Abstract
Practice guidelines suggest that treatment decisions in pulmonary arterial hypertension be informed by periodic assessment of patients’ clinical risk. Several tools, well validated for risk discrimination, such as the Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management calculator, were developed to assess pulmonary arterial hypertension patients’ risk of death based on multiple parameters, including functional class, hemodynamics, biomarkers, comorbidities, and exercise capacity. Using an online survey, we investigated the use of risk assessment tools by pulmonary hypertension healthcare providers in the United States. Of 121 survey respondents who make treatment decisions, 59% reported using risk assessment tools. The use of these tools was lower for non-physicians (48% vs. 65% physicians) and for practitioners at centers with 1 to 100 pulmonary arterial hypertension patients compared with centers with >100 patients (47% vs. 64%). Risk was most frequently assessed by decision makers at the time of diagnosis (cited by 54%) and at the time of worsening symptoms (cited by 42%), suggesting that use of pulmonary arterial hypertension risk assessment tools remains low. In our survey, non-physicians compared with physicians cited two major barriers to increased tool use: lack of education and training (20% vs. 4%) and lack of clarity on the best tool to use (30% vs. 18%). Information technology tools, such as electronic medical record integration and web or phone-based risk calculating applications, were cited most frequently as ways to increase the use of risk assessment tools.
Keywords: mortality risk assessment, multiparameter risk assessment, pulmonary arterial hypertension, REVEAL, risk assessment tools
In patients with pulmonary arterial hypertension (PAH), traditionally, symptom severity is classified according to World Health Organization or New York Heart Association functional class (FC), with FC I representing mild symptoms and FC IV indicating the most severe symptoms. Patients with FC I or II symptoms are viewed as having a low risk of near-term death or clinical worsening. However, several studies have disputed this assumption, suggesting that some patients with mild symptoms nonetheless have significant underlying disease that increases the risk of death or disease progression. In the Endothelin Antagonist Trial in Mildly Symptomatic PAH patients, a subset of patients with FC II symptoms were found to have more seriously compromised hemodynamic function, with mean pulmonary arterial pressure values similar to those seen in patients with FC III disease.1–4 In the French Network on Pulmonary Hypertension registry, 20% of the patients with FC I or II disease died within three years.5,6 Additionally, in the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL), clinical worsening was observed within one year in 401 of 925 patients (43%) with FC II disease and in 89 of 184 patients (48%) with FC I disease.7
These observations suggest that in PAH, underlying pathophysiologic changes may precede clinically apparent disease progression by months or even years.8 This hypothesis was supported by data from a prospective, observational study in which 22 patients with FC II or III symptoms were followed for up to eight years. Ten of these patients experienced disease progression during the study; among these patients, a gradual worsening of right ventricular function was observed. This deterioration of right ventricular function preceded clinical progression by several years, and importantly, it occurred without apparent worsening of symptoms or loss of exercise capacity.6,9
These findings provide a strong rationale for regularly assessing the individual risk level of PAH patients using a comprehensive approach that includes not only FC but also hemodynamic measurements, biomarkers of right ventricular function, comorbidities, and exercise testing. Several tools have been developed for assessing risk in this manner, including the REVEAL risk score calculator10,11 and several tools developed in PAH registry populations based on thresholds outlined in the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines.12 These include the Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA),13 the French Pulmonary Hypertension Registry (FPHR),14 and the Swedish PAH Register (SPAHR).15 In recent years, a number of authors have suggested that treatment decisions in PAH should be guided by the periodic assessment of risk using tools such as these, which may allow for better informed treatment decisions and earlier recognition of disease progression.6,12,16–19
In this paper, we briefly review the available risk assessment tools and review recommendations regarding their use. We present the findings of an online survey designed to document how risk tools are utilized in PAH clinic settings, including barriers to use and suggested strategies for overcoming these barriers.
Risk tools in PAH
Contemporary risk assessment tools stratify PAH patients into separate categories that predict their risk of mortality, with the low-risk group typically corresponding to a one-year mortality risk of <5%, the intermediate-risk group corresponding to a one-year mortality risk of 5% to 10%, and the high-risk group corresponding to a one-year mortality risk of >10%.12 The 2015 ESC/ERS guidelines for the management of pulmonary hypertension recommend that risk should be quantified at the time of diagnosis and periodically thereafter, and the goal of therapy should be to achieve and maintain a low-risk status.12 For patients who remain in the low-risk category, PAH therapy can often be continued unchanged. Patients in intermediate- or higher risk categories may require escalation of PAH therapy to achieve low-risk status. An individual’s risk status, as quantified by objective tools, can be improved by treatment, through improvements in symptoms, FC, exercise capacity, and hemodynamics.12,14,20,21
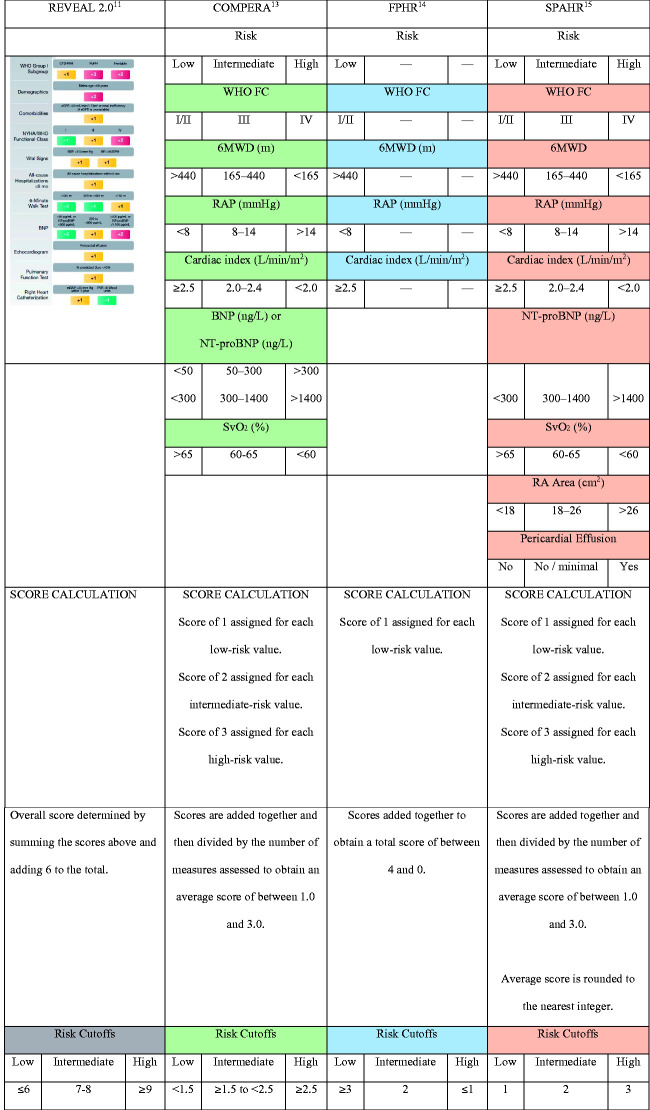
The 2015 ESC/ERS guidelines recommended expert assessment of risk, using specific cutoff values for 6-min walk distance, right atrial pressure, cardiac index, and several other clinical measures, which were derived from studies of prognostic factors in PAH. The cutoff values for 6-min walk distance, for example, were defined as >440 m (low-risk), 165–440 m (intermediate risk), and <165 m (high-risk); and the cutoff values for right atrial pressure were defined as <8 mmHg (low-risk), 8–14 mmHg (intermediate risk), and >14 mmHg (high-risk).12 This set of clinical measures and cutoffs, hereafter referred to as the “ESC/ERS guidelines,” suffers from one distinct limitation: the various measures for a given patient often do not fall uniformly within a single risk category, thereby requiring some degree of subjective judgment by the clinician to determine a patient’s overall risk status.16 Risk assessment tools such as COMPERA, FPHR, and SPAHR were developed to account for this multifactorial complexity by assigning point values to a patient’s individual variables, which are then used to derive an overall, objective risk score using various algorithms.13–15 First developed in 201210 and updated in 2019,11 the REVEAL risk score calculator which also assigns points, takes into account up to 13 patient variables but is not tied to the ESC/ERS guidelines. Instead, the thresholds were derived empirically from the REVEAL registry data set.
The components and scoring systems for COMPERA, FPHR, SPAHR, and REVEAL 2.0 (a recently updated version of REVEAL) are summarized in Fig. 1. Several studies have demonstrated the ability of these tools to identify subsets of PAH patients with differing rates of survival.16
Fig. 1.
Objective risk assessment tools in PAH.
6MWD: 6-min walk distance; BNP: brain natriuretic peptide; COMPERA: Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; FC: functional classification; FPHR: French Pulmonary Hypertension Registry; NT-proBNP: N-terminal fragment of pro-brain natriuretic peptide; NYHA: New York Health Association; PAH: pulmonary arterial hypertension; RA Area: right atrial area on cardiac magnetic resonance imaging; RAP: right atrial pressure; REVEAL: Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management; SPAHR: Swedish PAH Register; WHO: World Health Organization.
In a study of 504 patients with PAH who were risk-stratified according to REVEAL, patients assigned to the low-, average-, moderately high-, high-, and very high-risk categories had one-year survival rates of 95.1%, 91.5%, 84.6%, 76.3%, and 58.2%, respectively.10 The REVEAL 2.0 tool has recently shown improved discrimination between risk categories, relative to the original REVEAL tool.11 When the risk levels of 1588 PAH patients were stratified based on COMPERA, the low-, intermediate-, and high-risk groups had one-year mortality rates of 2.8%, 9.9%, and 21.2%, respectively.13 Similarly, when 1017 PAH patients were stratified into risk categories using FPHR, there were significant differences in the rates of transplant-free survival for the different risk groups, with a greater number of low-risk criteria at baseline associated with better outcomes.14 Finally, a recent analysis of 530 PAH patients using the SPAHR tool found one-year survival rates of 99%, 83%, and 74% for patients in the low-, intermediate-, and high-risk categories.15 Importantly, these tools can identify patients with elevated risk who may be overlooked when physician gestalt alone or a single variable is used in place of multiparametric risk assessment tools. A recent retrospective analysis of medical charts of 165 patients with FC II PAH compared the results of risk assessment by clinical gestalt to those of the COMPERA, modified non-invasive FPHR, and REVEAL 2.0 tools.22 Among the patients who were considered low-risk by physician gestalt, 11% to 36% were categorized as intermediate risk by the various risk tools, and 4% to 28% were categorized as high risk by the risk tools, showing that a significant percentage of these gestalt-stratified low-risk FC II patients were actually re-classified into intermediate- or high-risk categories when multiparametric tools were used.22
Given their demonstrated predictive power, formal risk tools continue to garner significant interest.6,16,19,23 The 2015 ESC/ERS guidelines recommended multifactorial assessment of risk.12 The latest update of the CHEST guideline and expert panel report for PAH stopped short of endorsing the use of risk tools in routine clinical practice.24 However, the recently published proceedings of the 6th World Symposium on Pulmonary Hypertension do prominently embrace the routine periodic use of formal risk tools, such as ESC/ERS, FPHR, SPAHR, COMPERA, and REVEAL 2.0.17,18 The 6th World Symposium on Pulmonary Hypertension recommendations are built around an algorithm in which treatment decisions (such as monotherapy vs combination therapy or oral vs parenteral therapy) are primarily based on the results of formal risk assessments. Follow-up intervals of three to six months are recommended in order to re-assess risk, determine treatment response, and consider possible escalation of therapy.18
Given the high level of interest in these risk tools, we sought to determine how widely they are being used in clinical practice settings within the United States. We hypothesized that tool use in clinical practice would be less frequent than commonly thought and that use would be greater in Pulmonary Hypertension Association (PHA)-accredited centers than non-accredited centers. We administered an online survey to PAH practitioners across the United States who are members of the PHA. The survey investigated how frequently risk assessment tools are used in the management of PAH patients, the barriers to tool use, and potential strategies for overcoming those barriers.
Methods
Survey design
This survey was designed to address several questions regarding the utilization of formal risk assessment tools in the management of PAH in clinical practice settings: (1) What percentage of PAH clinicians currently use formal tools to assess risk in their patients? (2) Does the use of risk assessment tools vary among different subgroups of healthcare providers? (3) Among clinicians who use formal risk assessment tools, how frequently are they used, and when are they used during the patient’s care pathway? (4) What are the primary barriers to widespread and frequent use of risk assessment tools? (5) What resources might help to overcome these barriers? The survey questionnaire was developed by the authors and is provided in the Supplementary Appendix (Supplemental Figure S1).
An invitation to participate in the survey was distributed, via email, to members of two professional networks associated with the PHA: the Pulmonary Hypertension Clinicians and Researchers network, which includes physicians and researchers, and the Pulmonary Hypertension Professional Network, which includes allied healthcare professionals such as nurse practitioners, physician assistants, registered nurses, and pharmacists. The invitation email included a hyperlink for accessing the survey online. The survey was conducted using the SurveyMonkey platform, and responses were collected between 7 February and 10 March 2019.
The survey was divided into three sections: the first section was designed to collect demographic information on respondents—provider type (e.g. physician, nurse practitioner, physician assistant), geographic location (US state), number of years treating PAH, volume of PAH patients followed at the practice, and PHA accreditation status of the center. This section included two screening questions: (a) Respondents were asked whether they make treatment decisions regarding PAH patients, and those who answered “yes” continued, while those who answered “no” exited the survey; and (b) Respondents were asked whether they use formal tools to assess clinical risk in their PAH patients, and those who answered “yes” continued into the second section, comprising a series of questions about which tools are used and how they are used, while those who answered “no” moved to the third section, with questions about the barriers to tool use and suggestions for facilitating tool use. The respondents who use risk tools were also asked to complete the third section.
Questions in the first section were multiple choice or yes/no, with respondents allowed to select one answer and allowed to write in their practice location. Questions in the second and third sections were largely in a multiple choice or “choose all that apply” format where respondents could select multiple answers (e.g. selecting each of the different risk tools that they use or selecting each of the issues that represent a significant barrier to tool use).
Statistical analyses
Only data from respondents who reported being PAH treatment decision makers (in the first screening question) were included in the results analysis. The analyses were further restricted to those treatment decision makers who identified themselves as physicians, nurse practitioners, or physician assistants. Respondents who answered “yes” to the first screening question but who did not respond to the second screening question (“do you use formal risk tools”) were included in the analyses and treated as non-tool users. Unless otherwise noted, percentages were calculated using the total number of treatment decision makers as the denominator. The survey architecture allowed participants to skip certain questions, continuing through the remainder of the survey. As a result, blank answers were treated as no response; thus, the percentages for some questions may not add up to 100. Two-sided 95% confidence intervals, Wilson method, were constructed around all the percentages reported. Because of the limited sample size, formal between-group statistical analysis was not conducted.
Results
Survey response and demographics
One hundred sixty-eight participants started the survey, of whom 138 (82%) identified themselves as PAH treatment decision makers and, therefore, continued the survey. Seventeen of these treatment decision makers identified themselves as registered nurses or nurse coordinators and were, therefore, excluded from the analysis, leaving a data set of 121 treatment decision makers who were physicians, nurse practitioners, or physician assistants. Response rates to questions about demographics (section 1) and tool use (section 2) ranged between 96.7% and 100%, while the response rate to the multiple-choice questions about barriers to tool use (section 3) was 86%. Distribution of decision-makers spanned across 34 states and the District of Columbia. One hundred and two respondents provided the name of their practice, 90 of which were unique. The highest number of respondents from a single practice was three, and this occurred for two different practice sites.
Demographics of the PAH treatment decision makers are summarized in Table 1. This included 77 physicians and 44 non-physicians, the latter comprising 41 nurse practitioners and 3 physician assistants. Overall, 28% of clinical decision makers reported working at practices with 100 or fewer PAH patients, 45% at practices with 101 to 300 PAH patients, and 26% at practices with >300 PAH patients. A total of 48% worked at PHA-accredited centers.
Table 1.
Demographic characteristics of survey participants (treatment decision-makers).
| All (n = 121) | Physicians (n = 77) | Non-physicians (n = 44) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Years treating PAH | |||
| 1–5 | 33 (27) | 17 (22) | 16 (36) |
| 6–10 | 31 (26) | 21 (27) | 10 (23) |
| 11–15 | 24 (20) | 14 (18) | 10 (23) |
| >15 | 32 (26) | 25 (32) | 7 (16) |
| Patient volume | |||
| 1–50 | 21 (17) | 14 (18) | 7 (16) |
| 51–100 | 13 (11) | 8 (10) | 5 (11) |
| 101–300 | 55 (45) | 34 (44) | 21 (48) |
| 301–500 | 19 (16) | 14 (18) | 5 (11) |
| >500 | 12 (10) | 7 (9) | 5 (11) |
| PHA-accredited (center) | |||
| Yes | 58 (48) | 37 (48) | 21 (48) |
| No | 61 (50) | 39 (51) | 22 (50) |
PAH: pulmonary arterial hypertension; PHA: Pulmonary Hypertension Association.
The majority of physicians (78%) had more than five years of experience treating PAH, with 32% having more than 15 years of experience. In contrast, non-physicians were generally less experienced, with 36% having one to five years of experience treating PAH, and only 16% having more than 15 years of experience. PAH patient volumes were similar between physicians and non-physicians, as were rates of affiliation with PHA-accredited versus non-accredited centers.
Use of risk tools
As shown in Table 2, 59% of treatment decision makers reported using formal tools to assess risk in their PAH patients. The rate of tool use was lower for non-physicians (48%) than for physicians (65%), marginally lower for treatment decision makers with fewer years of experience treating PAH (55%) for one to five years versus 61% for >5 years, and lower among those working at small practices (47% for 1 to 100 PAH patients vs. 64% for >100 patients).
Table 2.
Use of formal risk assessment tools within subgroups.
| Subgroup | Reported using formal risk tools, n (%) | |
|---|---|---|
| All treatment decision-makers (n = 121) | 71 (59) | |
| Credential | Physician (n = 77) | 50 (65) |
| Non-physician (n = 44) | 21 (48) | |
| Years treating PAH | 1–5 (n = 33) | 18 (55) |
| 6–10 (n = 31) | 21 (68) | |
| 11–15 (n = 24) | 13 (54) | |
| >15 (n = 32) | 19 (59) | |
| Patient volume | 1–100 (n = 34) | 16 (47) |
| 101–300 (n = 55) | 38 (69) | |
| >300 (n = 31) | 17 (55) | |
| PHA-accredited | Yes (n = 58) | 30 (52) |
| No (n = 61) | 40 (66) | |
PAH: pulmonary arterial hypertension; PHA: Pulmonary Hypertension Association.
The rate of tool use was unexpectedly lower at PHA-accredited centers (52%) than at non-accredited centers (66%), particularly among physicians (54% at accredited centers vs. 74% at non-accredited centers, Table 3). Tool use among non-physicians was similar at accredited and non-accredited centers (48% at accredited vs. 50% at non-accredited centers).
Table 3.
Use of formal risk assessment tools (physicians vs. non-physicians).
| Physician treatment decision makers |
Non-physician treatment decision makers |
|||
|---|---|---|---|---|
| All | Use formal risk tools | All | Use formal risk tools | |
| n | n (%) | n | n (%) | |
| All treatment decision makers | 77 | 50 (65) | 44 | 21 (48) |
| Years treating PAH | ||||
| 1–10 | 38 | 27 (71) | 26 | 12 (46) |
| >10 | 39 | 23 (59) | 17 | 9 (53) |
| Patient volume | ||||
| 1–100 | 22 | 12 (55) | 12 | 4 (33) |
| 101–300 | 34 | 28 (82) | 21 | 10 (48) |
| >300 | 21 | 10 (48) | 10 | 7 (70) |
| PHA-accredited center | ||||
| Yes | 37 | 20 (54) | 21 | 10 (48) |
| No | 39 | 29 (74) | 22 | 11 (50) |
PAH: pulmonary arterial hypertension; PHA: Pulmonary Hypertension Association.
In this study, accredited centers had higher PAH patient volumes than non-accredited centers. Among the treatment decision makers at accredited centers (n = 58), 40% reported volumes of >300 patients compared to 13% at non-accredited centers (n = 61).
As shown in Table 3, there were different trends for physicians compared to non-physicians in terms of how patient volume, center type, and experience related to tool use. Among physicians, 71% of those at centers with 1 to 300 patients reported using formal risk tools compared to 48% of those at centers with >300 patients. Among non-physicians, however, an opposite pattern was seen: 42% of those at centers with 1 to 300 patients reported using risk tools compared to 70% of those with >300 patients.
Patterns of tool use
Table 4 summarizes the use of risk tools at various milestones in patient care. The most common occasion for the use of risk tools was the time of PAH diagnosis (54%), followed by when symptoms of disease progression occurred (42%), when PAH medications were changed (34%), at repeat right heart catheterization (RHC, 33%), and at repeat echocardiogram (24%). Just 19% reported that they routinely use risk tools on all of these occasions.
Table 4.
PAH risk tools—patterns of use in the course of patient care.
| Time points in PAH patient care | |||||
|---|---|---|---|---|---|
| Time of diagnosis | Changing medications | Symptoms of disease progression | Repeat RHC | Repeat echo | Use formal risk tools n (%) |
| YES | 65 (54) | ||||
| YES | 41 (34) | ||||
| YES | 51 (42) | ||||
| YES | 40 (33) | ||||
| YES | 29 (24) | ||||
| YES | YES | YES | 36 (30) | ||
| YES | YES | YES | 28 (23) | ||
| YES | YES | YES | YES | YES | 23 (19) |
PAH: pulmonary arterial hypertension; RHC: right heart catheterization.
Of the decision makers who use risk tools (n = 71), 58% reported that they used more than one tool. REVEAL was the most commonly used tool (79%), followed by ESC/ERS guidelines (61%), and FPHR (17%). The choice of tools was similar among physicians (78% REVEAL, 58% ESC/ERS, 18% FPHR) and non-physicians (76%, 67%, and 14%, respectively). The most common reasons cited for using a particular tool were the tool was specified in treatment guidelines (32%), personal experience (31%), the tool was established at the practice site (16%), and the tool was recommended by other practitioners (11%).
Barriers to increased tool use
Decision makers were asked about the barriers that limit their use of risk tools. As shown in Table 5, the most commonly cited barriers were time constraints (cited by 46% of non-tool users vs. 42% of tool users), followed by lack of technology or electronic medical record (EMR) integration (37% non-tool users vs. 34% tool users). Respondents who do not use risk tools cited several types of barriers more frequently than their tool-using counterparts, including a lack of administrative or colleague support (28% vs. 15%) and a lack of education, training, or awareness (15% vs. 6%).
Table 5.
Most commonly cited barriers to increased use of risk tools.
| Subgroup | Time constraints | Lack of technology/ EMR integration | Lack of administrative/ colleague support | Complexity of tools and scoring systems | Lack of education/ awareness/training | Clarity on which tool to use |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| USE risk tools (n = 71) | 30 (42) | 24 (34) | 11 (15) | 9 (13) | 4 (6) | 15 (21) |
| DON’T USE risk tools (n = 46) | 21 (46) | 17 (37) | 13 (28) | 8 (17) | 7 (15) | 12 (26) |
| Physicians (n = 77) | 35 (45) | 29 (38) | 14 (18) | 14 (18) | 3 (4) | 14 (18) |
| Non-physicians (n = 44) | 16 (36) | 12 (27) | 10 (23) | 3 (7) | 9 (20) | 13 (30) |
EMR: electronic medical record; PAH: pulmonary arterial hypertension.
There were differences in the barriers cited by different subgroups (Table 5). Barriers cited more frequently by physicians than by other subgroups included a lack of technology or EMR integration (38% vs. 27% for non-physicians), time constraints (45% vs. 36% for non-physicians), and the complexity of tools and scoring systems (18% vs. 7% for non-physicians). Non-physicians were more likely to cite a lack of education, awareness, or training (20% vs. 4% for physicians) and a lack of clarity on the best tool to use (30% vs. 18% for physicians). There were also differences in the barriers cited by subgroups defined by years of experience treating PAH and characteristics of the treating center (Supplemental Table S1). Decision makers with one to five years of experience were more likely to cite a lack of clarity on the best tool to use (30% vs. 20% for >5 years). Decision makers at centers with 1 to 100 PAH patients were more likely to cite a lack of education, training, or awareness (18% vs. 7% for >100 patients), as were those at non-accredited centers (15% vs. 5% for accredited centers).
Facilitating tool use
Treatment decision makers were asked to suggest resources that would facilitate the regular use of risk tools in their PAH practice. The majority of suggestions pertained to technological interventions, such as EMR integration of risk tools (the most common suggestion), phone applications, online risk calculators, pop-up reminders, and the automated population of risk calculators with patient data. Overall, 63% of the suggestions offered by users of risk assessment tools fell into this category, as did 70% of the suggestions by non-users. Other common responses included education or literature reviews (16% of users, 7% of non-users), standard protocols, procedures, or guidelines for implementing risk tools (9% users, 4% non-users), additional staff assistance or time during visits (13% users, 7% non-users), and tools applicable to pediatric PAH patients (0% users, 11% non-users).
Discussion
This is, to our knowledge, the first study to investigate the use of PAH risk tools in clinical practice settings in the United States. Our survey garnered responses from a cohort of treatment decision makers (n = 121), which was diverse and well-balanced with respect to provider type, years of experience treating PAH, patient volume, center accreditation, and geographic location.
Our results indicate that risk assessment tools for PAH are currently used by fewer than 60% of treatment decision makers in the United States, with lower rates of use by providers in practices with 100 or fewer PAH patients. This low utilization of formal risk tools runs counter to clinical guidelines, which increasingly suggest that PAH treatment decisions should be guided by the periodic assessment of mortality risk using multiparametric score-based risk calculators.17,18
In our survey, we found that 90% of the treating providers not using a formal assessment tool reported that they assess PAH clinical risk by gestalt. This reliance on gestalt for assessing risk and guiding treatment decisions raises several concerns regarding the underestimation of risk using clinical gestalt alone. A recent analysis found that physician gestalt may underestimate risk in a subset of patients with elevated risk who would otherwise be identified if formal tools were used.22 In this analysis, up to 46% of patients judged to be low risk by gestalt were classified as intermediate risk by formal tools, and up to 28% of these ostensibly low-risk patients were classified as high-risk by the formal tools.22 Underestimation of risk may adversely affect outcomes in PAH patients.
In addition to documenting the percentage of clinicians who use risk tools in PAH, our results also provide some interesting insights into how often and when these tools are used. The survey provided two estimates of how frequently tools are used. A multiple-choice question, asking decision makers to select all of the occasions on which they use risk tools, found that 54% of respondents use tools at the time of diagnosis, while 19% use tools at all five of the occasions that were queried, including diagnosis, worsening symptoms, changes in medication, repeat RHC, and repeat echocardiogram (Table 4).
Our results identify several demographic factors that appear to influence the use of risk tools. Overall, the rate of tool use was lower among decision makers at low-volume practices with 1 to 100 PAH patients (Table 2). This finding is not unexpected. However, when decision makers were further subdivided into additional groups, a surprising observation emerged: two demographic factors—years of experience treating PAH and patient volume—appeared to influence tool use in different ways for physicians compared to non-physicians. Advance practice providers, working at accredited centers with larger patient volumes, were associated with a higher rate of tool use, and >10 years of experience treating PAH was also associated with a higher rate of tool use (albeit weakly, in the latter case). Among physicians, however, >10 years treating PAH was associated with lower tool use, and working at high-volume centers was also associated with lower tool use. The observation that tool use was 20 percentage points lower for physicians at PHA-accredited centers compared to those at non-accredited centers was also surprising. This may reflect the fact that physicians working at accredited centers have more years of experience treating PAH, and higher patient volumes—two factors which themselves are associated with lower rates of tool use by physicians. These observations could be interpreted in several ways.
First, it is possible that physicians with lower tool utilization rates at large accredited centers represent experienced PAH providers, who feel more comfortable in assessing risk based on experience and clinical gestalt. Alternatively, physicians with lower tool utilization rates at large centers could represent a subset of clinicians who have been practicing for longer, among whom the adoption of risk assessment tools has been less extensive. The lower rate of tool use by physicians at high-volume centers could also reflect differing divisions of labor at small versus large centers, rather than actual differences in patient care, since non-physician staff are more likely to carry out risk assessment at large centers (Table 3). Whether this is replacing formal risk assessment by physician providers cannot be determined with our data.
It is important to note, however, that while specific patterns of tool use were observed more frequently among practitioners at certain types of centers, these patterns reflect the behavior of the individual respondent. This behavior cannot be generalized to all practitioners at specific types of centers.
While this study finds that more than 40% of PAH treatment decision makers do not currently use risk tools, it also—importantly—offers insights regarding why the tools are not used and how their use might be encouraged.
Two groups with low rates of tool use are non-physicians and practitioners of any type with less experience treating PAH. It is worth noting, furthermore, that these two groups overlap substantially, with 36% of non-physicians reporting just one to five years of experience (compared to 22% for physicians). Educational programs targeted to these two overlapping groups might represent one viable strategy for increasing the uptake of risk tools by practitioners who are not currently using them. This is supported by the fact that a lack of education, awareness, and training was heavily cited by both groups as a major barrier to tool use, as was a related issue—a lack of clarity on which tool to use (Table 5). Educational programs targeted to practices with lower patient volumes could also provide benefits, since decision makers at clinics with 1 to 100 PAH patients were less likely to use risk tools and more likely to cite a lack of education as a barrier.
The barrier most frequently cited among all decision makers, and across all major subgroups, was that of time constraints. It is tempting, at face value, to view this purely as a reflection of workload, patient load, and staffing limitations; however, the survey results provide a more nuanced insight. Behind time constraints, the second-most frequently cited barrier was lack of technology and EMR integration. When respondents were prompted in a separate question to offer their own suggestions for how the use of risk tools might be increased, the great majority of these suggestions fell within a broad category, including EMR integration, online or phone-based calculators, pop-up reminders, and automatic pulling of patient data from medical records. These items share one major similarity: they all represent technologies of convenience, with the potential to save time during patient visits or medical charting. This finding suggests that many of the survey respondents who cited time constraints as a barrier may be receptive to interventions that streamline the time efficiency of risk assessment tools. Such a strategy could increase the uptake of risk tools by practitioners not currently using them, as well as increase the frequency with which current users deploy them.
EMR integration of risk tools involves at least two distinct functionalities, with varying levels of difficulty. Entry of risk assessment results into the EMR can be easily facilitated, using quick entry of predetermined text (sometimes referred to as “smart phrases” in EMRs) and thereby minimizing the manual entry of text needed to specify a particular risk tool and the resulting risk category. These same quick entry phrases and tables can be arranged, through hospital informational technology specialists, to carry over into each subsequent patient visit, thereby tracking risk assessment scores over time. The second functionality—automatically pulling patient variables from the EMR into a risk calculator—is more difficult, requiring backend coding, and customization of the EMR system itself. Phone applications, on the other hand, may be more readily available. The REVEAL risk tool was previously available as a smartphone application, and in this survey, multiple respondents expressed the hope that a similar application would become available for REVEAL 2.0. A more comprehensive online resource is also in development: The Pulmonary Hypertension Outcomes Risk Assessment (PHORA) web portal, which will allow clinicians to enter patient parameters, simultaneously generating scores based on several validated risk tools, including REVEAL 2.0, FPHR, and novel risk models based on machine learning. At our own institutions, we have increased the use of risk assessment by creating “smart phrases,” and through the use of low-technology convenience tools, such as printed and laminated copies of risk calculators.
One limitation of our study was the relatively small sample size (n = 121), which precluded formal statistical analysis. As a result, comparisons between different subgroups (e.g. the rate of tool use by physicians vs. non-physicians) were based on relatively small groups and may lack generalizability. Another limitation was that the process of inviting respondents via email to take part in an online survey could potentially introduce selection bias, in which email recipients with a greater interest in, or awareness of, PAH risk assessment tools are more likely to participate in the survey. This could cause the survey to overestimate rates of risk tool usage. With this potential limitation in mind, it is all the more noteworthy that just 59% of treatment decision makers reported using risk tools. Additionally, we aimed to make the survey responses anonymous by not requesting names or email addresses of respondents; we inadvertently diminished anonymity by asking for institutional affiliations. As the PAH community is small, this question may have led respondents to feel that their responses were not completely anonymous. Finally, since all participants in this study were practicing in the United States, there may be a bias toward using the REVEAL risk score calculator, since it was developed in the United States. Indeed 79% of respondents who used formal tools indicated that they use the REVEAL risk calculator. However, a notable proportion (61%) also reported the use of ESC/ERS Guidelines-based tool, and a majority (58%) reported using more than one tool.
In summary, the development of objective risk assessment tools represents a significant advancement in PAH care over the past few years. These tools have the potential to alert clinicians to early changes in disease severity to adjust treatment in a more timely manner. Results from this survey suggest that many practitioners who treat PAH still do not utilize these tools and that many of those who do use them do so relatively infrequently. Our data indicate that utilization of PAH risk assessment tools supported through targeted investments in education and technology aimed at improving convenience and saving time will increase usage.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020950186 for Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States by Melisa Wilson, Jennifer Keeley, Martha Kingman, Jiajing Wang and Fran Rogers in Pulmonary Circulation
Conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Melisa Wilson has served on the advisory boards and speakers bureaus for United Therapeutics, on the grant committee and speakers bureau for Bayer, and on the speakers bureau for Actelion. Jennifer Keeley has served on the advisory boards and speakers bureaus for Actelion and Bayer and has served as a consultant and speaker for Simply Speaking PAH, which is supported by United Therapeutics and Bayer. Martha Kingman has received consulting fees from and served on the advisory boards and speakers bureaus for Bayer, Gilead, Johnson & Johnson, United Therapeutics, and Arena Pharmaceuticals. Jiajing Wang has nothing to disclose. Fran Rogers has served on the speakers bureau for United Therapeutics and as a Consultant for Actelion.
Contributorship
The first draft was developed with the assistance of a medical writer in collaboration with MW, JK, MK, and FR. JW performed the data analysis. All authors critically edited the manuscript and provided final approval for manuscript submission.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for medical writing support was provided by Actelion Pharmaceuticals, Inc. Medical writing support was provided by Doug Fox and Holly Strausbaugh, PhD, on behalf of Twist Medical, LLC, and funded by Actelion Pharmaceuticals US, Inc. However, Actelion Pharmaceuticals, Inc., did not participate in the development of the manuscript, except for one courtesy review for accuracy only.
Guarantors
MW, JK, MK, JW, and FR.
ORCID iD
Melisa Wilson https://orcid.org/0000-0003-3359-0871
Supplemental material
Supplemental material for this article is available online.
References
- 1.Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Langleben D, Badesch D, et al. Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol 2006; 47: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 4.Olschewski H, Simommeau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 6.Gaine A, McLaughlin V. Pulmonary arterial hypertension: tailoring treatment to risk in the current era. Eur Respir Rev 2017; 26: 170095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost A, Badesch DB, Miller DP, et al. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest 2013; 144: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 8.Austin ED, Kawut SM, Gladwin MT, et al. Pulmonary hypertension: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 2014; 11: S178–S185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest 2015; 147: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 10.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 11.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 14.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 15.Kylhammar D, Kjellström B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. [DOI] [PubMed] [Google Scholar]
- 16.Benza RL, Farber HW, Selej M, et al. Assessing risk in pulmonary arterial hypertension: what we know, what we don’t. Eur Respir J 2017; 50: 1701353. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N, McLaughlin VV, Rubin LJ, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019. A; 53: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019. B; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weatherald J, Boucly A, Sahay S, et al. The low-risk profile in pulmonary arterial hypertension. Time for a paradigm shift to goal-oriented clinical trial endpoints? Am J Respir Crit Care Med 2018; 197: 860–868. [DOI] [PubMed] [Google Scholar]
- 20.Benza RL, Miller DP, Foreman AJ, et al. Prognostic implications of serial risk score assessments in patients with pulmonary arterial hypertension: a Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) analysis. J Heart Lung Transplant 2015; 34: 356–361. [DOI] [PubMed] [Google Scholar]
- 21.Leuchte HH, Ten Freyhaus H, Gall H, et al. Risk stratification strategy and assessment of disease progression in patients with pulmonary arterial hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 20–29. [DOI] [PubMed] [Google Scholar]
- 22.Sahay S, Tonelli A, Kung T, et al. Risk assessment in functional class (FC) II pulmonary arterial hypertension (PAH) patients: comparison of physician gestalt with ESC/ERS-guidelines and REVEAL 2.0. J Heart Lung Transplant 2019; 38: S95. [Google Scholar]
- 23.Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest 2019; 155: 565–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020950186 for Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States by Melisa Wilson, Jennifer Keeley, Martha Kingman, Jiajing Wang and Fran Rogers in Pulmonary Circulation