Abstract
Patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) following autologous stem cell transplant (ASCT) remain a management challenge with few reliably effective treatments. Lenalidomide, an immunomodulatory drug approved for patients with myelodysplastic syndrome with del(5q), multiple myeloma, and mantle cell lymphoma, has demonstrated some activity in patients with R/R cHL, though the toxicity of traditional doses and schedules has been a barrier to consistent use. Low dose continuous (LDC) schedules have emerged as promising, with a more favorable safety profile. We report herein that LDC schedules are associated with a far more tolerable toxicity profile, and exhibit at least equivalent efficacy in this patient population. We report that patients diagnosed with R/R cHL who previously underwent, or were not candidates for, ASCT and/or clinical trials, were administered daily LDC lenalidomide (20 mg orally with dose reduction for toxicity). Among the 19 patients included in this analysis, 11% of patients achieved a partial response (PR), with no documented complete responses (CR). A total of 12 (63%) patients maintained stable disease (SD), with 7 patients (37%) remaining in SD for more than 6 months. The clinical benefit rate (comprised of CR, PR, and SD for greater than 6 months) was 47% (7 out of 19 patients). The median progression-free survival and overall survival of all patients were 9.4 months (range, 4.6–14.4 months) and 90 months (range, 63.6–166.8 months), respectively. In general, the treatment was well tolerated, with grade 3 or 4 adverse events consisting of neutropenia (n = 4), and one case each of thrombocytopenia, fatigue, rash, creatinine elevation, aspartate transaminase/alanine transaminase elevation, and treatment related secondary malignancy. In a heavily treated R/R cHL patient population, daily LDC lenalidomide was associated with a high disease control rate with a favorable toxicity profile.
Keywords: lenalidomide, relapsed or refractory classical Hodgkin lymphoma
Introduction
Classical Hodgkin lymphoma (cHL) is a rare disease characterized by Reed-Sternberg cells that typically affects younger and older adults in a bimodal distribution.1 Front-line therapy for cHL usually consists of combination chemotherapy and is associated with a cure rate in about 70% of patients.2 For patients with relapsed or refractory disease (R/R), second-line cytoreductive chemotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) is considered the standard of care, for those patients considered transplant eligible.2 It has been estimated that 71% of patients with R/R cHL who progress after ASCT succumb to the disease within 1 year, with an estimated 90% of these patients dying within 2 years. These data would suggest that there is a need for effective, well-tolerated options for this setting.3 For patients who progress after ASCT, or are not eligible for ASCT, brentuximab vedotin and the anti-PD1 monoclonal antibodies nivolumab and pembrolizumab are approved for this indication.4–6 Other off-label options include low dose chemotherapy, gemcitabine-based treatment, and lenalidomide.2
Lenalidomide is an immunomodulatory drug (IMiD) originally approved for the treatment of lower risk myelodysplastic syndrome with deletion 5q.7 While the drug has also been approved for patients with multiple myeloma and mantle cell lymphoma, we presently have an incomplete understanding of its mechanism of action (MOA).8 Ito and colleagues have reported that the target of thalidomide was cereblon, which forms an E3 ubiquitin ligase complex.9 This mechanism of action was eventually confirmed to be a class effect, seen with other members in the class including lenalidomide and pomalidomide.10 Other experiences with conventionally dosed lenalidomide in this setting (25 mg daily for 21 of 28 consecutive days) have reported generally low overall response rate (ORR) with substantial toxicity.11–13 Given the goals of treatment in this patient population are focused on palliative control (that is a meaningful disease control rate) with minimal toxicity; there is a rationale to consider low dose continuous (LDC) schedules in patients with R/R cHL. Interestingly, compared with intermittent dosing schedules, LDC schedules have been associated with a shorter time interval between initiation of treatment and response, with a more favorable toxicity profile.14 Herein, we share the first experience with this schedule of lenalidomide in patients with R/R cHL.
Methods
Using data captured through the electronic medical record and pathology department database between 1 January 2010 and 31 October 2019, patients 18 years and older with a diagnosis of classical Hodgkin lymphoma according to the World Health Organization (WHO) classification were identified at New York Presbyterian Hospital – Columbia University Irving Medical Center. Patients who relapsed or were refractory after at least one prior systemic therapy and received LDC lenalidomide therapy were included in this series. Patients initially received 10 mg lenalidomide daily, being titrated up to 20 mg as tolerated. Dosing continued on a daily 30-day cycle. Using the 2007 Lugano criteria, responses were measured by either computed tomography (CT) or positron emission tomography/computed tomography (PET/CT) every 2–3 months.15
Treatment continued until disease progression or development of unacceptable adverse events at the lowest allowable administered dose (5 mg). Patients were considered eligible for the analysis if they had prior radiation therapy, autologous stem cell transplant, and/or allogeneic stem cell transplant. Overall survival was calculated from the time of diagnosis to death from any cause. Kaplan–Meier curves for overall survival were generated and compared based on the log-rank test. Data collection was completed and censored on 31 October 2019. Cox proportional hazard models were used to investigate the impact on overall survival by adjusting for age, gender, race, ethnicity, histology, and duration of treatment. The analysis was performed using SAS version 9.4. A p-value < 0.05 was considered statistically significant. The research reported herein was conducted and reported in accordance with the Declaration of Helsinki and approved by our Institutional Review Board (IRB AAAT0218). The need for informed consent was waived by our IRB because this study presented minimal risk.
Results
Patient characteristics and impact on survival
A total of 19 patients were included in the analysis and are described in Table 1. The median age of diagnosis and at initiation of treatment with lenalidomide were 28 years (range, 13–55 years) and 34 years (range, 27–61 years), respectively. The median number of prior therapies at the time of initiating LDC lenalidomide was 7.5 (range, 3–15), confirming this was a very heavily pretreated population. As shared in Table 1, the majority of patients were male (53%), white (84%), and not Hispanic (95%), with the majority of patients (63%) having a nodular sclerosis histology, nine (47%) having Stage 2 disease at diagnosis, and 63% having had prior exposure to radiation. A total of 13 patients (68%) underwent a prior ASCT, with 4 of those patients having had both an ASCT and allogeneic stem cell transplant.
Table 1.
Characteristics of patient population (n = 19).
| Characteristics | Value |
|---|---|
| Median age at treatment (range) | 27 years (34–61) |
| Race | |
| White (%) | 16 (84%) |
| Black (%) | 2 (10.5%) |
| Asian (%) | 1 (5%) |
| Ethnicity | |
| Not Hispanic (%) | 18 (95%) |
| Hispanic (%) | 1 (5%) |
| Histology | |
| Nodular sclerosis (%) | 12 (63%) |
| Not otherwise specified (%) | 6 (32%) |
| Mixed cellularity (%) | 1 (5%) |
| Stage at diagnosis | |
| Stage II | 9 (47%) |
| Stage III | 5 (26%) |
| Stage IV | 5 (26%) |
| Median lines of therapy (range) | 7.5 (3–15) |
| Prior therapies | |
| ABVD | 15 (79%) |
| BEACOPP or Stanford V or COPPABV | 4 (21%) |
| Platinum-based chemotherapy | 19 (100%) |
| Brentuximab vedotin | 13 (68%) |
| Histone deacetylase inhibitor | 7 (37%) |
| Immune checkpoint inhibitor | 0 |
| Prior transplant | |
| Autologous stem cell transplant (%) | 13 (63%) |
| Autologous and allogeneic transplant (%) | 4 (21%) |
| None (%) | 2 (10.5%) |
| Prior radiation therapy | |
| Yes (%) | 13 (63%) |
| No (%) | 6 (32%) |
ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; COPPABV, cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine.
Table 2.
Response rates for entire cohort (n = 19).
| Outcome | Number, n (%) |
|---|---|
| Complete response (CR) | 0 |
| Partial response (PR) | 3 (16%) |
| Stable disease (SD) | 11 (58%) |
| Stable disease >6 months (SD > 6 months) | 7 (37%) |
| Cytostatic ORR (CR + PR + SD > 6 months) | 10 (53%) |
| Progression of disease | 5 (26%) |
CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease.
Efficacy
Overall, the treatment was well tolerated. The median number of cycles administered was 5.9 (range, 1.6–28 cycles). Among the 19 patients, all of whom were considered evaluable, no patients achieved a complete response (CR), three patients achieved a partial response (PR; ORR of 16%), while 11 had stable disease (SD) as their best response (58%; with 37% sustaining SD for ⩾6 months). While the ORR was low, the clinical benefit rate (CR + PR + SD > 6 months) was 53% (Table 2). The median time to response in the three patients who achieved PR was 2.5 months (range, 2.3–7.6 months). Among the study population, 15 (79%) patients sustained a response or stable disease lasting 3 months or greater, while 7 (37%) patients had a response of 6 months or greater. One patient discontinued treatment after achieving a PR as they proceeded to ASCT. A total of 13 patients eventually discontinued treatment due to progression of disease. The median progression-free survival (PFS) and overall survival (OS) of the study population was 9.4 months (range, 4.6–14.4 months) and 90 months (range, 63.6–166.8 months), respectively (Figure 1A and B).
Figure 1.
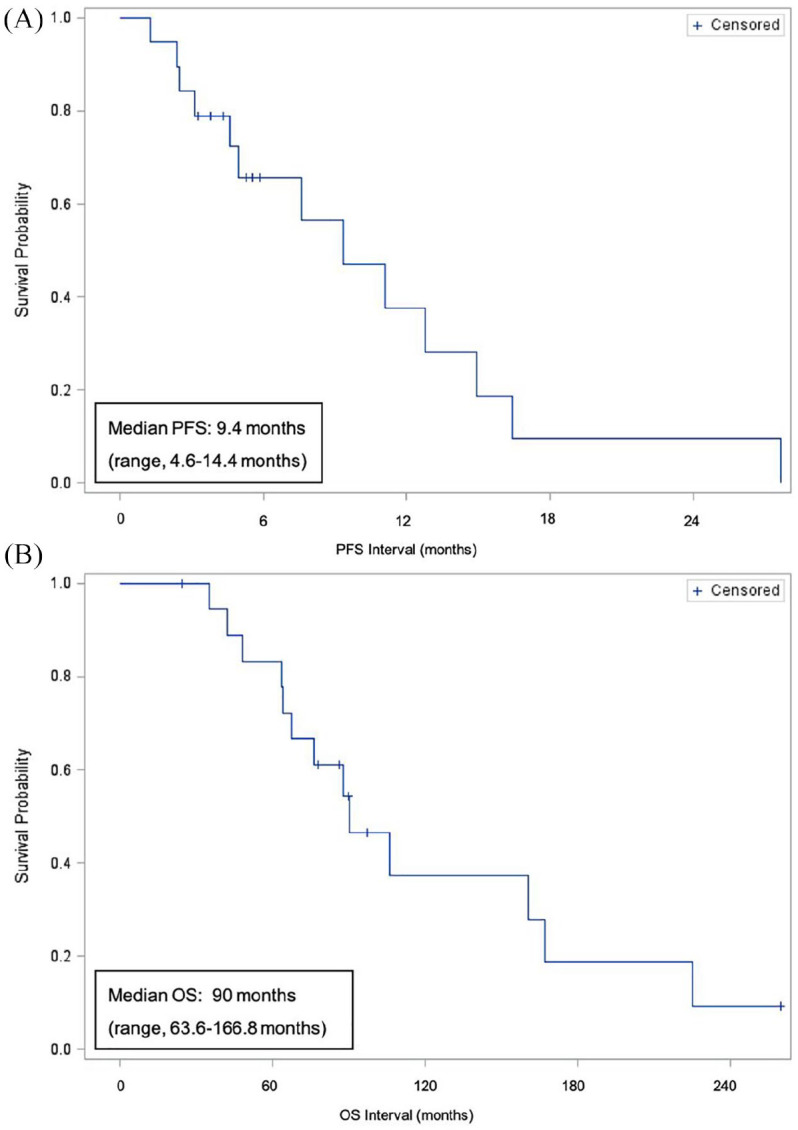
(A) PFS and (B) OS.
PFS, progression free survival; OS, overall survival.
Toxicity
The most common clinically significant adverse events that required dose interruptions or dose adjustments were: grade 3 or 4 neutropenia (n = 4); grade 2 neuropathy (n = 2); grade 2 diarrhea (n = 1); grade 3 rash, fatigue, liver toxicity, elevated creatinine (n = 1 each), and grade 4 thrombocytopenia (n = 1). Serious adverse events, including hospitalization for fever (n = 1) and pneumonia (n = 1), were also observed. Overall the LDC schedule was well tolerated, with 12 patients tolerating the 20 mg daily dose (of these patients, one patient who had tolerated 20 mg for 1 year of treatment required dose reduction to 10 mg for neutropenia), one patient who alternated between 10 mg and 20 mg daily, five patients who tolerated 10 mg daily, and one patient who required a dose reduction to 5 mg daily.
Four (21%) patients developed infections (pneumonia, upper respiratory infection, skin infection, and urinary tract infection), which were readily managed with antimicrobial therapy. While most toxicities were managed with supportive care and/or dose reductions, three patients discontinued treatment for the following reasons: interstitial nephritis (n = 1), exacerbation of neuropathy (n = 1), and liver toxicity (n = 1). One heavily treated patient, status post an ASCT, developed myelodysplastic syndrome-related acute myeloid leukemia 8 months after treatment discontinuation.
Discussion
Patients with R/R cHL have a poor prognosis. Combination chemotherapy can often improve survival but is often associated with a reduction in quality of life, regularly requiring blood tests, blood transfusions, and are not infrequently associated with life-limiting secondary myelodysplastic syndrome or secondary malignancies in heavily treated patients. Treatments that can produce disease control with minimal toxicity offer a meaningful strategy to manage these patients, placing a premium on quality of life. LDC lenalidomide is a relatively convenient oral option for patients who are heavily pretreated and not candidates for other more aggressive treatment approaches. Such an approach can provide time to freedom from progression and may be used as a bridge to definitive therapies. In addition, while difficult to establish with precise data, time off cytotoxic chemotherapy is likely associated with a re-sensitization to chemotherapy, which could be a strategy to diminish the impact of acquired drug resistance. The findings reported herein are consistent with other studies, supporting a clinically meaningful benefit for patients with few treatment options, an experience underscored in a recent case report.16
Regarding quality of life on lenalidomide, the most common grade 3 or 4 toxicity was neutropenia, which was often manageable with supportive care. Common adverse events such as infections and diarrhea were mild in this population, occurring in 4 (21%) and in 1 (5%) of the 19 patients. A case series published on 12 patients with R/R cHL treated with lenalidomide at a dose of 25 mg orally on days 1 through 21 of a 28-day cycle reported an ORR of 50%.13 Two follow-up phase II clinical trials have evaluated this treatment regimen in patients with R/R cHL, but administration was limited by toxicity.11,12 Kuruvilla and colleagues reported that, among the 14 evaluable patients, the ORR was 2/14 (14%), with five (36%) having to discontinue therapy due to toxicity, which included neutropenia, thrombocytopenia, anemia, and rash.11 In a phase II study of 36 evaluable patients with R/R cHL, Fehniger et al. also reported that intermittent dosing of lenalidomide achieved an ORR of 7/36 (19%) and cytostatic ORR of 33%.12 Four (11%) patients discontinued treatment due to toxicity, and the most common Grade 3 or 4 adverse events included neutropenia (47%), anemia (29%), and thrombocytopenia (18%).
Unfortunately, many patients with R/R cHL are not candidates for clinical trials given the cumulative toxicity from their previous treatment programs. Nowadays, virtually all of these patients have been exposed to the newer approved drugs like brentuximab vedotin and the PD-1 inhibitors, implying that the insertion of additional salvage therapies now creates an even more drug-resistant patient population, making additional lines of therapy more unlikely to provide clinical benefit. While LDC lenalidomide is not a cure for these heavily pretreated patients with cHL, it is notable that one patient who had five previous lines of therapy achieved a PR, allowing an ASCT. For patients who are likely not to be candidates for further myelosuppressive therapy given the accumulated secondary cytopenias resulting from their prior exposure, strategies like LDC lenalidomide may be an option, affording patients a break from frequent visits to the clinic or hospital with aggressive testing.
In addition to single agent use of novel agents, there are now a host of studies combining or attempting to combine existing agents in new combinations. For example, a trial exploring lenalidomide plus the histone deacetylase inhibitor panobinostat, have reported an ORR of 16.7% (n = 22 patients) with median PFS and OS of 3.8 and 16.4 months, respectively. With all caveats acknowledged, at first blush this does not appear substantially better then LDC lenalidomide. Other studies exploring lenalidomide in combination with vorinostat (ClinicalTrials.gov identifier: NCT01116154) or romidepsin (ClinicalTrials.gov identifier: NCT01742793), were both terminated because the response rate was considered similar to single agent lenalidomide, with what was felt to be an increase in toxicity.17 A plethora of other studies with lenalidomide as a single agent (ClinicalTrials.gov identifier: NCT00478959), and in combination with bendamustine (ClinicalTrials.gov identifier: NCT01412307), pembrolizumab (ClinicalTrials.gov identifier: NCT02875067), nivolumab (ClinicalTrials.gov identifier: NCT03015896), brentuximab vedotin (ClinicalTrials.gov identifier: NCT03302728), and temsirolimus (ClinicalTrials.gov identifier: NCT01076543) are now enrolling patients with R/R cHL. The present experiences suggests that LDC lenalidomide can be explored as a bridge or palliative option for R/R cHL who are ineligible or have exhausted available therapies.
Footnotes
Authors’ note: An earlier version of this work has been presented at the American Society of Hematology annual conference in 2013. HM and AS updated the data and wrote the manuscript. BC performed the statistical analysis. All authors critically edited and approved the final manuscript.
Conflict of interest statement: HM and BC have nothing to report.
FM received research funding from Seattle Genetics.
JKL received research support and advisory board service from Kymera Therapeutics, research support from Denovo Biopharma, advisory board service from Astex Pharmaceuticals, and Speakers’ Bureau from AstraZeneca.
CD received research funding from Bayer, Amgen, TG Therapeutics, Applied Therapeutics.
EM received research support from Merck, Celgene, Spectrum, and has a scientific advisory role with Mundipharma, Verastem, Spectrum, Myeloid Therapeutics.
OAO received research funding from Affimed, Agensys, Celgene, Merck, Seattle Genetics, Spectrum, TG Therapeutics, and Trillium; and has a scientific advisory role for Celgene (Data Safety Monitoring Committee), Mundipharma, and TG Therapeutics (Travel support only).
AS received research support from Affimed and consultancy fees from Seattle Genetics, Gilead, and Daiichi Sanko.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Helen Ma, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA.
Bin Cheng, Department of Statistics, Columbia University Irving Medical Center, New York, NY, USA.
Francesca Montanari, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA.
Jennifer K. Lue, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA
Changchun Deng, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA.
Enrica Marchi, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA.
Owen A. O’ Connor, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, New York, NY, USA
Ahmed Sawas, Center for Lymphoid Malignancies, Columbia University Irving Medical Center, 51 West 51st Street Suite 200, New York, NY 10019, USA.
References
- 1. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC, 2017, p.585. [Google Scholar]
- 2. Ansell SM. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am J Hematol 2018; 93: 704–715. [DOI] [PubMed] [Google Scholar]
- 3. Zagadailov EA, Corman S, Chirikov V, et al. Real-world effectiveness of brentuximab vedotin versus physicians’ choice chemotherapy in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplantation in the United Kingdom and Germany. Leuk Lymphoma 2018; 59: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 4. Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017; 35: 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 2012; 30: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol 2018; 36: 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah SR, Tran TM. Lenalidomide in myelodysplastic syndrome and multiple myeloma. Drugs 2007; 67: 1869–1881. [DOI] [PubMed] [Google Scholar]
- 8. Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol 2008; 26: 1544–1552. [DOI] [PubMed] [Google Scholar]
- 9. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012; 26: 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuruvilla J, Taylor D, Wang L, et al. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. American Society of Hematology Annual Meeting, San Francisco, CA, 2008. [Google Scholar]
- 12. Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood 2011; 118: 5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boll B, Borchmann P, Topp MS, et al. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Br J Haematol 2010; 148: 480–482. [DOI] [PubMed] [Google Scholar]
- 14. List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006; 355: 1456–1465. [DOI] [PubMed] [Google Scholar]
- 15. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 16. Mandac I, Kolonic SO. Lenalidomide induced good clinical response in a patient with multiple relapsed and refractory Hodgkin’s lymphoma. J Hematol Oncol 2010; 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maly JJ, Christian BA, Zhu X, et al. A phase I/II trial of panobinostat in combination with lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 2017; 17: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]


