Abstract
Objective
Our study aimed to explore the association between trimethylamine N-oxide and frailty in older adults with cardiovascular disease.
Patients and Methods
This cross-sectional study analyzed a total of 451 people aged 65 years or older who underwent comprehensive geriatric assessments. Frailty status was determined using a frailty index constructed with 48 variables according to the cumulative deficits model. Physical frailty and cognitive frailty were also assessed in detail. Fasting plasma TMAO was measured by mass spectrometry.
Results
The proportion of frail subjects was 29.9% (135/451). Plasma TMAO levels were significantly higher in frail patients than in nonfrail individuals (4.04 [2.84–7.01] vs 3.21 [2.13–5.03] µM; p<0.001). Elevated plasma TMAO levels were independently associated with the likelihood of frailty (OR 2.12, 95% CI 1.01–4.38, p=0.046). Dose–response analysis revealed a linear association between the TMAO concentration and the OR for frailty. A 2-unit increase in TMAO was independently correlated with physical frailty (OR 1.23, 95% CI 1.08–1.41, p for trend 0.002) and cognitive frailty (OR 1.21, 95% CI 1.01–1.45, p for trend 0.04).
Conclusion
Elevated circulating TMAO levels are independently associated with frailty among older adults with cardiovascular disease.
Keywords: older adults, frailty, TMAO, cardiovascular disease, physical frailty, cognitive frailty
Introduction
Frailty is defined as an age-related condition characterized by an accentuated vulnerability of an individual to physiological and environmental stressors. The presence of frailty has a negative impact on the prognosis of older adults, especially those with cardiovascular disease.1,2 Given the multifactorial etiology of frailty, it is generally accepted that declines in physical or cognitive reserve comprise the core domains of frailty.3 Although growing attention has been paid to frailty, the lack of valid biomarkers and effective intervention targets for frailty remains a problematic issue.4
Emerging evidence implies a crucial role of the gut microbiota in frailty among older adults.5,6 The gut microbiota may modulate aging-related alterations in physical performance, sarcopenia, and cognitive impairment, all of which are elements of frailty.7,8 Frail people have distinctive microbiota compositions with fewer and less diverse microorganisms compared to healthy people.9 Although the characteristics of the gut microbiota in frail people have been delineated, the instability and individuality of the gut microbiota preclude its use as an effective biomarker of frailty.10
Trimethylamine N-oxide (TMAO) is a stable metabolite of the gut microbiota and has attracted increasing interest because of its pathogenic role in cardiovascular disease.11,12 In the intestine, the microbiota metabolizes dietary choline and carnitine, which are found at high levels in red meat, into trimethylamine (TMA).13,14 Liver enzymes oxidize circulating TMA into TMAO. Importantly, TMAO also exerts an influence on the aging process. Experimental studies have shown that increasing TMAO levels can induce brain aging and age-associated arterial endothelial dysfunction by intensifying the inflammatory response and oxidative stress.15,16 Recently, a study showed that the gut microbiome of frail individuals had a higher representation of the bacterial CutC enzyme that catalyzes choline into TMA than nonfrail individuals, underlying a potential association between TMAO and frailty.17
Although potential mechanisms have emerged, the association between TMAO and frailty remains unclear. Therefore, we aimed to elucidate whether TMAO had an impact on frailty using cross-sectional data from a comprehensive geriatric assessment cohort. Moreover, we attempted to explore the relationship between TMAO and different phenotypes of frailty, such as physical frailty and cognitive frailty.
Patients and Methods
Study Design and Participants
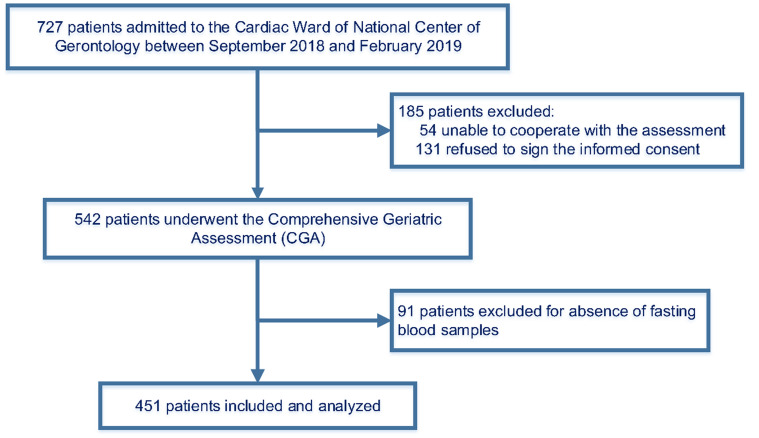
This cross-sectional study was based on data from a longitudinal study involving comprehensive geriatric evaluations of hospitalized older patients.18 A total of 727 patients (age 65 years and older) were included in our study. All patients were admitted to the cardiac ward of the National Center of Gerontology (Beijing, China) between September 2018 and February 2019. After obtaining written informed consent, trained personnel conducted a baseline survey and a series of comprehensive geriatric assessments for each participant. We excluded subjects who were unable to complete the questionnaire and patients without fasting blood samples. Ultimately, a total of 451 subjects were included in the final statistical analysis (Figure 1).
Figure 1.
Flow diagram of participant enrollment.
Frailty Assessment
Frailty Index
Frailty severity was measured using a frailty index (FI) following the deficit accumulation approach. The FI was based on 48 variables, which collectively included variables reflecting restricted activity, disability in activities of daily living, impairments in general cognition and physical performance, self-rated health, comorbidities, and depression/anxiety/insomnia (Table S1).18 All binary variables were recorded based on the convention that “0” indicated the absence of the deficit and “1” indicated the presence of the deficit. For variables that included a single intermediate response, we used an additional value of “0.5”. Consequently, the FI score was calculated as the proportion of deficits present out of all variables considered. According to previously determined cut-off points for the FI, study participants were assigned to binary categories of nonfrail (FI <0.25) and frail (FI ≥0.25) and ordinal categories of nonfrail (FI <0.12), prefrail (0.12≤ FI <0.25) and frail (FI ≥0.25).19
Physical Frailty
Physical frailty was defined according to the Cardiovascular Health Study (CHS) index criteria based on the following five conditions: weight loss, exhaustion, low activity, weakness, and slowness.20 Participants with three or more conditions were considered to have physical frailty. The Short Physical Performance Battery (SPPB) is an objective test of lower extremity function (balance, walking speed, repeated chair stands) and is utilized as a supplementary method to assess physical frailty. A score ≤ 9 on the SPPB was classified as physical frailty.21
Cognitive Frailty
Cognitive frailty was defined as a clinical manifestation characterized by the simultaneous presence of both physical frailty (CHS frailty criteria) and mild cognitive impairment (MCI), with the exclusion of concurrent Alzheimer’s disease or vascular dementia.22 Cognitive impairment was detected by using the Mini-Mental State Examination (MMSE) scale. The score ranges from 0 to 30, and low MMSE scores indicate poor cognitive function. To distinguish patients with MCI, a cut-off of ≤ 25 was used.23
Covariates
Demographic characteristics included age, sex, and education level. Tobacco smoking status was used to divide participants into two groups: current smokers or current nonsmokers (past smokers or never smokers). Anthropometric data, including weight, height, and brachial artery blood pressure, were collected at admission. History of chronic diseases was self-reported, which was reasonably accurate when compared to medical records. Information about prescribed medication in the past three months was also recorded in detail. Left ventricular ejection fraction (LVEF) was measured by experienced echocardiographers using the biplane modified Simpson’s method. All the above baseline medical information and measurement data were gathered and assessed by trained personnel.
Laboratory Testing
Fasting blood samples were collected with vacuum biochemical tubes on the second morning after admission. Blood samples were then centrifuged and frozen at −80°C until further analysis. Plasma TMAO was measured using stable isotope dilution ultra-performance liquid chromatography with online tandem mass spectrometry (UPLC-MS/MS; XEVO TQ-S Micro Mass Spectrometer, Waters Corporation, Milford, MA, USA) using a d9-(trimethyl)-labelled internal standard.12 The detailed measurement protocol of TMAO is provided in the supplementary materials. All laboratory testing for routine clinical parameters was performed in the Beijing Hospital Clinical Laboratories. The estimated glomerular filtration rate (eGFR) was estimated using the Cockcroft-Gault equation.24
Statistical Analysis
Continuous data are presented as the mean (SD) or median (interquartile range [IQR]) and were compared with the t-test or nonparametric test when appropriate. Categorical variables are presented as numbers (percent) and were compared between groups with chi-square tests. The groups (nonfrail, prefrail, and frail) were compared using the Kruskal–Wallis test with Bonferroni correction. LASSO regularization with logistic regression was performed to select confounding variables. Multivariable logistic regression analysis was used to examine the association of TMAO with frailty after adjustment for confounding variables, including age, sex, body mass index (BMI), history of diseases (diabetes, peripheral arterial disease (PAD), chronic kidney disease and stroke), left ventricular ejection fraction (LVEF), low-density lipoprotein cholesterol (LDL-C), and high-sensitivity C-reactive protein (hsCRP). Interactions between TMAO and covariates were tested by the likelihood ratio test using the multiplicative interaction term. Multivariable logistic regression analysis was used to examine the association between TMAO (per 2-unit increase), physical frailty, and cognitive frailty adjusted for age, sex, and BMI. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. The additional predictive value of TMAO for the risk of frailty was assessed with Harrell’s C statistics, the continuous net reclassification index (NRI) index and an integrated discriminatory index (IDI). A decision curve analysis (DCA) was used to estimate the clinical usefulness and net benefit of TMAO. All analyses were performed using R 3.6.1 (Vienna, Austria) and SAS version 9.4 (SAS, Cary, NC), and a two-tailed p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
The baseline characteristics of the 451 patients categorized by frailty status are described in Table 1. The overall sample included 451 subjects, of whom 29.9% were classified as frail, while 70.1% were classified as nonfrail. The proportions of participants with physical frailty and cognitive frailty were 21.5% (97/451) and 7.3% (33/451), respectively. Frail participants were significantly older, were more commonly female, were less educated, had lower BMI, LDL-C, eGFR and LVEF levels, and had a higher hsCRP level. Frail individuals were more likely to have hypertension, PAD, prior stroke, and diabetes than those without frailty. In contrast, history of coronary heart disease, current smoking, and medication history were similar between the two groups.
Table 1.
Baseline Characteristics of the Participants with and without Frailty
| Characteristics | Total (n=451) | Frailty Index | ||
|---|---|---|---|---|
| Frail Group (n=135) | Nonfrail Group (n=316) | p-value | ||
| Age, years | 75.2 (6.6) | 79.7 (6.4) | 73.3 (5.7) | <0.001 |
| Female, % | 212 (47.0) | 74 (54.8) | 138 (43.7) | 0.03 |
| Education level, % | 0.001 | |||
| Primary | 59 (13.1) | 29 (21.5) | 30 (9.5) | |
| Secondary | 223 (49.4) | 67 (49.6) | 156 (49.4) | |
| College | 169 (37.5) | 39 (28.9) | 130 (41.1) | |
| BMI, kg/m2 | 25.3 (3.3) | 24.8 (3.5) | 25.5 (3.2) | 0.05 |
| Current smoker, % | 44 (9.8) | 12 (8.9) | 32 (10.1) | 0.71 |
| History of diseases, % | ||||
| Hypertension | 331 (73.4) | 110 (81.5) | 221 (69.9) | 0.01 |
| Coronary heart disease | 313 (69.4) | 100 (74.1) | 213 (67.4) | 0.16 |
| Atrial fibrillation | 129 (28.6) | 46 (34.3) | 83 (26.2) | 0.09 |
| Peripheral arterial disease | 77 (17.1) | 39 (28.9) | 38 (12.0) | <0.001 |
| Prior stroke | 73 (16.2) | 38 (28.4) | 35 (11.1) | <0.001 |
| Diabetes mellitus | 152 (33.7) | 67 (49.6) | 85 (26.9) | <0.001 |
| Obesity | 37 (8.2) | 10 (7.5) | 27 (8.5) | 0.85 |
| Medications, % | ||||
| Antiplatelet drugs | 320 (71.0) | 96 (71.1) | 224 (70.9) | 0.96 |
| β-blocker | 243 (55.4) | 81 (60.4) | 162 (53.1) | 0.16 |
| ACEI/ARB | 200 (44.3) | 64 (47.4) | 136 (43.0) | 0.39 |
| Lipid-lowering agents | 378 (86.1) | 119 (88.8) | 259 (84.9) | 0.28 |
| Metformin | 82 (18.2) | 19 (14.2) | 63 (19.9) | 0.18 |
| MMSE<25, % | 90 (20.0) | 49 (36.6) | 41 (13.0) | <0.001 |
| SPPB score, % | <0.001 | |||
| 10–12 | 161 (35.8) | 15 (11.2) | 146 (46.2) | |
| 7–9 | 173 (38.4) | 43 (32.1) | 130 (41.1) | |
| 0–6 | 116 (25.8) | 76 (56.7) | 40 (12.7) | |
| eGFR<60 mL/min/1.73m2, % | 62 (13.8) | 37 (27.6) | 25 (7.9) | <0.001 |
| LDL-C, mmol/L | 2.1 (1.7–2.6) | 1.9 (1.6–2.4) | 2.2 (1.8–2.7) | <0.001 |
| hsCRP, mg/L | 1.0 (0.6–2.2) | 1.4 (0.7–3.8) | 1.0 (0.6–1.9) | 0.004 |
| LVEF<50%, % | 25 (5.6) | 14 (10.4) | 11 (3.5) | 0.003 |
| TMAO, μM | 3.4 (2.3–5.6) | 4.0 (2.8–7.0) | 3.2 (2.1–5.0) | <0.001 |
Note: Values are the mean (SD), median (interquartile range) or %.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MMSE, Mini-Mental State Examination; SBP, systolic blood pressure; SPPB, Short Physical Performance Battery; TMAO, trimethylamine N-oxide.
Comparison of TMAO Levels Between Groups
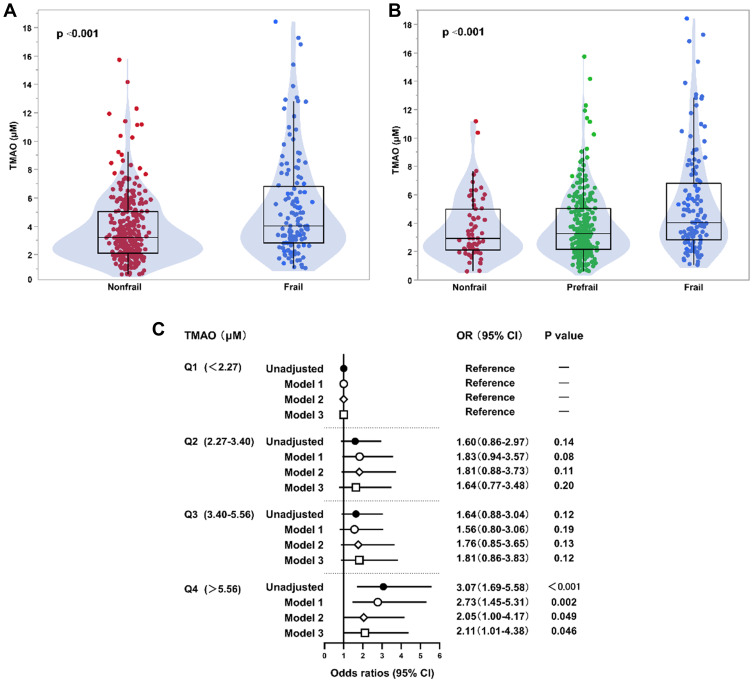
The median (IQR) TMAO concentrations in nonfrail and frail participants were 3.21 μM (IQR: 2.13–5.03 μM) and 4.04 μM (IQR: 2.84–7.01 μM), respectively. TMAO levels significantly differed with higher levels found among patients with frailty (p<0.001) (Figure 2A). Based on the three-category method used to define frailty, the median levels of TMAO in nonfrail, prefrail and frail subjects were 2.93 μM (IQR: 2.11–5.01 μM), 3.26 μM (IQR: 2.17–5.04 μM) and 4.04 μM (IQR: 2.84–7.01 μM), respectively. Differences in TMAO concentration among the three groups were significant (p<0.001) according to the Kruskal–Wallis test with Bonferroni correction. There was an increasing trend in the concentration of TMAO across categories of frailty (Figure 2B). Pairwise comparisons showed a significant difference in TMAO levels between the prefrail and frail groups (p<0.001) and between the nonfrail and frail groups (p<0.001). Although not statistically significant, the median TMAO concentrations in the prefrail group were higher than those in the nonfrail group.
Figure 2.
Association of TMAO levels with frailty in older adults with cardiovascular disease. (A) Box-Whisker-Violin plots of TMAO levels among older participants without and with frailty. (B) Box-Whisker-Violin plots of TMAO levels among nonfrail, prefrail and frail participants. (C) Forrest plots illustrating the odds of frailty among older participants according to quartiles of TMAO levels. Frailty was categorized into nonfrail and frail groups. Symbols represent the odds ratios (ORs), and the 5–95% confidence intervals (CIs) are indicated by line length. ORs and 95% CIs were calculated using binary logistic regression with adjustments. Model 1: age, sex, body mass index; Model 2: Model 1 plus history of diabetes, chronic kidney disease, prior stroke, and peripheral arterial disease; Model 3: Model 2 plus left ventricular ejection fraction, high-sensitivity C-reactive protein, and low-density lipoprotein cholesterol.
Association Between TMAO and Frailty
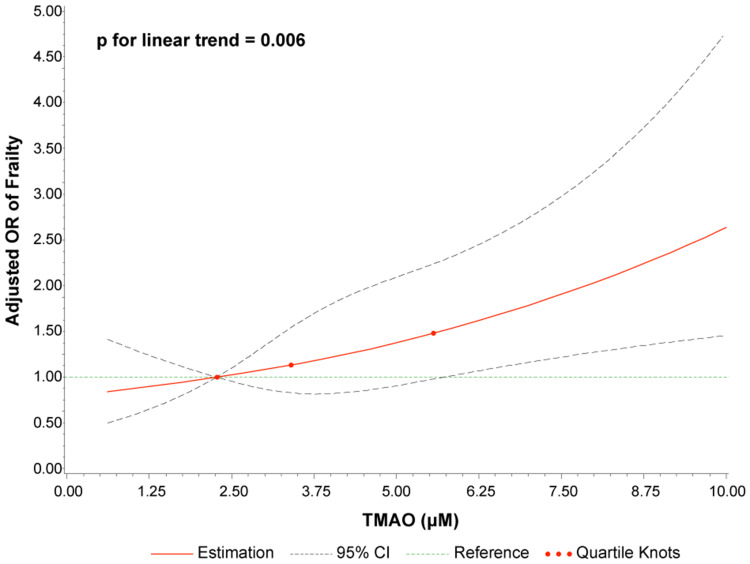
The frequency of frailty increased with increasing quartiles of TMAO levels as follows: 19.2% in quartile 1 (<2.27 μM), 28.1% in quartile 2 (2.27–3.40 μM), 28.6% in quartile 3 (3.40–5.56 μM), and 42.9% in quartile 4 (>5.56 μM) (p for trend <0.001). The results of the multivariable binary logistic regression models using quartiles of TMAO are shown in Figure 2C. Patients in the highest quartile (Q4) demonstrated a significant 3.07-fold increased risk of frailty (OR 3.07, 95% CI 1.69–2.97, p<0.001) compared with the risk in the lowest quartile of TMAO levels (Q1). After stepwise adjustment in the multivariable models, the association between TMAO and frailty remained significant (OR 2.11, 95% CI 1.01–4.38, p=0.046). The potential nonlinear association was assessed via 4-knot restricted cubic splines with the generalized linear model: the result suggested a positive linear association of TMAO levels with the OR of frailty (p for linear trend 0.006) (Figure 3).
Figure 3.
Dose–response relationship of TMAO concentration with the risk of frailty. Odds ratios (solid line) and 95% confidence intervals (dashed line) were adjusted for age, sex, body mass index, diabetes, peripheral arterial disease, chronic kidney disease, stroke, left ventricular ejection fraction, high-sensitivity C-reactive protein, and low-density lipoprotein cholesterol. The 25th percentile was set as the reference, and the other two knots’ positions were fitted at the 50th and 75th percentiles.
Abbreviation: CI, confidence interval; OR, odds ratio.
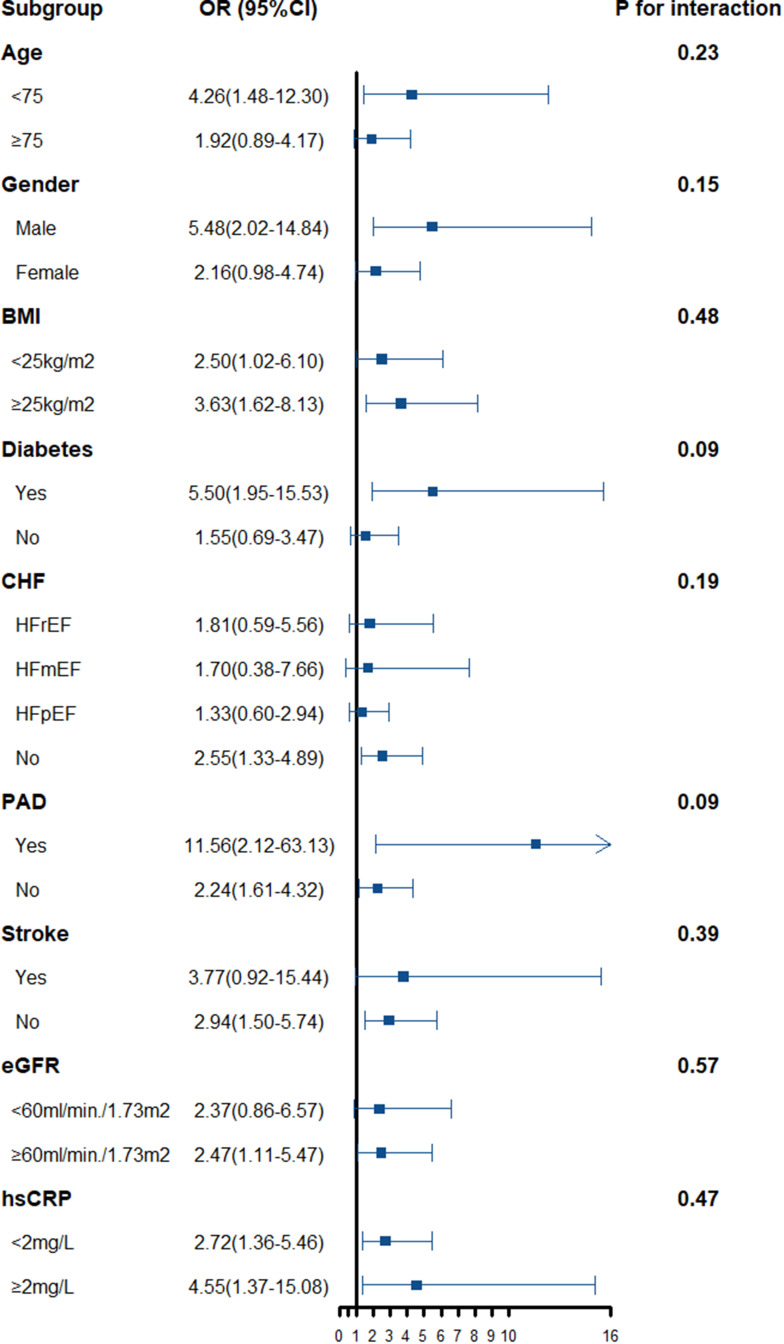
We further conducted stratified analyses according to age, sex, BMI, comorbidity status, renal function, and inflammatory marker level. The overall patterns of the TMAO–frailty associations were similar across all the above subgroups. The interaction analysis did not reveal any significant interaction effects of clinical subgroups on the association between TMAO levels and the likelihood of frailty. However, the TMAO-frailty associations tended to be stronger among individuals with a history of diabetes, PAD, or stroke, but the p-values for the interactions were not statistically significant (Figure 4). In heart failure with different ejection fraction subgroups, the trends of association between TMAO and frailty were without significant heterogeneity.
Figure 4.
Relationship between the TMAO concentration and the frailty risk stratified according to baseline characteristics. Bars represent 95% confidence intervals.
Abbreviations: BMI, body mass index; CHF, chronic heart failure; CI, confidence interval; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; HFrEF, heart failure with reduced ejection fraction; HFmEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; OR, odds ratio; PAD, peripheral arterial disease.
The addition of TMAO to the full multivariable model revealed that higher TMAO levels improved the area under the curve (AUC) for predicting frailty among older adults with cardiovascular disease (AUC: from 0.863 to 0.879, p = 0.027) (Figure S1A and B). The continuous NRI and IDI when adding TMAO to the fully adjusted model were 0.251 (95% CI 0.059–0.442, p=0.010) and 0.015 (95% CI 0.0003–0.03, p=0.045) (Table S2). Furthermore, on decision curve analysis, the full multivariable model with TMAO showed better net benefit and clinical usefulness compared with the model without TMAO (Figure S2).
Associations Between TMAO, Physical Frailty and Cognitive Frailty
Plasma TMAO levels were significantly higher in patients with physical frailty (CHS physical frailty criteria) than in nonfrail individuals (3.85 [2.54–6.94] vs. 3.30 [2.21–5.27] µM; p<0.001). Based on SPPB physical frailty criteria, participants with physical frailty still had significantly higher plasma TMAO concentrations than nonfrail participants (3.59 [2.36–5.83] vs. 3.10 [2.17–4.99] µM; p<0.001). A significantly higher plasma TMAO level was observed in patients with cognitive frailty than that in patients without (4.56 [2.81–7.59] vs. 3.38 [2.26–5.38] µM; p=0.004). There was a significant association between 2-unit increments in TMAO and physical frailty according to the criteria of the CHS and SPPB in both unadjusted and adjusted logistic regression analyses (Table 2). According to CHS physical frailty criteria, the likelihood of slowness, weakness, and low physical activity showed a significantly positive correlation with an increased TMAO level both before and after covariate adjustment. Weight loss showed a significant correlation with TMAO after full adjustment, but not in the crude model. Exhaustion did not show any association with TMAO. Furthermore, a strong association between each 2-unit increase in TMAO and cognitive frailty was observed in the crude analysis (OR 1.26, 95% CI 1.07–1.48, p=0.006). Although this relationship was attenuated to some extent by covariate adjustment, it remained statistically significant (OR 1.21, 95% CI 1.01–1.45, p=0.04). Moreover, the increase in TMAO levels was also independently correlated with calf circumference when comparing the lowest quartile of calf circumference (Q1) to the highest quartile (Q4) (OR 1.15, 95% CI 1.03–1.29, p=0.02).
Table 2.
Association Between Physical Frailty, Cognitive Frailty and Calf Circumference by Univariate and Multivariate Logistic Regression Analysis
| TMAO (per 2 units)a | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P for Trend | OR | 95% CI | P for Trend | |
| CHS physical frailty | 1.29 | (1.13,1.47) | <0.001 | 1.23 | (1.08,1.41) | 0.002 |
| Weight loss | 1.16 | (0.99,1.36) | 0.07 | 1.18 | (1.00,1.40) | 0.047 |
| Exhaustion | 1.08 | (0.96,1.21) | 0.23 | 1.05 | (0.93,1.19) | 0.40 |
| Low activity | 1.31 | (1.15,1.50) | <0.001 | 1.29 | (1.12,1.47) | <0.001 |
| Slowness | 1.34 | (1.13,1.59) | 0.006 | 1.38 | (1.15,1.65) | 0.008 |
| Weakness | 1.38 | (1.09,1.73) | 0.008 | 1.58 | (1.05,2.38) | 0.04 |
| SPPB physical frailty | 1.26 | (1.11,1.43) | <0.001 | 1.19 | (1.05,1.36) | 0.009 |
| Cognitive frailty | 1.26 | (1.07,1.48) | 0.006 | 1.21 | (1.01,1.45) | 0.04 |
| Calf circumference (Q1/Q4)b | 1.12 | (1.01,1.25) | 0.04 | 1.15 | (1.03,1.29) | 0.02 |
Notes: aEach point corresponds to a 2-unit increment in TMAO. bComparing the first and fourth quartiles of calf circumference. Logistic regression adjusted for sex, age, and body mass index.
Abbreviations: OR, odds ratio; CI, confidence interval; CHS, Cardiovascular Health Study; SPPB, Short Physical Performance Battery; TMAO, trimethylamine N-oxide.
Discussion
To our best knowledge, our study demonstrated for the first time that the gut microbiota-generated metabolite TMAO levels are independently associated with frailty among older patients with cardiovascular disease. In detail, strong correlations between TMAO levels, physical frailty and cognitive frailty were elucidated. Moreover, our results proved that the risk of frailty increases linearly with the increase of TMAO concentration. Our findings suggest that TMAO may serve as a potential biomarker and therapeutic target for older patients with cardiovascular disease.
We now realize that microbiota-dependent metabolism may produce metabolites with potential adverse cardiovascular impact, such as TMAO, which can promote atherosclerosis and increase thrombosis risk.25 Recent experimental studies demonstrate that TMAO may lead to cardiovascular aging and brain aging by inducing oxidative stress and endothelial cell senescence.15,16 As a condition of aging-related vulnerability to stressors, frailty has a potential link to TMAO metabolism supported by a recent bioinformatic study.17 This study demonstrated that choline consumption and TMA production are characteristic microbial metabolism of frail subjects. Furthermore, from the perspective of genome-metabolism interactions, the study revealed a higher abundance of the microbial CutC enzyme catalyzing choline-TMA conversion in the gut microbiota of frail people. However, these bioinformatic results only offered indirect evidence for the relationship between TMAO and frailty. Based on the established link, our study directly demonstrated that TMAO is dose-dependently associated with frailty in older adults (Figure 3). The difference in frailty risk across TMAO quartiles is highly evident in participants in the fourth quartile (Q4), in which participants had a twofold higher OR of frailty than those in the lowest quartile (Q1). To avoid bias caused by different cardiovascular diseases, we carried out a sensitivity analysis indicating that the associations between TMAO and frailty remained evident and independent (Figure 4). It is thus conceivable that frailty was prone to occur in elevated plasma TMAO levels regardless of the effects of different cardiovascular diseases. Taken together, our findings provided additional support for an aging-related effect of elevated systemic levels of TMAO.
In the subgroup analysis, we observed that heterogeneity existed in diabetes, PAD, or stroke subgroups, while further interaction tests were with no statistical significance, indicating these comorbidities may not contribute to the association between frailty and TMAO (Figure 4). Previous studies have shown that TMAO is a predictor of poor prognosis in patients with heart failure.26,27 Although we found that the association between TMAO and frailty was more evident in the chronic heart failure subgroup, the results showed high heterogeneity (OR 4.67, 95% CI 0.61–35.49, p for interaction 0.70). Regarding the different predictive values of TMAO on distinct types of heart failure,27,28 the heart failure patients were divided by different levels of LVEF. Further subgroup analysis indicated that intra-group heterogeneity reduced and all subtypes of heart failure had no significant effect on the association between TMAO and frailty. This intriguing difference could be attributed to that chronic heart failure is not the leading cause of increased TMAO in frail patients. Another possible explanation for this difference is that the sample size of this post hoc subgroup analysis is limited to reach significance. Further large sample research is required to identify the influence of heart failure on the relationship between TMAO and frailty.
The present study suggests that physical frailty was positively correlated with an increase in TMAO using different diagnostic criteria of CHS and SPPB. To date, these findings could be explained by two existing theories, the gut-muscle axis and microbiota-associated physical performance. First, impaired physical performance is mainly due to the age-related degenerative loss of skeletal muscle mass and strength. These age-related manifestations can also be referred to as sarcopenia, which represents the process of the frailty-to-disability transition and can be affected by the gut microbiota and metabolites.29 Short-chain fatty acids (SCFAs) and indoxyl sulfate, as metabolites of the gut microbiota similar to TMAO, have also been shown to play an essential role in physical frailty and sarcopenia.30,31 The above mechanism is consistent with our findings that the increase in TMAO was associated with a smaller calf circumference and lower grip strength. This suggests that increased TMAO might negatively correlate with the mass and function of skeletal muscle, indicating a potential linking mechanism between TMAO and sarcopenia. Second, growing evidence has shown that the gut microbiota has an effect on physical performance. A meta-omics analysis showed that Veillonella atypica could enhance exercise performance via the lactate metabolism pathway among elite athletes.7 Some species within the gut microbiota, such as Enterobacteriaceae, Lachnospiraceae and Lactobacillaceae,32,33 are associated with slow gait speed among the older population. All of these taxa are enriched in TMA synthesis pathways, indicating that TMAO may correlate with microbiota-associated physical performance.34 In addition to these previous findings, our findings revealed that the circulating TMAO concentration was strongly inversely associated with gait speed, suggesting that TMAO is associated with physical performance.
In our study, we demonstrated that a higher TMAO concentration was associated with worse cognitive frailty. These findings add to the growing evidence that TMAO can induce a decline in cognition. In mice, TMAO deteriorates brain aging by promoting neuron senescence and aggravates cognitive dysfunction by damaging synapses and inhibiting the mTOR signaling pathways which are associated with learning and memory abilities.15 Likewise, several clinical studies also emphasize the adverse impact of TMAO on cognitive function in Alzheimer’s disease and poststroke patients.35,36 Our results are consistent with these prior findings and highlight the effect of TMAO on the overall condition of the older population by focusing on cognitive frailty because studies have shown that older adults with cognitive frailty have a worse prognosis than those with cognitive impairment only.37 Currently, only long-term physical activity can reduce cognitive frailty,38 and neither a biomarker nor a targeted intervention for cognitive frailty has been proposed. Therefore, considering TMAO as a predictor of cognitive frailty can provide a novel prospect for future research on improving cognitive frailty.
The significance of our study is highlighted by the ability of TMAO to be modified with diet adjustment and pharmacological agents. Imbalanced diet, specially a low-fiber/high-fat diet contributes to the formation of the frail microbiome and an increase of plasma TMAO.8,39 Dietary transition to a high-fiber/low-fat diet can serve to lower the levels of circulating TMAO levels and reduce frailty.13,40 Moreover, fat-soluble vitamins, broad-spectrum antibiotics and microbe-targeted inhibitors have been reported to lower plasma TMAO concentration in intervention trials.41,42 As a latest breakthrough, a family of second-generation choline-TMA lyase inhibitors, halomethylcholines, have been depicted as mechanism-based drugs that are more potent in lowering plasma concentrations of TMA and TMAO without observable toxicity in the preclinical trial.43 All these advances inspire us of the potential of targeting TMAO for improving frailty status in future studies.
Limitations
We note several limitations of the present study. First, this was a single-center study conducted in a tertiary medical that recruits patients at the point of admission; therefore, we could not exclude selection bias for patients who accepted comprehensive geriatric assessment (especially with relatively preserved cognitive, renal and heart function). Second, considering the diverse definition of frailty, although we sought to evaluate it with multiple methods, the different definitions might affect the results. Third, all associations were assessed in a cross-sectional manner, and blood samples were collected only at enrollment. Therefore, conclusions about the temporal relationships between TMAO and changes in frailty over time cannot be inferred from our results. Finally, we did not have data on the dietary habits of participants that might affect the circulating TMAO levels. A further multicenter cohort study with dietary intake information should be performed to explore the association between plasma TMAO and frailty in a long term.
Conclusion
The circulating TMAO concentration is independently associated with frailty in older adults with cardiovascular disease. Higher levels of TMAO are associated with the presence of frailty, and this positive correlation exhibits a linear dose–response relationship. Moreover, physical frailty and cognitive frailty are significantly associated with plasma TMAO levels. These findings provide a novel perspective linking the gut microbiota metabolites to the pathogenesis of frailty. Further studies are warranted to explore the use of TMAO as a modifiable target to promote healthy aging.
Acknowledgments
The authors thank Dr. Peter Hu and Dr. Rachel Hu for their assistance in the revision of this manuscript. We thank Dr. Yao-Dan Liang and Dr. Meng-Ge Zhou for the helpful discussion and advice.
Data Sharing Statement
Data will be available upon request from the corresponding authors.
Ethics Approval and Informed Consent
The study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Hospital (No. 2018BJYYEC-121-02). This study was monitored by the Peking University Clinical Research Institute, which was entrusted by the Beijing Municipal Science & Technology Commission. Our clinical trial registration number is ChiCTR1800017204 and the date of registration is 2018/7/18.
Disclosure
Dr. Hua Wang reports grants from Beijing Municipal Science & Technology Commission, grants from CAMS Innovation Fund for Medical Sciences, grants from the Chinese Academy of Medical Sciences, during the conduct of the study. The other authors report no conflicts of interest in this work.
References
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 2.Vitale C, Jankowska E, Hill L, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21(11):1299–1305. doi: 10.1002/ejhf.1611 [DOI] [PubMed] [Google Scholar]
- 3.Pilotto A, Custodero C, Maggi S, Polidori MC, Veronese N, Ferrucci L. A multidimensional approach to frailty in older people. Ageing Res Rev. 2020;60:101047. doi: 10.1016/j.arr.2020.101047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saedi AA, Feehan J, Phu S, Duque G. Current and emerging biomarkers of frailty in the elderly. Clin Interv Aging. 2019;14:389–398. doi: 10.2147/CIA.S168687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108 Suppl 1(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350(6265):1214–1215. doi: 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- 7.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104–1109. doi: 10.1038/s41591-019-0485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi: 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 9.O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cell Mol Life Sci. 2018;75(1):119–128. doi: 10.1007/s00018-017-2673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8(1):8. doi: 10.1186/s13073-016-0262-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30(12):1700–1705. [DOI] [PubMed] [Google Scholar]
- 12.Tan Y, Zhou J, Liu C, et al. Association between plasma trimethylamine N-oxide and neoatherosclerosis in patients with very late stent thrombosis. Can J Cardiol. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–594. doi: 10.1093/eurheartj/ehy799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassambai S, Salzano A, Yazaki Y, et al. Impact of acute choline loading on circulating trimethylamine N-oxide levels. Eur J Prev Cardiol. 2019;26(17):1899–1902. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Ke Y, Zhan R, et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell. 2018;17(4):e12768. doi: 10.1111/acel.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, Chen Y, Gua C, Li X. Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol. 2017;8:350. doi: 10.3389/fphys.2017.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh TS, Das M, Jeffery IB, O’Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife. 2020;9:e50240. doi: 10.7554/eLife.50240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang YD, Zhang YN, Li YM, et al. Identification of frailty and its risk factors in elderly hospitalized patients from different wards: a Cross-Sectional Study in China. Clin Interv Aging. 2019;14:2249–2259. doi: 10.2147/CIA.S225149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiukui H, Jun L, Birong D, Xiaoying L. Chinese experts consensus on assessment and intervention for elderly patients with frailty. Chin J Geriatr. 2017;036(3):251–256. [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 21.Da Camara SM, Alvarado BE, Guralnik JM, Guerra RO, Maciel AC. Using the short physical performance battery to screen for frailty in young-old adults with distinct socioeconomic conditions. Geriatr Gerontol Int. 2013;13(2):421–428. doi: 10.1111/j.1447-0594.2012.00920.x [DOI] [PubMed] [Google Scholar]
- 22.Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J Nutr Health Aging. 2013;17(9):726–734. [DOI] [PubMed] [Google Scholar]
- 23.Roppolo M, Mulasso A, Rabaglietti E. Cognitive frailty in Italian community-dwelling older adults: prevalence rate and its association with disability. J Nutr Health Aging. 2017;21(6):631–636. doi: 10.1007/s12603-016-0828-5 [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 25.Yao ME, Liao PD, Zhao XJ, Wang L. Trimethylamine-N-oxide has prognostic value in coronary heart disease: a meta-analysis and dose-response analysis. BMC Cardiovasc Disord. 2020;20(1):7. doi: 10.1186/s12872-019-01310-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salzano A, Cassambai S, Yazaki Y, et al. The gut axis involvement in heart failure: focus on trimethylamine N-oxide. Heart Fail Clin. 2020;16(1):23–31. doi: 10.1016/j.hfc.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Schuett K, Kleber ME, Scharnagl H, et al. Trimethylamine-N-oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2017;70(25):3202–3204. doi: 10.1016/j.jacc.2017.10.064 [DOI] [PubMed] [Google Scholar]
- 28.Salzano A, Israr MZ, Yazaki Y, et al. Combined use of trimethylamine N-oxide with BNP for risk stratification in heart failure with preserved ejection fraction: findings from the DIAMONDHFpEF study. Eur J Prev Cardiol. 2019;2047487319870355. [DOI] [PubMed] [Google Scholar]
- 29.Buford TW, Carter CS, VanDerPol WJ, et al. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40(3):257–268. doi: 10.1007/s11357-018-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poggiogalle E, Lubrano C, Gnessi L, et al. The decline in muscle strength and muscle quality in relation to metabolic derangements in adult women with obesity. Clin Nutr. 2019;38(5):2430–2435. doi: 10.1016/j.clnu.2019.01.028 [DOI] [PubMed] [Google Scholar]
- 31.Enoki Y, Watanabe H, Arake R, et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep. 2016;6:32084. doi: 10.1038/srep32084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barichella M, Severgnini M, Cilia R, et al. Unraveling gut microbiota in parkinson’s disease and atypical parkinsonism. Mov Disord. 2019;34(3):396–405. doi: 10.1002/mds.27581 [DOI] [PubMed] [Google Scholar]
- 33.Morita E, Yokoyama H, Imai D, et al. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11(4):868. doi: 10.3390/nu11040868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rath S, Heidrich B, Pieper DH, Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5(1):54. doi: 10.1186/s40168-017-0271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogt NM, Romano KA, Darst BF, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):124. doi: 10.1186/s13195-018-0451-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu C, Li G, Lv Z, et al. Association of plasma trimethylamine-N-oxide levels with post-stroke cognitive impairment: a 1-year longitudinal study. Neurol Sci. 2020;41(1):57–63. doi: 10.1007/s10072-019-04040-w [DOI] [PubMed] [Google Scholar]
- 37.Yi C, Lin J, Cao P, et al. Prevalence and prognosis of coexisting frailty and cognitive impairment in patients on continuous ambulatory peritoneal dialysis. Sci Rep. 2018;8(1):17305. doi: 10.1038/s41598-018-35548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Hsu F-C, Trombetti A, et al. Effect of 24-month physical activity on cognitive frailty and the role of inflammation: the LIFE randomized clinical trial. BMC Med. 2018;16(1):185. doi: 10.1186/s12916-018-1174-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JE, Miller M, Rhyne J, Wang Z, Hazen SL. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr Metab Cardiovasc Dis. 2019;29(5):513–517. doi: 10.1016/j.numecd.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 40.Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;gutjnl–2019–319654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Organ CL, Li Z, Sharp TE, et al. Nonlethal inhibition of gut microbial trimethylamine N-oxide production improves cardiac function and remodeling in a murine model of heart failure. J Am Heart Assoc. 2020;e016223. [DOI] [PMC free article] [PubMed] [Google Scholar]