Abstract
Background:
Epidemiological studies have suggested that vitamin D deficiency is associated with the development of type 2 diabetes (T2DM) and is related to diabetes complications. This study was undertaken to determine the relationship between diabetes complications and cardiovascular risk factors with vitamin D3 and its metabolites: 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), 25-hydroxyvitamin D3 (25(OH)D3), 24,25-dihydroxyvitamin D3 (24,25(OH)2D3); and 25-hydroxy-3epi-vitamin D3 (3epi25(OH)D3).
Methods:
750 Qatari subjects, 460 (61.3%) with and 290 (38.7%) without T2DM, who were not taking vitamin D3 supplements, participated in this cross-sectional, observational study. Plasma concentrations of vitamin D3 and its metabolites were measured by liquid chromatography tandem mass spectrometry analysis.
Results:
T2DM subjects had lower concentrations of all vitamin D3 metabolites (p < 0.001) except 3epi25(OH)D3 (p < 0.071). Males had higher concentrations of all vitamin D3 metabolites (p < 0.001). In the T2DM subjects, lower 25(OH)D3 was associated with retinopathy (p < 0.03) and dyslipidemia (p < 0.04), but not neuropathy or vascular complications; lower 1,25(OH)2D3 was associated with hypertension (p < 0.009), dyslipidemia (p < 0.003) and retinopathy (p < 0.006), and coronary artery disease (p < 0.012), but not neuropathy; lower 24,25(OH)2D3 concentrations were associated with dyslipidemia alone (p < 0.019); 3epi25(OH)D3 associated with diabetic neuropathy alone (p < 0.029). In nondiabetics, 25(OH)D3, 1,25(OH)2D3 and 24,25(OH)2D3 were associated with dyslipidemia (p < 0.001, p < 0.001, p < 0.015, respectively) and lower 1,25(OH)2D3 was associated with hypertension (p < 0.001). Spearman’s correlation showed 1,25(OH)2D3 to be negatively correlated to age and diabetes duration.
Conclusions:
Different diabetes complications were associated with differing vitamin D parameters, with diabetic retinopathy related to lower 25(OH)D3 and 1,25(OH)2D3 levels, hypertension significantly associated with lower 1,25(OH)2D3, while dyslipidemia was associated with lower 25(OH)D3, 1,25(OH)2D3 and 24,25(OH)2D3. While 25(OH)D metabolites were lower in females, there was not an exaggeration in complications.
Keywords: diabetes complications, type 2 diabetes, vitamin D3, vitamin D3 epimers, vitamin D3 metabolites
Background
Accumulating evidence suggests that vitamin D deficiency increases the risk of type 2 diabetes (T2DM).1,2 Deficiency of vitamin D is associated with both insulin resistance and beta cell dysfunction.3 As long ago as 1980, impaired insulin secretion was demonstrated in isolated perfused rat pancreas in the setting of vitamin D deficiency.4 Later reports suggested that vitamin D deficiency could contribute to metabolic syndrome and T2DM.5,6 Vitamin D appears to exert its antidiabetic effect through modulation of hepatic glucose and lipid metabolism versus activation of calcium and the adenosine monophosphate–activated protein kinase (AMPK) pathway, and through promotion of beta cell function and survival.7
A large prospective study of women, with 20 years of follow up, reported an inverse relationship between vitamin D concentrations and the onset of diabetes.8 Vitamin D deficiency in T2DM has also been correlated with microvascular complications of retinopathy, neuropathy and nephropathy,9,10 though there is still some debate.
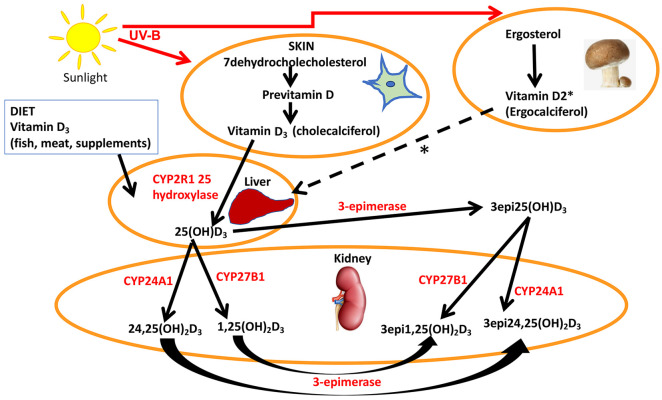
Vitamin D3 (cholecalciferol) is endogenously produced by UV-B irradiation of 7-dehydrocholesterol, while ergosterol is derived from the diet (primarily from mushrooms and fungi) and converted to previtamin D2 (ergocalciferol) by UV-B light, though both are hydroxylated to 25(OH)D3 and 25(OH)D2, respectively, by multiple 25-hydroxylases in the liver (Figure 1).11 25(OH)D is transported to the kidney and converted to either the active 1,25(OH)2D by 1 alpha hydroxylase, or to 24,25(OH)2D, which is also active, by the 24 alpha hydroxylase present in the renal tubular cells (Figure 1).12 It has recently been reported that extrarenal tissues may also convert 25(OH)D to 1,25(OH)2D, although, notably, activation in renal and non-kidney tissues is regulated differently with macrophage production of 1,25(OH)2D through the type 2 interferon response.13 1,25(OH)2D binds to the vitamin D receptor (VDR) but, to exert its effect (which may take several hours), it heterodimerizes with the retinoid X receptor;12 however, reports suggest alternative, more rapid mechanisms of action through binding to membrane VDR or through the 1,25D-membrane-associated, rapid response steroid-binding protein receptor with activation of protein kinases A and C.14 Obesity can exacerbate vitamin D deficiency through decreased bioavailability due to deposition of vitamin D in the body fat compartments.15 In many countries, vitamin D2 is available as a pharmaceutical and a supplement to counter vitamin D deficiency.16
Figure 1.
A simplified schematic representation of the synthesis and metabolism of vitamin D.
7-dehydrocholesterol in the skin is converted to previtamin D3 by UV-B and then is thermally isomerized to vitamin D3. Transport of vitamin D3 from the skin to the liver is mediated by vitamin D binding protein (DBP) where vitamin D3 is hydroxylated at position 25 to 25(OH)D3, DBP then transports 25(OH)D3 to the kidney. 25(OH)D3/DBP is filtered by the glomeruli and 25(OH)D3 is taken up into the tubular cells, following DBP binding to megalin, a transmembrane protein. 25(OH)D3 undergoes a second hydroxylation step by the 1-alpha-hydroxylase Cyp27B1, converting to the active 1α,25 (OH)2D3, while 24 hydroxylase Cyp27A1 converts to 24,25(OH)2D3. Keratinocytes contribute to the 3-epimerase activity, but the exact sites of activity remain unknown. 3epi25(OH)D3 is converted by Cyp27A1 to 24,25(OH)D3 and Cyp27B1 to 1,25(OH)D3, in equal measure. Ergosterol (provitamin D2) is plant-based and is converted to ergocalciferol by UV-B light that then follows the same pathway as vitamin D3 that is depicted with the dotted line * to the liver to form 25(OH)D2 and then transported to the kidney and epimerized to the D2 metabolites.
While vitamin D deficiency is a global issue,17 cultural norms dictating full-body coverage in parts of the world such as the Middle East magnify the issue of vitamin D deficiency in these regions.18
In Qatar, T2DM is a particularly serious health issue affecting approximately 20% of the population, a figure 2–3 times higher than the world average.19 As a consequence, the burden associated with diabetes complications in Qatari patients is tremendous. A total of 43% of patients referred to dialysis have diabetic nephropathy.20 A significant proportion of the total patient population presenting with cardiac pathologies in Qatar have T2DM: 57% of patients presenting with acute heart failure, and 30% of atrial fibrillation patients.21
The aim of this study was specifically to answer the question as to whether vitamin D deficiency was associated with diabetes retinopathy. In addition, given the pervasively low vitamin D status in the Middle East, we hypothesized that full-body coverage for women would lead to lower vitamin D parameter concentrations compared to men and lead to increased diabetes complications.
Methods
Study population
A total of 750 Qatari patients, 460 (61.3%) with T2DM, were recruited from the Hamad outpatient clinic, along with 290 (38.7%) nondiabetic participants, who were relatives accompanying the T2DM patients to their clinic visits from July 2013 to July 2015, as part of a study investigating gene expression and genomics in individuals with diabetes (Table 1). None of the diabetic patients were taking vitamin D3 supplements, though all had been prescribed vitamin D2 supplements.
Table 1.
Demographic data and vitamin D3 levels in the control (n = 290) and type 2 diabetic (n = 460) groups.
| Control |
Diabetes |
p value | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age (years) | 44.5 (38.0–53.0) | 56.0 (48.0–62.0) | <0.001 |
| BMI (kg/m2) | 30.1 (26.7–35.1) | 32.4 (28.6–37.2) | <0.001 |
| Hypertensive – n (%) | 71 (24.5) | 320 (69.6) | <0.001 |
| HbA1c (%) | 5.6 (5.3–5.9) | 7.9 (6.7–9.5) | <0.001 |
| Glucose (mmol/L) | 5.2 (4.7–5.7) | 8.6 (6.4–12.2) | <0.001 |
| 1,25(OH)2D3 (ng/ml) | 0.043 (0.025–0.066) | 0.025 (0.013–0.044) | <0.001 |
| 25(OH)D3 (ng/ml) | 8.82 (4.88–14.47) | 6.49 (3.40–13.57) | <0.001 |
| 24,25(OH)2D3 (ng/ml) | 0.38 (0.21–0.66) | 0.29 (0.18–0.56) | 0.001 |
| 3epi25(OH)D3 (ng/ml) | 0.47 (0.23–0.93) | 0.39 (0.22–0.75) | 0.071 |
BMI, body mass index; HbA1c, glycosylated hemoglobin A1c; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 3epi25(OH)D3, 25-hydroxy-3epi-vitamin D3; IQR, interquartile range.
Inclusion criteria were male or female Qataris, aged 30 years or older. The diagnosis of T2DM was made according to the WHO guidelines;22 for inclusion in the T2DM group, at least one of the following was required: fasting plasma glucose >7 mmol/L, HbA1c >6.5%, or a diagnostic glucose tolerance test. Inclusion in the nondiabetic control group required a normal glucose tolerance test. Patients were excluded from the study if they had a diagnosis of type 1 diabetes or any active form of diabetes, such as gestational diabetes or diabetes secondary to steroid treatment. All diabetes patients had retinal photography, a clinical foot examination, blood pressure measurement and urine analyzed for the albumin:creatinine ratio collected at their outpatient visit. Dyslipidemia was defined as a total cholesterol greater than 190 mg/dl (>4.9 mmol/L) and/or fasting triglycerides greater than 150 mg/dl (>1.7 mmol/L) untreated, or if patients were under treatment.
The study was approved by Weill Cornell IRB (IRB# 13-00063) and all participants provided written informed consent. The conduct of the trial was in accordance with ICH GCP and the Declaration of Helsinki.
Study design
Following an overnight fast, blood samples were collected, and weight and blood pressure were measured at the baseline visit. Fasting venous blood was collected into fluoride oxalate and serum gel tubes. Samples were separated by centrifugation at 2000 g for 15 min at 4°C, and the blood, serum and plasma aliquots were stored at −80°C within 1 h of collection. Overnight urine samples were collected, and aliquots were stored at −80°C until batch analysis. Blood pressure was measured using an automated device (NPB-3900; Nellcor Puritan Bennett, Pleasanton, CA, USA) during each study visit. Blood pressure measurements were performed after the participants had been seated quietly for at least 5 min and with the right arm supported at heart level. Three readings were taken, each at least 2 min apart, and then the average of the readings was calculated.
Serum vitamin D and metabolite measurements
Serum vitamin D concentrations were quantified using isotope-dilution liquid chromatography tandem mass spectrometry (LC-MS/MS). A total of 25µl of internal standards (d6-1calcitriol (1.5 ng/ml), d6-25OHD3 (50 ng/ml) and d6-epi-25(OH)D3 (20 ng/ml)) was added into each microcentrifuge tube containing 250 µl of calibration standards, quality control or serum samples, and kept for 30 min to reach binding equilibrium. The samples were diluted with 250 µl of pretreatment solution (isopropanol and water; 50:50 v/v) and left to stand for at least 15 min to displace binding protein.
Pretreated samples of 300 µl were loaded onto ISOLUTE® supported liquid extraction (SLE+) columns (Biotage), followed by elution with 1.8 ml of n-heptane (2 × 900 µl) into a collection tube already containing 200 µl of 0.25 mg/ml PTAD solution in ethyl acetate and heptane (8:92 v/v). The eluate was evaporated to dryness using turbovap under nitrogen gas heated at 38°C. Once dried, 50 µl of reconstituted solution consisting of methanol and deionized water, 70:30 v/v, and 0.006% methylalamine were added into all tubes. The derivatized extracts were transferred into LC insert vials and 10 µl from each was injected into the LC-MS/MS system. Data for the 25(OH)D3 and metabolite validation are shown in Supplemental Table 1.
Diabetes-related complications
Coronary artery disease (CAD) was defined as a history of myocardial infarction or angina, confirmed by coronary angiography. Peripheral arterial disease (PAD) was defined as a history of claudication or rest pain with evidence of artery stenosis on ultrasound or lower limb angiography. Stroke was defined as a sudden onset neurological deficit lasting more than 24 h.
Diabetic retinopathy was diagnosed by fundoscopy. Diabetic neuropathy (DN) was diagnosed based on the vibration perception threshold (Neurothesiometer NU-1, Horwell, UK) of the great toe being >25 V.
Study outcomes
Statistical analyses
A pilot study of 270 diabetes patients was previously undertaken in this population based on previous literature9 that suggested that 460 total patients, of whom 40% had retinopathy, would be required at α = 0.05 to detect a standard deviation difference of 0.27 in mean vitamin D levels with 80% power, considered a small to moderate-sized effect.
Data trends were visually and statistically evaluated for normality. Non-parametric tests (Mann–Whitney U) were applied to data that violated the assumptions of normality when tested using the Kolmogorov–Smirnov test. Bonferroni correction was applied to account for multiple testing. Statistical analysis was performed using SPSS for Windows, version 24.0. All values are given as mean ± SD or as mean with 95% confidence interval (CI) unless specified. Correlations between vitamin D and its metabolites were undertaken with Spearman’s correlation.
Results
Baseline characteristics
Diabetes patients were older than controls (median age (range) 55.2 (9.9) and 46.1 (10.8) years, respectively, p < 0.001)) and diabetes patients had a greater body mass index (BMI) than controls (median age (range) 32.4 (44) and 30.1 (34.8) years, respectively, p < 0.001)), and concentrations of 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3 were significantly different to controls (p < 0.001), though 3epi25(OH)D3 did not differ (p < 0.071) (Table 1). The diabetes complications for the T2DM cohort are shown in Table 2.
Table 2.
The relationship of vitamin D3 and its epimers to diabetes complications in the group of participants (n = 460) with type 2 diabetes.
| 1,25(OH)2D3 (ng/dl) median (IQR) | p value | 25(OH)D3 (ng/dl) median (IQR) | p value | 24,25(OH)2D3 (ng/dl) median (IQR) | p value | 3-epi-25(OH)D3 (ng/dl) median (range) | p value | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male (n = 227) | 0.030 (0.014–0.050) | <0.001 | 8.3 (4.4–15.3) | <0.001 | 0.35 (0.20–0.65) | 0.002 | 0.511 (0.284–0.839) | 0.003 |
| Female (n = 233) | 0.020 (0.011–0.037) | 4.7 (2.9–10.3) | 0.26 (0.16–0.44) | 0.304 (0.190–0.579) | ||||
| Hypertension | ||||||||
| No (n = 140) | 0.033 (0.016–0.049) | 0.009 | 7.0 (3.5–14.5) | 0.465 | 0.31 (0.20–0.65) | 0.059 | 0.330 (0.208–0.746) | 0.390 |
| Yes (n = 320) | 0.023 (0.012–0.041) | 6.4 (3.4–13.4) | 0.27 (0.18–0.53) | 0.415 (0.228–0.744) | ||||
| Dyslipidemia | ||||||||
| No (n = 109) | 0.031 (0.015–0.052) | 0.003 | 7.9 (4.0–16.1) | 0.041 | 0.34 (0.22–0.65) | 0.019 | 0.340 (0.203–0.732) | 0.719 |
| Yes (n = 351) | 0.023 (0.012–0.041) | 6.2 (3.3–12.5) | 0.27 (0.18–0.53) | 0.402 (0.223–0.752) | ||||
| Diabetic retinopathy | ||||||||
| No (n = 300) | 0.029 (0.014–0.046) | 0.006 | 6.3 (3.3–13.9) | 0.031 | 0.29 (0.19–0.62) | 0.207 | 0.350 (0.208–0.691) | 0.369 |
| Yes (n = 160) | 0.021 (0.012–0.035) | 7.1 (3.6–13.5) | 0.29 (0.18–0.47) | 0.513 (0.262–0.853) | ||||
| Diabetic Neuropathy | ||||||||
| No (n = 439) | 0.028 (0.014–0.046) | 0.084 | 6.4 (3.4–13.9) | 0.518 | 0.29 (0.19–0.53) | 0.948 | 0.349 (0.208–0.708) | 0.029 |
| Yes (n = 21) | 0.023 (0.012–0.035) | 7.1 (3.5–13.5) | 0.30 (0.17–0.62) | 0.471 (0.265–0.757) | ||||
| PAD | ||||||||
| No (n = 378) | 0.024 (0.013–0.043) | 0.334 | 6.4 (3.4–13.5) | 0.355 | 0.29 (0.19–0.55) | 0.705 | 0.381 (0.219–0.746) | 0.812 |
| Yes (n = 82) | 0.030 (0.017–0.054) | 8.8 (3.6–15.3) | 0.32 (0.14–0.65) | 0.352 (0.251–0.794) | ||||
| CAD | ||||||||
| No (n = 378) | 0.027 (0.014–0.046) | 0.012 | 6.5 (3.4–13.9) | 0.978 | 0.29 (0.19–0.56) | 0.347 | 0.364 (0.209–0.740) | 0.196 |
| Yes (n = 82) | 0.020 (0.012–0.033) | 6.4 (4.0–12.0) | 0.26 (0.16–0.59) | 0.429 (0.287–0.757) | ||||
| Stroke | ||||||||
| No (n = 439) | 0.026 (0.014–0.044) | 0.052 | 6.5 (3.4–14.0) | 0.264 | 0.29 (0.19–0.58) | 0.085 | 0.385 (0.223–0.746) | 0.716 |
| Yes (n = 21) | 0.015 (0.010–0.023) | 5.5 (3.4–8.4) | 0.22 (0.14–0.46) | 0.346 (0.211–0.744) | ||||
1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; CAD, coronary artery disease; IQR, interquartile range; PAD, peripheral arterial disease.
Vitamin D metabolite measurements
Vitamin D3 concentrations are lower in T2DM
Concentrations of 25(OH)D3, and its active form 1,25(OH)2D3, and its metabolite 24,25(OH)2D3 were lower in the T2DM patients (median (ng/dl) 6.45, 0.02 and 0.29, respectively) compared with nondiabetic controls (median (ng/dl) 8.82, 0.04 and 0.38, respectively: p < 0.001), but 3epi25(OH)D3 did not differ (p = 0.07) (Table 1).
Gender differences in vitamin D3 concentrations
In T2DM, concentrations of 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3 and 3epi25(OH)D3 were all lower in females versus males (p < 0.01) (Table 2).
Concentrations of 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3 were also lower in nondiabetic females versus nondiabetic males (p < 0.01) (Table 3). Despite the lower vitamin D parameters in females, there was no difference in diabetes complications between males and females (data not shown).
Table 3.
The relationship of vitamin D3 and its epimers to complications in the group of nondiabetic control participants (n = 290).
| 1,25(OH)2D3 (ng/dl) median (range) |
p value | 25(OH)D3 (ng/dl) median (range) |
p value | 24,25(OH)2D3 (ng/dl) median (IQR) |
p value | 3epi25(OH)D3 (ng/dl) median (IQR) |
p value | |
|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||
| Male | 0.048 (0.031–0.069) | 0.001 | 10.68 (6.96–15.78) | <0.001 | 0.44 (0.26–0.69) | 0.007 | 0.24 (5.17) | 0.081 |
| Female | 0.036 (0.017–0.059) | 6.21 (3.38–10.82) | 0.30 (0.17–0.58) | 0.12 (7.15) | ||||
| Hypertension | ||||||||
| No | 0.047 (0.030–0.070) | 0.001 | 9.11 (5.14–15.18) | 0.350 | 0.39 (0.20–0.68) | 0.557 | 0.17 (5.17) | 0.868 |
| Yes | 0.032 (0.021–0.047) | 7.96 (4.37–13.12) | 0.37 (0.23–0.55) | 0.19 (7.15) | ||||
| Dyslipidemia | ||||||||
| No | 0.047 (0.029–0.068) | 0.001 | 9.40 (5.92–15.74) | <0.001 | 0.41 (0.20–0.69) | 0.015 | 0.55 (0.26–1.02) | 0.148 |
| Yes | 0.035 (0.020–0.054) | 7.49 (3.62–12.63) | 0.33 (0.21–0.54) | 0.36 (0.21–0.84) | ||||
1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; IQR, interquartile range.
Relationship of vitamin D3 metabolite concentrations with diabetic complications
In this T2DM Qatari cohort, lower concentrations of 1,25(OH)2D3 (p < 0.006) and lower levels of 25(OH)D3 were weakly associated with diabetic retinopathy (p < 0.031), as shown in Table 2.
Spearman’s correlation showed 1,25(OH)2D3 to be negatively correlated to age and diabetes duration (R2 –0.301, p < 0.01; –0.16, p < 0.01, respectively); 25(OH)D3 was negatively correlated to age (R2 –0.08, p < 0.024), with no correlation evident for 24,25(OH)2D3 or 3epi25(OH)D3.
There was no difference in the frequency of diabetic complications between males and females.
Relationship of vitamin D3 metabolite concentrations with cardiovascular complications
In this T2DM Qatari cohort, lower concentrations of 1,25(OH)2D3 were associated with hypertension (p < 0.009), dyslipidemia (p < 0.003) and coronary heart disease (p < 0.01). Lower levels of 25(OH)D3 were weakly associated with dyslipidemia (p < 0.041) and low concentrations of 24,25(OH)2D3 were associated with dyslipidemia (p < 0.02), as shown in Table 2.
Relationship of vitamin D3 metabolite concentrations in nondiabetic participants
In nondiabetic participants, concentrations of 25(OH)D3, 1,25(OH)2D3 and 24,25(OH)2D3, but not 3epi25(OH)D3, were lower in females versus males (p < 0.01) (Table 3).
Lower concentrations of 1,25(OH)2D3 were associated with hypertension (p < 0.001) and dyslipidemia (p < 0.001). Lower levels of 25(OH)D3 were associated with dyslipidemia (p < 0.001), as were low concentrations of 24,25(OH)2D3, as shown in Table 3.
eGFR and vitamin D3 concentrations
We considered the possibility that 25(OH)D3 metabolite concentrations were merely reflecting the eGFR in these patients. However, the lack of correlation between eGFR and 1,25-dihydroxyvitamin D (1,25(OH)2D3), 25-hydroxyvitamin D3 (25(OH)D3) and 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) demonstrated that this was not the case (Table 4).
Table 4.
Correlation of eGFR with levels of vitamin D and its epimers in patients with diabetes.
| Diabetic + control participants | ||
|---|---|---|
| eGFR |
||
| R | p value | |
| 1,25(OH)2D3 | 0.141 | 0.001 |
| 25(OH)D3 | −0.028 | 0.461 |
| 24,25(OH)2D3 | 0.096 | 0.012 |
| Diabetic patients | ||
| eGFR |
||
| R | p value | |
| 1,25(OH)2D3 | 0.067 | 0.242 |
| 25(OH)D3 | −0.032 | 0.507 |
| 24,25(OH)2D3 | 0.148 | 0.002 |
| Controls | ||
| eGFR |
||
| R | p value | |
| 1,25(OH)2D3 | 0.108 | 0.119 |
| 25(OH)D3 | −0.112 | 0.076 |
| 24,25(OH)2D3 | −0.063 | 0.324 |
eGFR, estimated glomerular filtration rate; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3.
Discussion
This study was specifically powered on retinopathy, and both 25(OH)D3 and the active form 1,25(OH)2D3 were highly associated with diabetic retinopathy. The association of vitamin D with diabetes complications is controversial and unclear, with some studies suggesting that it is associated with microvascular complications9,10 and others that it is not.23 An association specifically with diabetic retinopathy was reported,9,24 and low concentrations of vitamin D have been associated with development of microvascular complications in T2DM,25 which is in accord with the results reported here, and may, in fact, be predictive of diabetic complication risks.26 This discrepancy between studies may be due to measuring total vitamin D rather than the D3 form specifically, and perhaps contributed to by having less precise measurement of vitamin D levels than the gold standard measurement employed here. Of note, vitamin D deficiency is not related to seasonal variability in the Middle East, likely due to the population remaining covered throughout the year.27
Only low 3epi25(OH)D3 levels were found with DN and otherwise 3epi25(OH)D3 did not relate to any other complication. Little is known about the epimers of vitamin D, and the assumption has been that they are biologically less potent.28,29 It is therefore unclear if this is a random chance observation or of specific significance that needs further clarification on the role of vitamin D epimers in disease, and specifically in diabetes.
The metabolite 24,25(OH)2D3 was highly associated with dyslipidemia, and may just reflect 25(OH)D3 concentrations that were also highly associated with dyslipidemia; however, 24,25(OH)2D3 may not be an inactive metabolite, as it has been shown to induce non-genomic signaling pathways and to suppress Apo A-1 in Hep G cells,30 and it may have a physiological role in growth plate formation;11 therefore, a direct effect on lipid metabolism cannot be excluded.
Hypertension specifically was associated with lower 1,25(OH)2D3 levels for those with and without diabetes. No relationship of 25(OH)D with hypertension was found in a meta-analysis,31 in accordance with the findings here for 25(OH)D3, 24,25(OH)2D3 or the 3epi25(OH)D3. This suggests that only low 1,25(OH)2D3 is related to hypertension and has therefore been missed in the past; however, as 1,25(OH)2D2 was not measured, we do not know if this is specifically related to the 1,25(OH)2D3 metabolite.
Dyslipidemia was significantly associated with lower 25(OH)2D3, 1,25(OH)2D3 and 24,25(OH)2D3 for both participants with and without diabetes in accord with what others have reported.32
Lower 1,25(OH)2D3 levels were found with CAD, though this was not seen for 25(OH)D3, 24,25(OH)2D3 or 3epi25(OH)D3. Vitamin D has been associated epidemiologically with CAD, though a systematic review concluded that there was no effect on vascular function.33 This study was not powered for this outcome and, therefore, future studies are needed to determine if this is a true association, although vitamin D3 supplements seem to be of limited therapeutic impact.33 It is of note that no vitamin D3 metabolite was associated with either PAD or stroke; however, vitamin D has been associated with increased risk of PAD34 and stroke in some studies.35
The lower 25(OH)D3 concentrations in the Qatari T2DM females versus males are also in accord with other studies,36 though this is not a universal finding.9 However, despite the lower vitamin D levels in women, they did not have a higher rate of diabetes.
The strength of this study was the number of participants, all of whom were vitamin D3 supplement naive, and the use of state-of-the-art measurement of vitamin D3 metabolites in this homogeneous population. However, this was a cross-sectional observational study that could not address the underlying causality or mechanisms, and interventional vitamin D3 studies are needed. All of the participants were Qatari, but the controls without T2DM were recruited from the relatives that accompanied the patients to clinic, which may have resulted in a source of bias. Vitamin D deficiency is not related to seasonal variability in the Middle East, likely due to the population remaining covered throughout the year,27 and therefore would not have been a confounder.
Conclusion
These data showed that both 25(OH)D3 and the active form 1,25(OH)2D3 were highly associated with diabetic retinopathy for which the study was powered, but in addition a relationship between 1,25(OH)2D3 concentrations and hypertension and CAD was found: 25(OH)D3 and 24,25(OH)2D3 levels were associated with dyslipidemia, giving further support to the evidence that vitamin D metabolites may have a role in the development of diabetes as well as cardiovascular complications.
Supplemental Material
Supplemental material, R1_Supp_Table_1_Vit_D_Metabolites for Association of vitamin D3 and its metabolites in patients with and without type 2 diabetes and their relationship to diabetes complications by Alexandra E. Butler, Soha R. Dargham, Aishah Latif, Haira R. Mokhtar, Amal Robay, Omar M. Chidiac, Amin Jayyousi, Jassim Al Suwaidi, Ronald G. Crystal, Charbel Abi Khalil and Stephen L. Atkin in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: AEB researched the data and wrote the manuscript. SRD performed the statistical analysis. AL and HRM performed the vitamin D measurements. AR, OMC, AJ and JAS researched the data. RGC, SLA and CAK designed the study and contributed to the discussion. SLA is the guarantor of this work.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Dr. Abi Khalil’s lab is funded by the Qatar National Research Fund (QNRF), NPRP Grant 9-169-3-024. The funding source did not have a role in the design of the study, decision to publish or writing of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: The study was approved by Weill Cornell IRB (IRB# 13-00063) and all participants provided written informed consent. The conduct of the trial was in accordance with ICH GCP and the Declaration of Helsinki.
Consent to publish: All authors agree to publish this work.
ORCID iD: Alexandra E. Butler  https://orcid.org/0000-0002-5762-3917
https://orcid.org/0000-0002-5762-3917
Availability of data and materials: All data are available upon reasonable request by contacting the corresponding author.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alexandra E. Butler, Diabetes Research Center (DRC), Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University (HBKU), Qatar Foundation (QF), PO Box 34110, Doha, Qatar.
Soha R. Dargham, Weill Cornell Medicine-Qatar, Doha, Qatar
Aishah Latif, Antidoping Laboratory Qatar, Doha, Qatar.
Haira R. Mokhtar, Antidoping Laboratory Qatar, Doha, Qatar
Amal Robay, Weill Cornell Medicine-Qatar, Doha, Qatar.
Omar M. Chidiac, Weill Cornell Medicine-Qatar, Doha, Qatar
Amin Jayyousi, Hamad Medical Corporation, Doha, Qatar.
Jassim Al Suwaidi, Hamad Medical Corporation, Doha, Qatar.
Ronald G. Crystal, Department of Genetic Medicine, Weill Cornell Medicine, New York, USA
Charbel Abi Khalil, Weill Cornell Medicine-Qatar, Doha, Qatar; Department of Genetic Medicine, Weill Cornell Medicine, New York, USA.
Stephen L. Atkin, Weill Cornell Medicine-Qatar, Doha, Qatar; Royal College of Surgeons in Ireland, Busaiteen, Bahrain.
References
- 1. Nakashima A, Yokoyama K, Yokoo T, et al. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes 2016; 7: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Husemoen LL, Thuesen BH, Fenger M, et al. Serum 25(OH)D and type 2 diabetes association in a general population: a prospective study. Diabetes Care 2012; 35: 1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiu KC, Chu A, Go VL, et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004; 79: 820–825. [DOI] [PubMed] [Google Scholar]
- 4. Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984; 73: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angellotti E, Pittas AG. The role of vitamin D in the prevention of type 2 diabetes: to D or not to D? Endocrinology 2017;158: 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berridge MJ. Vitamin D deficiency and diabetes. Biochem J 2017; 474: 1321–1332. [DOI] [PubMed] [Google Scholar]
- 7. Leung PS. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients 2016; 8: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006; 29: 650–656. [DOI] [PubMed] [Google Scholar]
- 9. Bajaj S, Singh RP, Dwivedi NC, et al. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J Endocrinol Metab 2014; 18: 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wan H, Wang Y, Zhang K, et al. Associations between vitamin D and microvascular complications in middle-aged and elderly diabetic patients. Endocr Pract 2019; 25: 809–816. [DOI] [PubMed] [Google Scholar]
- 11. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 2014; 21: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016; 96: 365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams JS, Rafison B, Witzel S, et al. Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol 2014; 144 Pt A: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 2003; 4: 46–56. [DOI] [PubMed] [Google Scholar]
- 15. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000; 72: 690–693. [DOI] [PubMed] [Google Scholar]
- 16. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69: 842–856. [DOI] [PubMed] [Google Scholar]
- 17. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81: 353–373. [DOI] [PubMed] [Google Scholar]
- 18. Chakhtoura M, Rahme M, Chamoun N, et al. Vitamin D in the Middle East and North Africa. Bone Rep 2018; 8: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alhyas L, McKay A, Balasanthiran A, et al. Prevalences of overweight, obesity, hyperglycaemia, hypertension and dyslipidaemia in the Gulf: systematic review. JRSM Short Rep 2011; 2: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shigidi MM, Fituri OM, Chandy SK, et al. Peritoneal dialysis, an expanding mode of renal replacement therapy in Qatar. Saudi J Kidney Dis Transpl 2011; 22: 587–593. [PubMed] [Google Scholar]
- 21. Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci 2006; 1084: 1–29. [DOI] [PubMed] [Google Scholar]
- 22. Deckers JG, Schellevis FG, Fleming DM. WHO diagnostic criteria as a validation tool for the diagnosis of diabetes mellitus: a study in five European countries. Eur J Gen Pract 2006; 12: 108–113. [DOI] [PubMed] [Google Scholar]
- 23. Joergensen C, Hovind P, Schmedes A, et al. Vitamin D levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care 2011; 34: 1081–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mutlu U, Ikram MA, Hofman A, et al. Vitamin D and retinal microvascular damage: the Rotterdam study. Medicine (Baltimore) 2016; 95: e5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008; 31: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herrmann M, Sullivan DR, Veillard AS, et al. Serum 25-hydroxyvitamin D: a predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care 2015; 38: 521–528. [DOI] [PubMed] [Google Scholar]
- 27. Sedrani SH, Elidrissy AW, El Arabi KM. Sunlight and vitamin D status in normal Saudi subjects. Am J Clin Nutr 1983; 38: 129–132. [DOI] [PubMed] [Google Scholar]
- 28. Kamao M, Tatematsu S, Hatakeyama S, et al. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem 2004; 279: 15897–15907. [DOI] [PubMed] [Google Scholar]
- 29. Molnar F, Sigueiro R, Sato Y, et al. 1α,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor. PLoS One 2011; 6: e18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wehmeier K, Onstead-Haas LM, Wong NC, et al. Pro-inflammatory signaling by 24,25-dihydroxyvitamin D3 in HepG2 cells.J Mol Endocrinol 2016; 57: 87–96. [DOI] [PubMed] [Google Scholar]
- 31. Qi D, Nie XL, Wu S, et al. Vitamin D and hypertension: prospective study and meta-analysis. PLoS One 2017; 12: e0174298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rashidbeygi E, Rahimi MH, Mollahosseini M, et al. Associations of vitamin D status and metabolic dyslipidemia and hypertriglyceridemic waist phenotype in apparently healthy adults. Diabetes Metab Syndr 2018; 12: 985–990. [DOI] [PubMed] [Google Scholar]
- 33. Beveridge LA, Khan F, Struthers AD, et al. Effect of vitamin D supplementation on markers of vascular function: a systematic review and individual participant meta-analysis. J Am Heart Assoc 2018; 7: pii: e008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rapson IR, Michos ED, Alonso A, et al. Serum 25-hydroxyvitamin D is associated with incident peripheral artery disease among white and black adults in the ARIC study cohort. Atherosclerosis 2017; 257: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheerah HA, Eshak ES, Cui R, et al. Relationship between dietary vitamin D and deaths from stroke and coronary heart disease: the Japan collaborative cohort study. Stroke 2018; 49: 454–457. [DOI] [PubMed] [Google Scholar]
- 36. Scragg R, Sowers M, Bell C, et al. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care 2004; 27: 2813–2818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, R1_Supp_Table_1_Vit_D_Metabolites for Association of vitamin D3 and its metabolites in patients with and without type 2 diabetes and their relationship to diabetes complications by Alexandra E. Butler, Soha R. Dargham, Aishah Latif, Haira R. Mokhtar, Amal Robay, Omar M. Chidiac, Amin Jayyousi, Jassim Al Suwaidi, Ronald G. Crystal, Charbel Abi Khalil and Stephen L. Atkin in Therapeutic Advances in Chronic Disease