the culture after 30 days of incubation with fibrous cellulose revealed numerous cells of strain PL17T being attached to micro-fibers of cellulose (Fig. 2). Cell counting revealed a substantial increase by two orders of magnitude in cell abundance between the incubation start (8.16 ± 1.43 × 107 cells ml−1) and 30 days of incubation with cellulose (1.32 ± 0.14 × 109 cells ml−1). By contrast, no increase in cell abundance was observed in control incubations without cellulose (cell density after 30 days of incubation was 8.78 ± 1.52 × 107 cells ml−1).
Fig. 2.
Specific detection of cells of strain PL17T grown on liquid medium with fibrous cellulose for 30 days by FISH: phase-contrast image (a); epifluorescent micrographs of in situ hybridizations with Cy3-labeled probes PLA46 and PLA886 (b); DAPI stained cells (c). Bar, 10 μm.
Strain PL17T was resistant to ampicillin, chloramphenicol, lincomycin, novobiocin and streptomycin, but sensitive to gentamicin, kanamycin, neomycin, rifampicin and tetracycline.
Genome characteristics and phylogenetic analysis
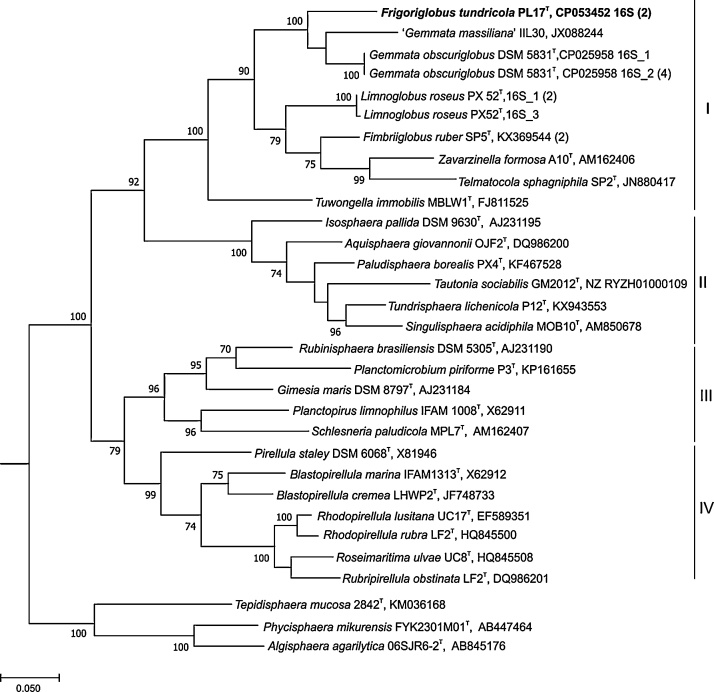
The genome of strain PL17T consists of a 9,832,702 bp chromosome and a circular plasmid of 24,691 bp. The G + C content of the chromosomal DNA is 67.4 mol% and the corresponding value for the plasmid is 62.3 mol%. A total of 8,923 potential protein-coding genes and 83 tRNA genes were predicted. The genome harbors one rRNA operon with a canonical gene order (16S–23S–5S) and no tRNA genes between the 16S and 23S rRNA genes, and an “unlinked” operon with 16S rRNA gene located distantly from a 23S-5S rRNA gene pair. The presence of unlinked rRNA operons in many bacteria including Gemmata species has recently been reviewed [6]. The linked and unlinked 16S rRNA gene copies in strain PL17T were identical and were most similar to that of Gemmata obscuriglobus UQM2246T, showing 94.5% sequence identity (Fig. 3).
Fig. 3.
16S rRNA gene-based maximum parsimony tree showing the phylogenetic position of strain PL17T in relation to other members of the family Gemmataceae (I) and some members of the families Isosphaeraceae (II), Planctomycetaceae (III) and Pirellulaceae (IV). All 16S rRNA gene copies revealed in the genomes of the family Gemmataceae are included in the tree. The number of identical 16S rRNA gene copies is shown in parenthesis. Bar, 0.1 substitutions per nucleotide position. The root (not shown) was composed of five 16S rRNA gene sequences from anammox planctomycetes (AF375994, AF375995, AY254883, AY257181, and AY254882).
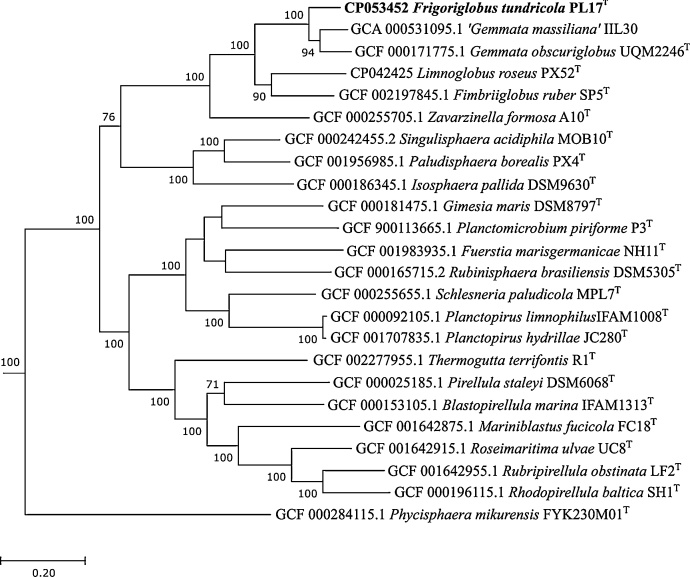
The phylogenomic analysis confirmed that strain PL17T is most closely related to members of the genus Gemmata (Fig. 4). Following the proposed minimal standards for the use of genome data for the taxonomy of prokaryotes [10], we determined both the overall genome similarity and the average nucleotide identity (ANI) between strain PL17T and Gemmata obscuriglobus UQM2246T. Strain PL17T shared 22.4 ± 2.5% overall genome similarity with Gemmata obscuriglobus UQM2246T, while the ANI value calculated for the genomes of these planctomycetes was 80%. The average amino acid sequence identity (AAI) between the PL17T genome and the genomes of G. obscuriglobus and G. massiliana, was in the range of 69–71%, a value slightly above the recently proposed AAI threshold for members of the same genus (65% AAI; Konstantinidis et al., 2017 [20]). However, taking into account the < 95% identity of 16S rRNA sequences of strain PL17T and known members of the genus Gemmata (Fig. 3), PL17T should be considered as a member of a novel genus within the family Gemmataceae.
Fig. 4.
Phylogenomic tree inferred from the concatenation of 120 single-copy, phylogenetically informative bacterial marker genes showing the phylogenetic position of strain PL17T. The tree was reconstructed using the Genome Taxonomy Database toolkit (Parks et al., 2018 [37]), release 04-RS89. The significance levels of interior branch points obtained in maximum-likelihood analysis were determined by bootstrap analysis (100 data re-samplings). Bootstrap values of over 70% are shown. The root (not shown) was composed of 14 genomes of members of the Candidate division Poribacteria. Bar, 0.2 substitutions per amino acid position.
Fatty acid and lipid composition
The major fatty acids (FA) detected in strain PL17T were C16:1ω5c and C18:0 (Table 1). Notably, the C16:1ω5c FA, which is one of the most abundant FAs in strain PL17T, Gemmata obscuriglobus UQM2246T, Zavarzinella formosa A10T, Telmatocola sphagniphila SP2T, and Tuwongella immobilis MBLW1T [21], [24], [42], is absent in Fimbriiglobus ruber SP5T and Limnoglobus roseus PX52T [22], [23]. The fatty acid βOH-C16 comprised 12.9% of total FAs in strain PL17T and was also present in the acid hydrolysate of Limnoglobus roseus PX52T (11.6%), Fimbriiglobus ruber SP5T (8.2%) and Telmatocola sphagniphila SP2T (4.9%). The βOH-fatty acids are derived from hydrolysis of intact polar ornithine lipids and differ in composition. Trimethylornithine was the major component of the IPL profile of strain PL17T (Table 1), although minor amounts of dimethylphosphatidylethanolamine, mono- and dimethylornithine were also detected. The presence of polyunsaturated hydrocarbon n-C31:9 is characteristic for all members of this family. Parkeol, an unusual C30 sterol, is present as well (Table 1). Biosynthesis of parkeol is confirmed by the presence of a small operon encoding squalene monooxygenase and 2,3-oxidosqualene cyclase in the genome (GenPept, QJX00199.1 and QJX00198.1). Parkeol is very uncommon in bacteria and has previously only been reported in Gemmata obscuriglobus DSMZ 5831 [38]. This chemotaxonomical trait of strain PL17T confirms its close relationship with other Gemmata species.
Table 1.
Fatty acids and hydrocarbons (%; normalized on their sum) present in the acid hydrolysate of cell material of strain PL17T (1) in comparison to those in Gemmata obscuriglobus DSM 5831T (2). Only fatty acids comprising ≥0.5% of the total are shown. Major components (>5%) are given in bold type face.
| Fatty acids | 1 | 2 |
|---|---|---|
| C14:0 | 1.1 | |
| anteisoC15:0 | 2.1 | |
| C16:1ω5 | 10.3 | 16.1 |
| C16:0 | 1.1 | 1.2 |
| isoC17:0 | 0.8 | |
| anteisoC17:0 | 0.9 | |
| C17:0 | 1.1 | |
| C18:1ω9 | 0.6 | 0.8 |
| C18:1ω7 | 1.9 | 1.6 |
| C18:1ω5 | 0.9 | 1.5 |
| C18:0 | 47.9 | 48.3 |
| isoC19:0 | 1.5 | |
| anteisoC19:0 | 0.6 | |
| C19:0 | 0.5 | 1.4 |
| C20:0 | 5.3 | 2.1 |
| Hydroxy-fatty acids | ||
| iso-βOH-C15:0a | 1.2 | |
| βOH-C16:1a | 12.9 | 1.7 |
| iso-βOH-C17:1a | 5.1 | |
| anteiso-βOH-C17:1a | 1.3 | |
| iso-βOH-C17:0a | 1.4 | |
| βOH-C18:1a | 4.0 | |
| ωOH-C24 | 2.8 | |
| (ω-1)OH-C28:1 | 1.3 | |
| (ω-1)OH-C28:0 | 1.5 | |
| (ω-1)OH-C30:1 | 0.8 | |
| Sterols | ||
| Parkeol | 0.9 | 1.7 |
| Hydrocarbons | ||
| n-C31:9 | 7.8 | 6.0 |
| IPLs | ||
| PG | + | |
| OL | + | + |
| DMO | + | |
| PC | ++ | |
| TMO | +++ | +++ |
Mainly present as lyso-ornithine lipids.
Genome-encoded metabolism and cell biology
All genes encoding metabolic pathways common for chemo-organotrophic bacteria, such as glycolysis, the citrate cycle, the pentose-phosphate pathway, and oxidative phosphorylation were present in the genome of strain PL17T. This planctomycete has the genomic potential for synthesis of all amino acids. The number of ABC transporters based on PROKKA predictions is 20. Also, one fructose-type sugar-specific subunit of phospho-transferase system could be found in PL17T. All genes essential for chemotaxis, including cheA, cheB, cheR, cheC, cheY and cheW, were identified in the genome of strain PL17T. The high number of identified genes responsible for flagellar assembly (24 genes) agrees well with the fact that strain PL17T produces motile swarmer cells.
Genomic determinants of cellulolytic and xylanolytic capabilities
Homology analysis of all proteins potentially encoded in the genome of strain PL17T was performed in order to select only the proteins from fifteen CAZy families (see the corresponding list of families in Methods) containing at least one enzyme with experimentally characterized cellulase activity (EC 3.2.1.4; [48]). Three proteins falling in the GH5 (GenPept, QJX00361.1), GH10 (QJX00392.1), and GH74 (QJW93300.1) families of glycoside hydrolases were identified. All three proteins were predicted to contain an N-terminal signal peptide indicating their export and extracellular operation.
The GH10 family of glycoside hydrolases includes mainly endo-β-xylanases (EC 3.2.1.8 and 3.2.1.32) and few cellulases only [27]. The identified protein (GenPept, QJX00392.1) has a high similarity with three proteins represented in the GH10 family list at the CAZy database: QGJ72167.1 (E-value = 4e–87), QDV27060.1 (3e−60), and AQT68668.1 (3e−48). However, many other hits correspond to so-called NC list which includes proteins not yet assigned to a family. Usually proteins from this list show only a distant similarity to experimentally characterized glycoside hydrolases. Multiple sequence alignment of the identified protein (QJX00392.1) and its closest homologues from other planctomycetes revealed that both catalytically essential Glu residues (nucleophile and proton donor) are absent, suggesting that this group of planctomycetal GH10 proteins does not have any enzymatic activity [32], [33].
The GH74 family of glycoside hydrolases includes mainly xyloglucanases (EC 3.2.1.151); the only known cellulase is from Thermotoga maritima (GenPept, AAD35393.1). The identified protein (QJW93300.1) has a low similarity with proteins of the GH74 family: AFY66524.1 (E-value = 5e–13), APO44368.1 (2e−09), AFY32698.1 (4e−09), AEY66994.1 (9e−09). However, it is closely related (E-value = 0.0) to two proteins from the NC list: VIP00677.1 and QHL87682.1. Comparison of the identified protein (QJW93300.1) and the T. maritima cellulase showed only 26% of sequence identity. These results suggest that the identified GH10 and GH74 proteins (QJX00392.1 and QJW93300.1), most likely, do not have the cellulase activity.
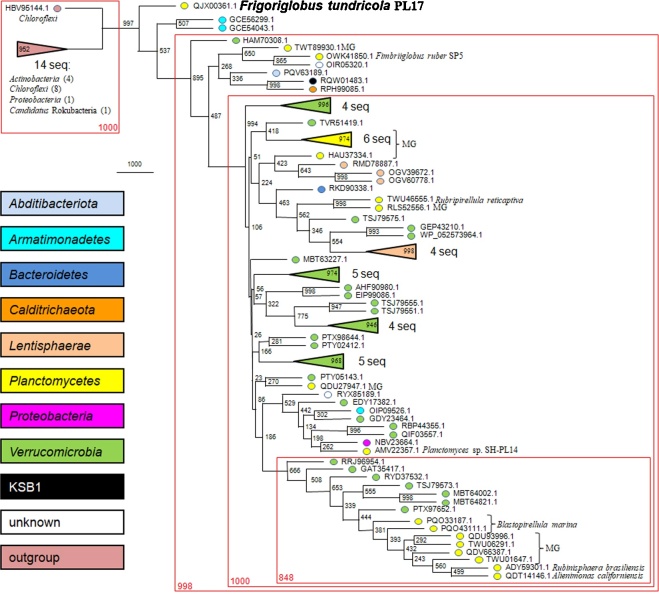
The GH5 family of glycoside hydrolases includes experimentally characterized proteins with 28 types of enzymatic activities; the cellulase activity is one of the most represented. This family is divided into 56 subfamilies with more specific spectrum of enzymatic activities [3], [27]. The identified protein (GenPept, QJX00361.1) has rather high similarity with many proteins from the GH5 family: QDT14146.1 (E-value = 3e–30), QIF03557.1 (1e−29), ADY59301.1 (2e−29), QDV66387.1 (8e−29), QDU93996.1 (1e−28), AMV22357.1 (8e−28), QDU27947.1 (3e−27), etc. However, none of these proteins are included into any subfamily at the CAZy database. These proteins, therefore, compose a new subfamily, which does not include any experimentally characterized members. The identified protein (QJX00361.1) consists of three domains: the N-terminal catalytic TIM-barrel-type GH5 domain (PF00150), the internal Ig-like-fold domain (PF16640), and the C-terminal β-propeller-fold VCBS-like domain (similar to PF13517). Phylogenetic analysis of the GH5 domains from the identified protein and its closest homologues demonstrated that planctomycetal proteins do not form a single cluster. They are more or less randomly distributed at the phylogenetic tree (Fig. 5). The protein from PL17T is the most divergent representative of a distinct cluster which has 99.7% and 100% of bootstrap support at MP- and NJ-tree, respectively. This cluster contains mainly representatives of three poorly studied bacterial phyla: Verrucomicrobia, Planctomycetes, and Lentisphaerae. Several other phyla, such as Abditibacteriota, Armatimonadetes, Calditrichaeota, Bacteroidetes, and Proteobacteria, are significantly less represented. The tree topology suggests that several lateral gene transfers between Planctomycetes and Verrucomicrobia occurred during the evolution.
Fig. 5.
The maximum parsimony phylogenetic tree of the studied subfamily of the glycoside hydrolase family GH5. Statistical significance of tree nodes was assessed by bootstrap analysis; the number of supporting pseudoreplicas out of 1000 is indicated at each node. For each of seven stable clusters, bootstrap support is indicated inside the triangle and the number of proteins is indicated near the triangle. Phylogenetic affiliation of proteins is indicated by colors. Organism names are indicated for members of the Planctomycetes only. MG, planctomycetal metagenomes. Composition of the outgroup is indicated. Four very stable clusters identified by the NJ-analysis are defined by brown rectangles.
The above described protein (QJW93300.1) from the GH74 family may potentially be responsible for the observed ability of strain PL17T to hydrolyse xylan. Short xylo-oligomers, produced through hydrolysis of xylan by xylanases, could be cleaved into monomeric xylose by signal-peptide containing β-xylosidase of GH39 family (GenPept, QJW93461.1). Xylose probably is imported into the cell via ABC-type transporters and further metabolized via the isomerase pathway, as suggested by the presence of genes encoding xylose isomerase and xylulose kinase, which convert xylose to xylulose-5-P. The presence of xylulose 5-phosphate phosphoketolase suggests that xylulose-5-P could than enter the phosphoketolase pathway usually employed by heterolactic acid bacteria for fermentation.
Overall, the presence of several dozen of putative glycoside hydrolases of unknown functions is consistent with a broad range of polysaccharides that could support the growth of strain PL17T.
In summary, strain PL17T is phylogenetically (Fig. 3, Fig. 4) and phenotypically (Table 2) distinct from other described members of the family Gemmataceae, i.e., members of the genera Gemmata, Limnoglobus, Fimbriiglobus, Zavarzinella, Telmatocola and Tuwongella. We, therefore, propose to classify it as representing a novel genus and species of planctomycetes, Frigoriglobus tundricola gen. nov., sp. nov. Descriptions of these novel genus and species are given in Table 3.
Table 2.
Major characteristics that distinguish strain PL17T, Gemmata obscuriglobus DSM 5831T[14], [21], Limnoglobus roseus PX52T[23], Fimbriiglobus ruber SP5T[22], Telmatocola sphagniphila SP2T[24], Zavarzinella formosa A10T[21] and Tuwongella immobilis MBLW1T[42].
| Characteristic | PL17T | G. obscuriglobus | L. roseus | F. ruber | T. sphagniphila | Z. formosa | T. immobilis |
|---|---|---|---|---|---|---|---|
| Cell shape | Spherical | Spherical to ovoid | Spherical | Spherical | Spherical | Ellipsoidal | Spherical |
| Cell size (μm) | 1.8–2.6 | 1.4–3.0 × 1.4–3.0 | 1.6–2.3 | 1.6–2.8 × 1.4–2.8 | 1.2–2.0 | 2.5–3.2 × 2.0–2.5 | 2.2–3.1 |
| Motile swarm cells | + | + | + | − | − | + | − |
| Rosette formation | − | − | − | − | + | + | − |
| Stalk formation | − | − | + | − | + | + | − |
| Colony color | Pink | Rose | Pink | Dark pink to red | Pink | Pink | Pink |
| Salinity tolerance | <0.5% | <0.6% | <0.5% | <0.1% | <0.1% | <0.6% | <0.4% |
| pH growth range | 4.2–6.8 | 7.8–8.8 | 5.0–7.5 | 4.0–6.8 | 4.0–7.0 | 3.8–7.2 | 6.0–10.5 |
| pH optimum | 5.0–5.5 | ND | 6.5 | 5.5–6.0 | 5.0–5.5 | 5.5–6.0 | 7.5–8.0 |
| Temperature range, °C | 4–28 | 16–35 | 10–30 | 10–30 | 10–30 | 10–30 | 20–40 |
| Temperature optimum, °C | 15–22 | ND | 20–25 | 20–25 | 20–26 | 20–25 | 32–36 |
| Major fatty acids | 18:0, βOH-C16:1,16:1ω5c, | 18:0, 16:1ω5c | 18:1ω7c, 18:0, βOH-C16:0 | 20:1ω9c, 16:0 | 16:1ω5c, 18:1ω5c, 18:0 | 18:1ω5c,16:1ω5c, 18:0 | 16:1ω5c, 16:0, 18:0 |
| Oxidase test | − | − | − | − | − | + | + |
| Catalase test | + | + | + | + | + | + | − |
| Urease test | + | − | − | − | − | − | + |
| Carbon sources | |||||||
| Sucrose | + | + | + | + | + | + | − |
| Xylose | + | + | + | + | + | + | − |
| Lactose | + | + | + | + | − | + | |
| Mannose | + | + | + | + | + | − | ND |
| Sorbose | − | − | − | − | − | + | ND |
| Raffinose | − | − | + | + | + | + | ND |
| N-acetylglucosamine | + | + | + | + | + (<0.01%) | + | + |
| Xylose | + | + | + | + | + | + | − |
| Pyruvate | − | − | − | + | − | + | ND |
| Chondroitin sulfate | + | + | − | − | − | + | ND |
| Xylan | + | ND | + | + | + | + | ND |
| Pectin | − | + | − | − | − | + | ND |
| Cellulose | + | − | − | − | + | − | ND |
| Carboxymethylcellulose | + | − | − | − | + | − | ND |
| Enzymatic activities | |||||||
| α-Galactosidase | − | + | − | − | − | − | ND |
| Cystine arylamidase | − | − | − | − | + | + | |
| Valine arylamidase | + | − | + | + | + | + | |
| N-acetyl-β-glucosaminidase | + | + | − | + | w | + | |
| α-Mannosidase | − | + | − | + | − | − | |
| DNA G + C content, mol% | 67.4* | 64–67* | 65.6* | 64.2* | 58.5 | 59* | 58.1* |
ND, not determined.
Data are based on genome sequence analysis.
Table 3.
Descriptions of Frigoriglobus gen. nov. and Frigoriglobus tundricola sp. nov.
| Genus name | Frigoriglobus | |
| Species name | Frigoriglobus tundricola | |
| Genus status | gen. nov | |
| Genus etymology | Fri.go.ri.glo′bus. L. neut. n. frigus, -oris, cold; N.L. masc. n. globus, a ball; N.L. masc. n. Frigoriglobus a ball growing in the cold | |
| Type species of the genus | Frigoriglobus tundricola | |
| Specific epithet | – | tundricola |
| Species status | – | sp. nov. |
| Species etymology | – | tun.dri′co.la. N.L. fem. n. tundra tundra, a cold treeless region; L. suffix -cola (from L. masc. or fem. n. incola) inhabitant, dweller; N.L. masc. n. tundricola tundra inhabitant |
| Description of the new taxon and diagnostic traits | Spherical cells that occur singly, in pairs, in short chains or in shapeless aggregates. Reproduce by budding. Daughter cells are motile. Chemoheterotrophic aerobes. Moderately acidophilic and psychrotolerant. Sensitive to NaCl. The major fatty acids are C16:1ω5, C18:0, βOH-C16:1; the major polar lipid is trimethylornithine; a characteristic lipid is parkeol. The genus is a member of the phylum Planctomycetes, class Planctomycetia, order Gemmatales, family Gemmataceae. | Colonies are pink. Mature spherical cells are 1. 8–2.6 μm in diameter. Catalase and urease – positive, cytochrome oxidase – negative. Dissimilatory nitrate reduction and glucose fermentation are negative. Do not produce indole from triptophane. Carbon sources include cellobiose, glucose, galactose, fructose, lactose, leucrose, maltose, mannose, melibiose, ribose, sucrose, trehalose, xylose, salicin and N-acetylglucosamine. Capable of hydrolyzing aesculin, arabinogalactan, chondroitin sulfate, dextrin, laminarin, lichenan, locust bean gum, starch, xanthan gum, xylan and gelatin. Possess weak cellulolytic potential and capable of slow growth on carboxymethylcellulose and fibrous cellulose. Cannot utilize melizitose, raffinose, rhamnose, sorbose, fucose, glycerol, gluconate, methanol, ethanol, galacturonate, acetate, benzoate, caproate, citrate, formate, formaldehyde, fumarate, glutarate, galacturonate, lactate, malate, succinate, pyruvate, propionate, tartrate, adonitol, dulcitol, mannitol, sorbitol, alanine, arginine, asparagine, aspartate, cysteine, cystine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, norleucine, ornithine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine and valine. Cannot hydrolyze casein, chitosan, chitin and pectin. Shows the following enzyme activities: alkaline and acid phosphatase, esterase, esterase lipase, leucyne arylamidase, valine arylamidase, phosphohydrolase, β-glucosidase, N-acetyl-β-glucosaminidase, α-glucosidase, β-galactosidase (API ZYM test). The following enzyme activities are not present: lipase, trypsin, chymotrypsin, cystine arylamidase, α-galactosidase, β-glucuronidase, α-fucosidase and α-mannosidase. Resistant to ampicillin, chloramphenicol, lincomycin, novobiocin and streptomycin, but sensitive to gentamicin, kanamycin, neomycin, rifampicin and tetracycline. Growth occurs at pH 4.2–6.8 (optimum, pH 5.0–5.5) and at temperatures between 4 and 28 °C (optimum 15–22 °C). NaCl inhibits growth at concentrations above 0.5% (w/v). The G + C content of the DNA is 67.4 mol% |
| Country of origin | – | Russian Federation |
| Region of origin | – | Nadym region, Yamalo-Nenets Autonomous Okrug |
| Date of isolation | – | 20/07/2016 |
| Source of isolation | – | Shallow littoral wetland in a forested tundra |
| Sampling date | – | 21/07/2015 |
| Latitude | – | 65°35′03″N |
| Longitude | – | 73°04′20″E |
| Genome accession number | – | GenBank: CP053452 (chromosome) and CP053453 (plasmid) |
| Genome status | – | Complete |
| Genome size | – | 9.83 |
| GC mol% | – | 67.4 |
| Number of strains in study | – | 1 |
| Information related to the Nagoya Protocol | – | Not applicable |
| Designation of the type strain | – | PL17T |
| Strain collection numbers | – | CECT 9407T = VKM B-3467T |
Funding
This study was supported by the Russian Science Foundation (project No 16-14-10210). JSSD received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement n° 694569 – MICROLIPIDS). JSSD also receives funding from the Soehngen Institute for Anaerobic Microbiology (SIAM) though a gravitation grant from the Dutch ministry for Education, Culture and Science (grant number 024.002.002).
References
- 1.Aghnatios R., Cayrou C., Garibal M., Robert C., Azza S., Raoult D., Drancourt M. Draft genome of Gemmata massiliana sp. nov, a water-borne Planctomycetes species exhibiting two variants. Stand. Genomic Sci. 2015;10:120. doi: 10.1186/s40793-015-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apweiler R., Bairoch A., Bougueleret L., Altairac S., Amendolia V., Auchincloss A., Argoud-Puy G., Axelsen K., Baratin D., Blatter M.C., Boeckmann B., Bolleman J., Bollondi L., Boutet E., Quintaje S.B., Breuza L., Bridge A., DeCastro E., Ciapina L., Coral D., Coudert E., Cusin I., Delbard G., Dornevil D., Roggli P.D., Duvaud S., Estreicher A., Famiglietti L., Feuermann M., Gehant S., Farriol-Mathis N., Serenella Ferro S., Gasteiger E., Gateau A., Gerritsen V., Gos A., Gruaz-Gumowski N., Hinz U., Hulo C., Hulo N., James J., Jimenez S., Jungo F., Junker V., Kappler T., Keller G., Lachaize C., Lane-Guermonprez L., Langendijk-Genevaux P., Lara V., Lemercier P., Le Saux V., Lieberherr D., de Oliveira Lima T., Mangold V., Martin X., Masson P., Michoud K., Moinat M., Morgat A., Mottaz A., Paesano S., Pedruzzi I., Phan I., Pilbout S., Pillet V., Poux S., Pozzato M., Redaschi N., Reynaud S., Rivoire C., Roechert B., Schneider M., Sigrist C., Sonesson K., Staehli S., Stutz A., Sundaram S., Tognolli M., Verbregue L., Veuthey A.L., Yip L., Luiz Zuletta L., Apweiler R., Alam-Faruque Y., Antunes R., Barrell D., Binns D., Bower L., Browne P., Wei Mun C., Dimmer E., Eberhardt R., Fedotov A., Foulger R., Garavelli J., Golin R., Horne A., Huntley R., Jacobsen J., Kleen M., Kersey P., Laiho K., Leinonen R., Legge D., Lin Q., Magrane M., Martin M.J., O’Donovan C., Orchard S., O’Rourke J., Patient S., Pruess M., Sitnov A., Stanley E., Corbett M., di Martino G., Donnelly M., Luo J., van Rensburg P., Wu C., Arighi C., Arminski L., Barker W., Chen Y., Hu Z.Z., Hua H.K., Huang H., Mazumder R., McGarvey P., Natale D.A., Nikolskaya A., Petrova N., Suzek B.E., Vasudevan S., Vinayaka C.R., Su Yeh L., Zhang J. The Universal Protein resource (UniProt) 2009. Nucleic Acids Res. 2009;37(Suppl. 1):D169–D174. doi: 10.1093/nar/gkn664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspeborg H., Coutinho P.M., Wang Y., Brumer H., Henrissat B. Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5) BMC Evol. Biol. 2012;12:186. doi: 10.1186/1471-2148-12-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auch A.F., von Jan M., Klenk H.-P., Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand. Genomic Sci. 2010;2(1):117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auch A.F., Klenk H.-P., Göker M. Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand. Genomic Sci. 2010;2(1):142–148. doi: 10.4056/sigs.541628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer T.E., Albertsen M., Edwards A., Kirkegaard R.H., Rocha E.P.C., Fierer N. Unlinked rRNA genes are widespread among bacteria and archaea. ISME J. 2020;14:597–608. doi: 10.1038/s41396-019-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brümmer I.H.M., Felske A.D.M., Wagner-Döbler I. Diversity and seasonal changes of uncultured Planctomycetales in river biofilms. Appl. Environ. Microbiol. 2004;70(9):5094–5101. doi: 10.1128/AEM.70.9.5094-5101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley D.H., Huangyutitham V., Nelson T.a., Rumberger A., Thies J.E. Diversity of Planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl. Environ. Microbiol. 2006;72(7):4522–4531. doi: 10.1128/AEM.00149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun J., Oren A., Ventosa A., Christensen H., Arahal D.R., da Costa M.S., Rooney A.P., Yi H., Xu X.W., De Meyer S., Trujillo M.E. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 11.Dedysh S.N., Derakshani M., Liesack W. Detection and enumeration of methanotrophs in acidic Sphagnum peat by 16S rRNA fluorescence in situ hybridization, including the use of newly developed oligonucleotide probes for Methylocella palustris. Appl. Environ. Microbiol. 2001;67:4850–4857. doi: 10.1128/AEM.67.10.4850-4857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dedysh S.N., Ivanova A.A. Planctomycetes in boreal and subarctic wetlands: diversity patterns and potential ecological functions. FEMS Microbiol. Ecol. 2019;95(2):fiy227. doi: 10.1093/femsec/fiy227. [DOI] [PubMed] [Google Scholar]
- 13.Dedysh S.N., Kulichevskaya I.S., Beletsky A.V., Ivanova A.A., Rijpstra W.I.C., Damsté J.S.S., Mardanov A.V., Ravin N.V. Lacipirellula parvula gen. nov., sp. nov., representing a lineage of planctomycetes widespread in low-oxygen habitats, description of the family Lacipirellulaceae fam. nov. and proposal of the orders Pirellulales ord. nov., Gemmatales ord. nov. and Isosphaerales ord. nov. Syst. Appl. Microbiol. 2020;43(1):126050. doi: 10.1016/j.syapm.2019.126050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzmann P.D., Skerman V.B. Gemmata obscuriglobus, a new genus and species of the budding bacteria. Antonie van Leeuwenhoek. 1984;50(3):261–268. doi: 10.1007/BF02342136. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt P. American Society for Microbiology; 1981. Manual of Methods for General Bacteriology. [Google Scholar]
- 16.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova A.A., Wegner C.-E., Kim Y., Liesack W., Dedysh S.N. Metatranscriptomics reveals the hydrolytic potential of peat-inhabiting Planctomycetes. Antonie van Leeuwenhoek. 2018;111:801–809. doi: 10.1007/s10482-017-0973-9. [DOI] [PubMed] [Google Scholar]
- 18.Ivanova A.O., Dedysh S.N. Abundance, diversity, and depth distribution of Planctomycetes in acidic northern wetlands. Front. Microbiol. 2012;3:5. doi: 10.3389/fmicb.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanehisa M., Sato Y., Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016;428(4):726–731. doi: 10.1016/j.jmb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinidis K.T., Rosselló-Móra R., Amann R. Uncultivated microbes in need of their own taxonomy. ISME J. 2017;11:2399–2406. doi: 10.1038/ismej.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulichevskaya I.S., Baulina O.I., Bodelier P.L.E., Rijpstra W.I.C., Sinninghe Damsté J.S., Dedysh S.N. Zavarzinella formosa gen. nov., sp. nov., a novel stalked, Gemmata-like planctomycete from a Siberian peat bog. Int. J. Syst. Evol. Microbiol. 2009;59(2):357–364. doi: 10.1099/ijs.0.002378-0. [DOI] [PubMed] [Google Scholar]
- 22.Kulichevskaya I.S., Ivanova A.A., Baulina O.I., Rijpstra W.I.C., Damsté J.S.S., Dedysh S.N. Fimbriiglobus ruber gen. nov., sp. nov., a Gemmata-like planctomycete from Sphagnum peat bog and the proposal of Gemmataceae fam. nov. Int. J. Syst. Evol. Microbiol. 2017;67(2):218–224. doi: 10.1099/ijsem.0.001598. [DOI] [PubMed] [Google Scholar]
- 23.Kulichevskaya I.S., Naumoff D.G., Miroshnikov K.K., Ivanova A.A., Philippov D.A., Hakobyan A., Rijpstra W.I.C., Damsté J.S.S., Liesack W., Dedysh S.N. Limnoglobus roseus gen. nov., sp. nov., a novel freshwater planctomycete with a giant genome from the family Gemmataceae. Int. J. Syst. Evol. Microbiol. 2020;70(2):1240–1249. doi: 10.1099/ijsem.0.003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulichevskaya I.S., Serkebaeva Y.M., Kim Y., Rijpstra W.I.C., Damsté J.S.S., Liesack W., Dedysh S.N. Telmatocola sphagniphila gen. nov., sp. nov., a novel dendriform planctomycete from northern wetlands. Front. Microbiol. 2012;3:146. doi: 10.3389/fmicb.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagesen K., Hallin P., Rødland E.A., Stærfeldt H.H., Rognes T., Ussery D.W. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laslett D., Canback B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004;32(1):11–16. doi: 10.1093/nar/gkh152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(D1):490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011;17.1:10–12. [Google Scholar]
- 29.Moore E.K., Hopmans E.C., Rijpstra W.I.C., Villanueva L., Dedysh S.N., Kulichevskaya I.S., Wienk H., Schoutsen F., Sinninghe Damsté J.S. Novel mono-, di-, and trimethylornithine membrane lipids in northern wetland planctomycetes. Appl. Environ. Microbiol. 2013;79(22):6874–6884. doi: 10.1128/AEM.02169-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naumoff D.G. Phylogenetic analysis of a protein family. Zbio. 2006;1 Art. 3. [Google Scholar]
- 31.Naumoff D.G., Carreras M. PSI protein classifier: a new program automating PSI-BLAST search results. Mol. Biol. 2009;43:652–664. [PubMed] [Google Scholar]
- 32.Naumoff D.G. GH10 Family of glycoside hydrolases: structure and evolutionary connections. Mol. Biol. 2016;50(1):132–140. doi: 10.7868/S0026898415060208. [DOI] [PubMed] [Google Scholar]
- 33.Naumoff D.G., Ivanova A.A., Dedysh S.N. Phylogeny of β-xylanases from Planctomycetes. Mol. Biol. 2014;48(3):439–447. doi: 10.1134/S0026893314030145. [DOI] [PubMed] [Google Scholar]
- 34.Neef A., Amann R., Schlesner H., Schleifer K.H. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology. 1998;144(12):3257–3266. doi: 10.1099/00221287-144-12-3257. [DOI] [PubMed] [Google Scholar]
- 35.Overbeek R., Begley T., Butler R.M., Choudhuri J.V., Chuang H.-Y., Cohoon M., de Crécy-Lagard V., Diaz N., Disz T., Edwards R., Fonstein M., Frank E.D., Gerdes S., Glass E.M., Goesmann A., Hanson A., Iwata-Reuyl D., Jensen R., Jamshidi N., Krause L., Kubal M., Larsen N., Linke B., McHardy A.C., Meyer F., Neuweger H., Olsen G., Olson R., Osterman A., Portnoy V., Pusch G.D., Rodionov D.A., Rückert C., Steiner J., Stevens R., Thiele I., Vassieva O., Ye Y., Zagnitko O., Vonstein V. The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res. 2005;33(17):5691–5702. doi: 10.1093/nar/gki866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pankratov T.A., Kirsanova L.A., Kaparullina E.N., Kevbrin V.V., Dedysh S.N. Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. Int. J. Syst. Evol. Microbiol. 2012;62:430–437. doi: 10.1099/ijs.0.029629-0. [DOI] [PubMed] [Google Scholar]
- 37.Parks D.H., Chuvochina M., Waite D.W., Rinke C., Skarshewski A., Chaumeil P.A., Hugenholtz P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018;36:996–1004. doi: 10.1038/nbt.4229. [DOI] [PubMed] [Google Scholar]
- 38.Pearson A., Budin M., Brocks J.J. Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc. Natl. Acad. Sci. U.S.A. 2003;100(26):15352–15357. doi: 10.1073/pnas.2536559100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollet T., Tadonléké R.D., Humbert J.F. Spatiotemporal changes in the structure and composition of a less-abundant bacterial phylum (Planctomycetes) in two perialpine lakes. Appl. Environ. Microbiol. 2011;77(14):4811–4821. doi: 10.1128/AEM.02697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravin N.V., Rakitin A.L., Ivanova A.A., Beletsky A.V., Kulichevskaya I.S., Mardanov A.V., Dedysh S.N. Genome analysis of Fimbriiglobus ruber SP5T, a planctomycete with confirmed chitinolytic capability. Appl. Environ. Microbiol. 2018;84(7) doi: 10.1128/AEM.02645-17. AEM. 02645-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-R L., Konstantinidis K. The enveomics collection: a toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Prepr. 2016;4:e1900v1. doi: 10.7287/peerj.preprints.1900. [DOI] [Google Scholar]
- 42.Seeger C., Butler M.K., Yee B., Mahajan M., Fuerst J.A., Andersson S.G.E. Tuwongella immobilis gen. nov., sp. nov., a novel non-motile bacterium within the phylum Planctomycetes. Int. J. Syst. Evol. Microbiol. 2017;67(12):4923–4929. doi: 10.1099/ijsem.0.002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 44.Sinninghe Damsté J.S., Rijpstra W.I.C., Hopmans E.C., Weijers J.W.H., Foesel B.U., Overmann J., Dedysh S.N. 13,16-Dimethyl octacosanedioic acid (iso-diabolic acid), a common membrane-spanning lipid of Acidobacteria subdivisions 1 and 3. Appl. Environ. Microbiol. 2011;77(12):4147–4154. doi: 10.1128/AEM.00466-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staley J.T., Fuerst J.A., Giovannoni S., Schlesner H. The Order Planctomycetales and the Genera Planctomyces, Pirellula, Gemmata, and Isosphaera. In: Balows A., Trüper H.G., Dworkin M., Harder W., Schleifer K.-H., editors. The Prokaryotes: A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. Springer New York; New York, NY: 1992. pp. 3710–3731. [Google Scholar]
- 46.Wang J., Jenkins C., Webb R.I., Fuerst J.A. Isolation of Gemmata-like and Isosphaera-like planctomycete bacteria from soil and freshwater. Appl. Environ. Microbiol. 2002;68(1):417–422. doi: 10.1128/AEM.68.1.417-422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13(6):e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu S., Wu S. Processivity and the mechanisms of processive endoglucanases. Appl. Biochem. Biotechnol. 2020;190:448–463. doi: 10.1007/s12010-019-03096-w. [DOI] [PubMed] [Google Scholar]