Abstract
Background:
Patients with type 2 diabetes mellitus (T2DM) remain at increased cardiovascular residual risk and endothelial dysfunction, even after optimizing metabolic control and treatment by sodium-glucose-2 transporter inhibitors (SGLT2-is). The present study was based on the hypothesis that proprotein convertase subtilisin/kexin 9 inhibitor (PCSK9i) therapy may mitigate endothelial dysfunction in T2DM patients who are on regular treatment by SGLT2-i.
Methods:
The EXCEED-BHS3 is a prospective, single-center, investigator-blinded, open-label, randomized clinical trial. Participants (n = 110) will be randomized (1:1) to either empagliflozin 25 mg/day alone or empagliflozin 25 mg/day plus evolocumab 140 mg every 2 weeks in addition to optimal medical care. The primary endpoint was defined as the change in the 1-min flow-mediated dilation (FMD) after 16 weeks of treatment. The secondary endpoint is the FMD change after ischemia/reperfusion injury protocol (reserve FMD) after 16 weeks of treatment. Exploratory outcomes comprise the change in FMD and reserve FMD after 8 weeks of treatment and the change after 16 weeks of treatment in the following parameters: plasma levels of nitric oxide, vascular cell adhesion molecule-1 and isoprostane, high-density lipoprotein (HDL) and low-density lipoprotein subfractions profile, HDL function, blood pressure, body mass index, waist circumference and adipokines.
Conclusion:
This will be the first study to evaluate the add-on effect of PCSK9i on endothelial function of T2DM patients under regular use of empagliflozin.
Trial registration:
ClinicalTrials.gov identifier: NCT03932721
Keywords: diabetes mellitus, endothelial function, evolocumab, sodium-glucose transporter 2 inhibitors
Background
Patients with type 2 diabetes (T2DM) have a 10-year shorter life expectancy, mostly due to increased incidence of macro and microvascular disease.1 Vascular structural and functional damage result from a set of mechanisms, from which stand out insulin resistance, inflammation and oxidative stress.2
Statins therapy in association with blood glucose and blood pressure control has been considered the central pillar for attenuating cardiovascular (CV) risk in T2DM patients. Recently, proprotein convertase subtilisin/kexin 9 inhibitors (PCSK9is) emerged as highly effective cholesterol-lowering agents that reduce the CV risk even in patients with T2DM on statins.3 In spite of the intensive decrease in low-density lipoprotein (LDL) cholesterol, these T2DM patients using PCSK9-i had a CV event rate >7%/year.3 In parallel, sodium-glucose transporter-2 inhibitors (SGLT2-is) also reduce CV risk, regardless of glucose control.4–6 Still, patients with T2DM using SGLT2-i remain at a CV event rate ⩾3%/year, particularly those in secondary prevention.4–6 Although the combined use of both classes of drugs can be additive in mitigating cardiovascular disease, there are no data available to support this hypothesis.
Endothelial dysfunction is one of the earliest manifestations of both CV disease and T2DM, and is notably sensitive to the above mentioned pathophysiological mechanisms related to T2DM.7,8 Consistently, improved endothelial function is one of the earliest signs after preventive therapies.8 Thus, endothelial function change has been used as an investigation tool to support hypotheses and justify more expensive clinical trials, aimed at clinical outcomes.9 Flow-mediated dilation (FMD) became the method of choice to assess endothelial function due to non-invasiveness, close correlation with coronary endothelial function10 and its association with long-term incidence of coronary events.11 A substantial evolution of the method has been observed in recent years, largely obtained by improvements of the technology of image capture by ultrasound, automatic detection of luminal edges, use of probe-holders and blind offline analysis of exam recordings.12 At the same time, the concomitant assessment of plasma biomarkers of endothelial function strengthens the accuracy of the investigation.
A beneficial effect of SGLT2-i on FMD has been indicated in some studies in patients with type 1 or type 2 diabetes mellitus.13–17 Although data from animal models indicated that the lack of PCSK9 would benefit endothelial cells,18 to the best of our knowledge, this is the first clinical trial to assess the impact of PCSK9-i on endothelial function. If a beneficial effect of PCSK9-i on the endothelium of patients with T2DM is proven, this effect will be clinically relevant only if these patients are under optimal medical treatment. Thus, the study was designed to test whether there is benefit in adding therapy with PCSK9-i in patients using SGLT2-i.
Methods
Study design and population
EXpanded Combination of Evolocumab plus Empagliflozin on Diabetes Trial (EXCEED-BHS3 Trial) is a prospective, single-center, randomized, investigator-blinded, open-labeled phase-4 trial, registered at the clinicaltrials.gov site under the number NCT03932721. Patients will be voluntarily screened after radio and internet advertisements. Clinical and laboratory analyses will be performed by the Atherosclerosis and Vascular Biology Laboratory (Atherolab) situated at the Clinical Research Center (CRC) at the University of Campinas (UNICAMP), Brazil. The description of the study design protocol follows the recommendation of SPIRIT statements.19
Selection criteria
Patients with T2DM regardless of the presence of macro- and microvascular complications, aged between 40 and 70 years, BMI <40 kg/m2 must meet the following inclusion criteria after a run-in phase: (i) glycated hemoglobin (HbA1c) between 7% and 9% and no change in the use or dose of antidiabetic drugs for the last 12 weeks before randomization, (ii) LDL cholesterol (LDL-C) between 70 and 100 mg/dL in use of statin with or without ezetimibe for at least 120 days, (iii) blood pressure (BP) <140/90 mmHg and, for those with hypertension, no change in the antihypertensive drugs in the last 12 weeks, and (iv) FMD between 1% and 12%.
Exclusion criteria are: (i) glucagon-like peptide-1 agonist receptor, SGLT2-i or thiazolidinediones therapy in the last 4 months, (ii) plasma triglycerides higher than 500 mg/dL, (iii) uncontrolled BP after use of three-drug classes, (iv) active smoking or smoking cessation in the 6 months before randomization, (v) childbearing potential or pregnant woman, (vi) heart failure symptoms or left ventricle ejection fraction <50%, as shown by echocardiography, (vii) major cardiovascular events, such as myocardium infarction or stroke, within 6 months prior to randomization, (viii) C-reactive protein (CRP) higher than 5 mg/dL, (ix) estimated glomerular filtration rate <60 ml/min per 1.73 m2, (x) alcohol abuse, (xi) allergy to the study drugs.
Clinical care protocol
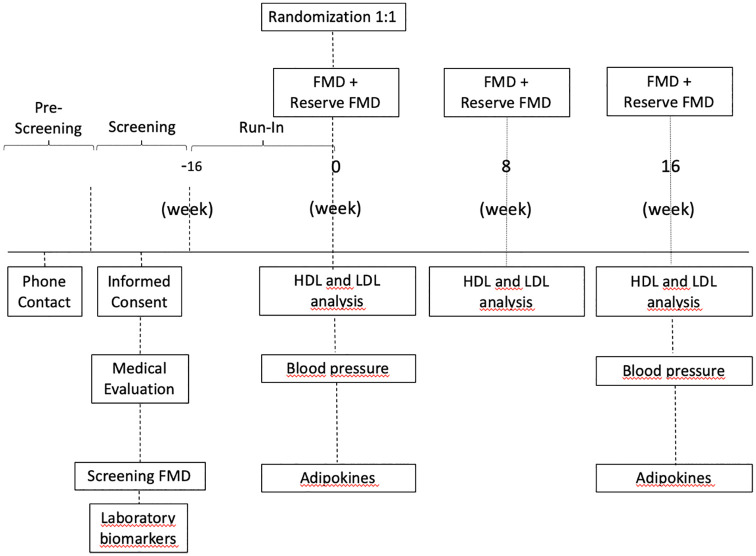
Figure 1 illustrates the EXCEED study protocol. The volunteers from the metropolitan region of Campinas, Brazil, will undergo preliminary evaluation by a telephone interview by the study coordinator. After fulfillment of the initial criteria, the patients will be invited to a medical appointment during which an informed consent will be signed and screening FMD performed. If the FMD values are in the range of inclusion, blood samples will be collected for HbA1c, creatinine, urea, CRP, glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, glycemia, thyroid-stimulating hormone, total cholesterol (TC), high-density lipoprotein (HDL) and triglycerides (TG) measurements. Additionally, plasma aliquots will be stored in liquid nitrogen for posterior analysis of adiponectin, leptin, resistin, retinol binding protein 4 (RBP4), vascular cell adhesion molecule 1 (VCAM-1) and isoprostane.
Figure 1.
Study flow diagram with timeline for each outcome.
FMD, flow-mediated dilation; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Selected patients will enter the run-in phase to undergo adjustments of glycemia, blood pressure (BP) and LDL-C, as specified in the inclusion criteria. For the glycemic target (HbA1c 7–9%), the first antidiabetic treatment will be metformin 500–2000 mg/day (Glifage XR, Merck S.A., Brazil). If needed, sitagliptin 50–100 mg/day will be added (Nimegon, Schering-Plough S.A., Brazil) and as the third option gliclazide 30–120 mg/day (Azukon MR, Torrent-Pharma S.A., Brazil).20 After randomization and addition of empagliflozin, hypoglycemic symptoms will be systematically investigated and if observed and confirmed by glycemia, antidiabetic therapy will be adjusted, initially by reducing the dose or withdrawing gliclazide. If the adverse event persists despite the change in the therapy, the patient will be excluded from the study.
BP control will be attained as recommended by the American Diabetes Association.1 The first choice will be losartan 25–100 mg/day (Aradois, Biolab-Sanus S.A., Brazil). If needed, hydrochlorothiazide 25 mg/day (Clorana, Sanofi S.A., Brazil) will be prescribed and if BP remains uncontrolled hydralazine 25–100 mg/day would also be added (Apresolina, Novartis S.A., Brazil). After randomization, if there is a report of symptomatic hypotension proven by measured blood pressure, the diuretic will be suspended. Statins will be prescribed to target LDL-C between 70 and 100 mg/dL (Sinvacor, Baldacci S.A., Brazil, or Rosucor, Torrent Pharma S.A., Brazil).
BP
Oscillatory automatic BP measurement will be carried out twice during each visit, using the OMRON HEM-705CP device (Omron Healthcare, Japan), after at least 3 min of quiet resting. All patients will undergo a 24-h ambulatory blood pressure monitoring (24 h-AMBP) at the day of randomization and again at the end of the study (SpaceLabs, model 90207-8Q, USA) in the same arm using the same diameter cuff.
Analysis of endothelial function: FMD and reserve FMD
FMD will be evaluated in the same arm, at randomization, in the eighth and 16th weeks by two experienced operators. Exam preparation includes a 12-h fast, and at least 24 h without ingesting xanthine, fatty or salt-enriched foods. Nitrate-derived vasoactive drugs will be withdrawn at least 5 days before the exam.
After the volunteer has been resting at least 15 min in a quiet room with controlled temperature between 22 and 25°C, FMD will be performed using high-resolution ultrasound (Epiq CVX, Philips, Eindhoven, The Netherlands), according to the previously published guidelines.12 The brachial artery will be scanned transversally slightly above the antecubital fossa. A 6–8 cm longitudinal scan of the artery will be searched to find the best temporal, axial and lateral resolution and to identify the proximal and distal intima layer, aiming to avoid artifacts. The ultrasound probe will then be fastened to a probe holder (Quipu, Pisa, Italy) to avoid movement, while the volunteer will remain motionless during the whole test.
The images will be continually recorded using a video capture device (Epiphan’s DVI2USB 3.0™, Epiphan Video, Ottawa, Canada) connected to a dedicated computer. A semi-automatic edge-detecting software will evaluate the baseline, ischemia, hyperemia and dilation phases (LabVIEW 6.02, National Instruments). FMD is defined as the percentage of greatest dilation compared with the median baseline in 1 min after deflating the cuff. Other parameters to be evaluated include velocity-time integral in anterograde and retrograde flow (determined by Doppler), dilation area under the curve (compared with baseline area under the curve), ischemia area under the curve (compared with baseline area under the curve), time to maximum dilation, time to recover to baseline diameter, time to return to basal flow, and shear-rate (4 times velocity divided by arterial diameter).
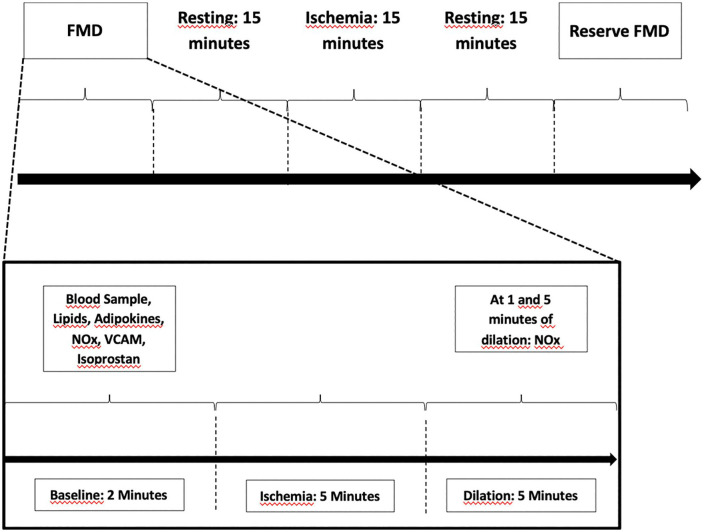
Two FMD assessments will be performed on the same day, separated by 15 min of resting and 15 min of ischemia-reperfusion injury, followed by blood samples collection (Figure 2). The second FMD is intended to estimate the vasomotor endothelial reserve after ischemia and reperfusion injury.
Figure 2.
FMD protocol.
FMD, flow-mediated dilation; NOx, nitrite and nitrate; VCAM, vascular cell adhesion molecule.
During each FMD protocol, blood samples will be collected for nitrite and nitrate (NOx) quantification at baseline, 1 and 5 min after brachial artery cuff deflation (Figure 2). Blood will be collected at baseline, eighth and 16th weeks to assess the levels of endothelial biomarkers. VCAM-1 and isoprostane in plasma samples will be evaluated at both baseline FMD and FMD reserve.
Laboratory methodology
Resistin and RBP4 (Abundant Serum Markers 26-Plex Human ProcartaPlex™ Panel, USA); omentin (BioVendor, BioCompare), VCAM-1 (Invitrogen™ eBioscience™ ProcartaPlex Human VCAM-1 Simplex kit, USA) and isoprostane (8-Isoprostane ELISA Kit, Cayman Chemical Company, USA) will be assessed by enzyme-linked immunosorbent assay (ELISA). Samples will be prepared following the manufacturer’s recommendations and absorbance measured using a microplate reader (Biotek EPOCH 2). Martin’s formula21 will be used to calculate LDL-C during the run-in phase. At randomization and at the end of the trial, lipid profile will be measured by gradient ultracentrifugation.
Continuous density gradient ultracentrifugation (CDGU) will be used to assess very low-density lipoprotein, LDL (subfractions 1–5) and HDL (subfractions 2a, 2b, 3a, 3b and 3c), using a rotor SW41Ti (Beckman Coulter, Brea, CA, USA). TC will be measured in each lipoprotein fraction by enzymatic methods (Cholesterol, Biotechnique, Minas Gerais, Brazil) in the biochemical analyzer SX-140 (Sinnowa, São Paulo, Brazil).
For nitrite and nitrate (NOx) assessment, whole blood will be collected in sodium heparin tube, stored immediately on ice, centrifuged at 3500 rev/min, at 4°C, for 3 min and serum aliquots stored in liquid nitrogen until analysis. Both will be measured by chemiluminescence (model NOA, Sievers Instruments, Boulder, CO, USA).
HDL cholesterol efflux capacity
HDL cholesterol efflux capacity (HDL CEC) will be evaluated using HDL isolated from plasma by CDGU as extracellular acceptors. Total HDL will be prepared by mixing all five HDL subfractions. HDL CEC will be evaluated by a standardized, widely used radioisotopic cell-based technique as previously described.22 In detail, total CEC will be evaluated in the J774 murine macrophage cell model (J774A.1 ATCC® TIB-67™), seeded in 48-well plates in 10% fetal calf serum (FCS) containing Dulbecco’s modified Eagle’s medium (DMEM) (both FCS and DMEM from Lonza, Verviers, Belgium) in the presence of antibiotics (penicillin–streptomycin from Thermo Fisher Scientific, Waltham, MA, USA). Cells will be labeled with 2 µCi/mL (1,2-3H) cholesterol (PerkinElmer, Waltham, MA, USA) for 24 h in the presence of 2 µg/mL of an inhibitor of the cholesterol esterifying enzyme acyl-coenzyme A: cholesterol acyltransferase (Sandoz 58035; Sigma-Aldrich, Milan, Italy), in order to allow all intracellular cholesterol to be in the free form. J774 cells will be incubated in the presence of a cAMP analogue (cpt-cAMP 0.3 mM; Sigma Aldrich), to induce the expression of the cholesterol transporter ATP-binding cassette A1,23 in 0.2% bovine serum albumin (BSA)-containing medium for 18 h (BSA from Sigma-Aldrich). Cells will be then exposed for 4 h to 12.5 µg/mL (in protein) HDL isolated from patients before and after treatment. Total HDL CEC will be expressed as the percentage of radiolabeled cholesterol released into the medium over total radioactivity incorporated by cells before the addition of extracellular acceptors (T0 cells). To check for adequate cell responsiveness, lipid-free human 10 µg/mL apoA-I (Sigma-Aldrich) and 12.5 µg/mL of HDL isolated from a standard serum obtained from a pool of normolipidemic subjects will be tested in each assay. The obtained CEC values will be used to normalize the experiments between each other in order to correct for the inter-assay variability. Sample counting will be performed in a β-counter with automatic calibration, through an internal radioactive standard. To compare data from different experiments, a correction factor for the normalization will be obtained by dividing the efflux value of the standard HDL obtained in the first assay by the value of the standard HDL obtained in a given experiment.
HDL anti-inflammatory activity
Human umbilical vein endothelial cells (HUVECs) will be cultured in their own medium (DMEM, Gibco) supplemented with growth factors in a humidified atmosphere (37°C, 5% CO2), grown until confluence and kept at rest in medium containing 0.5% fetal bovine serum (FBS). For the assays, the cells will be resuspended in 6 mL of DMEM and trypsinized. Then, the trypsin will be neutralized with 6 mL of DMEM + 10% FBS, by centrifugation for 2 min at 1500 rev/min. After assessing cell viability, 0.1 to 0.3 × 105 cells/mL will be placed in each well with DMEM + 10% FBS with a total of 12 mL per well and incubated until confluence.
Initially, the cells will be incubated with HDL (final concentration of 25 μg total protein/mL) for 1 h. Then, TNF-α (5 ng/mL) will be added and the HUVECs will be incubated for 3 h in a humidified atmosphere (37°C, 5% CO2). At the end, 700 μL of the supernatant will be removed to assess the levels of VCAM-1 (human VCAM-1 ELISA kit, ECM340, Millipore, MA, USA).
Antioxidant activity of HDL
Total protein, TC, TG, phospholipids, free cholesterol (FC) and ApoA-I contents will be determined using commercially available assays. The cholesteryl ester will be calculated by multiplying the difference between the TC and the FC by 1.67.24 To verify the antioxidant activity of HDL, LDL (10 mg TC/dL) will be oxidized using 2,2'-azobis-(2-amidinopropane) hydrochloride (AAPH). Initially, PBS treated with Chelex 500 ion-exchange resin at 1 g/L to remove transition metal ions will be added into microtubes. Then, LDL and HDL will be added to the respective tubes and, later, AAPH (final concentration 1 mM). After homogenization, they will be transferred to a UV-Star microplate (Greiner Bio-One, Brazil) read in an Epoch 2 microplate reader (Biotek).
Drug dispensing, adherence and adverse effects
Patients will return monthly to a medical appointment for drug adherence evaluation and drug dispensing.
The incidence of adverse events (AEs) will be investigated every 15 days either by telephone call or during appointments. Those patients reporting AEs by the telephone interview will be asked to come for a medical appointment.
Data management plan
The clinical report form will be based on the Research Electronic Data Capture (REDCap) platform to store all clinical visits and exams during the trial.25 Medical history and physical examination will be obtained during the following visits: screening, run-in, randomization, and every 4 weeks until the end of the study. The recorded videos of the entire FMD proceeding will be processed in MP4 format and stored in a dedicated storage rack of the UNICAMP data center. The investigators will perform a systematic routine of evaluation for medical assessments, ensuring homogeneity in data collection and case management. The principal investigator, Andrei C Sposito, is responsible for the data obtained and stored in this research and, together with the BHS-group, is also responsible for all analyses of the trial.
Randomization and follow-up
Randomization will be performed by a research team member, not involved with FMD execution, using built-in software from REDCap, stratified by sex (male or female) and age (40–59 years old or 60–70 years old). Patients will be randomized 1:1 into the following study arms: (i) empagliflozin 25 mg/day, in addition to optimal medical treatment or (ii) empagliflozin 25 mg/day plus evolocumab 140 mg every 2 weeks. Although there are similar decreases in cardiovascular events by empagliflozin 10 mg or 25 mg daily,4 we opted for the highest dose, based on the reported improvement of endothelial dysfunction, as estimated by FMD, with the higher dose.13
After randomization, the patients in the evolocumab group will return to the clinical research center every 15 days to receive this drug
Objectives and endpoints
The primary endpoint is the change in 1-min FMD from baseline to 16 weeks of treatment. The secondary endpoint is the change from baseline to 16 weeks in 1-min reserve FMD. Exploratory outcomes are: (i) change from baseline to 8 weeks in 1-min FMD, (ii) change from baseline to 8 weeks in 1-min reserve FMD, (iii) change from baseline to 16 weeks in NO bioavailability, VCAM-1 and isoprostane, (iv) change from baseline to 16 weeks in LDL and HDL subfractions, (vii) change from baseline to 16 weeks in HDL function, (viii) change from baseline to 16 weeks in BP, (ix) change in the body mass index (BMI) and waist circumference; (x) change in adipokines.
Sample size and statistical analysis
To the best of our knowledge, there is no previous study on the effect of anti-PCSK9 monoclonal antibodies on FMD. We selected a study with a high-dose statin that achieved a reduction in LDL-C close to the effect we will have after evolocumab treatment (±60%), in which FMD increased by 3.6 ± 2%.26 These data were hence used as estimate for sample size calculation and considering a two-tailed α value of 0.05 and β value of 95%, 53 patients will be required in each arm (G-Power Version 3.1.9.4). Taking into consideration the dropout possibility, we will include 55 patients in each arm.
Covariance analysis will be performed to evaluate a change in FMD at the 16th week of treatment. The assumptions of the analysis of covariance (ANCOVA) model (linearity, distribution of normality, and equality of variances) will be verified using histograms, residual scatter plots and normal probability graph. Age, sex and baseline FMD will be included as covariates in ANCOVA models. Adjustment of baseline values is necessary to allow regression to possible biases. Non-normal continuous variables will be transformed to correct for dispersions. We will check whether there is an interaction between the change in the outcomes and the covariables age, sex, drugs in use, duration of diabetes and the previous manifestation macro- or microvascular. If there is a significant interaction, we will perform ANCOVA adjusted for the identified variable. If the variables still persist as non-normal even after transformation, they will be analyzed using the Kruskal–Wallis test. The gatekeeper approach will be used for the analysis of secondary outcomes. All exploratory outcomes will be assessed with a 95% confidence interval.
Discussion
The emergence of SGLT2i has profoundly improved the landscape of T2DM prognosis, reducing both major adverse cardiovascular events (MACEs) and mortality.27 Accordingly, recent guidelines now recommend SGLT2-i as a treatment of choice in high-risk patients.28 In parallel, a large number of data have supported the intensive LDL-lowering treatment as a key piece in the prevention of MACE in T2DM.28 Recently, the FOURIER trial,3 with a large number of T2DM patients enrolled into a lipid-lowering study, demonstrated that the even more intensive reduction in LDL-C (about 30 mg/dL) by adding evolocumab onto statin or statin plus ezetimibe treatment (~5% of the cases) can further reduce 15% of the risk of MACE.3
In spite of this substantial advance, the residual risk remains very high in patients with T2DM. Hence, the EXCEED trial was designed to verify whether the combined use of these two therapies can play an additive role in mitigating micro- and macrovascular function. As a fundamental step in the consolidation of plausibility that will serve as a basis for clinical studies with hard endpoints, EXCEED was designed using FMD as a surrogate outcome. The choice of this outcome was based on the precociousness of its change with therapy and the strong association with the incidence of MACEs.
Consistent with the new guidelines12 for conducting FMD, we will continuously capture the diameter of the brachial artery and the flow velocity during 5 min after deflating the cuff. In this way, we intend to discern any prolonged effects of evolocumab on the test, particularly on microvascular function. Indeed, the analysis of flow velocity 5 min after cuff deflation has been shown to be a better predictor of cardiovascular events than the isolated value of FMD in 1 min.29
Endothelial dysfunction commonly found in T2DM patients is exacerbated when ischemia and reperfusion (I/R) injury occurs and is one of the reasons for the greater extent of myocardial infarction.30 From the mechanistic standpoint, imbalance between constricting and relaxing factors derived from the endothelium, such as NO and endothelin-1, reduces arterial blood flow after reperfusion and aggravates ischemia.31 In this sense, we include as a secondary endpoint the assessment of reserve FMD after I/R to check whether there is benefit in this specific clinical condition.
In addition to assessing the impact of treatment with evolocumab on macro- and microvascular function, the EXCEED trial will assess its effect on the proportion of LDL and HDL subfractions. Well-validated HDL functions such as antioxidant, anti-inflammatory activity32 and the capacity to mediate cholesterol efflux33 will also be evaluated. The set of these analyses will help to estimate whether at least in part the benefit of the treatment stems from the qualitative change of lipid profile.
A large number of studies have indicated that changes in lipid metabolism itself can modulate several mechanisms involved in blood pressure regulation, such as endothelium-dependent vasodilation, secretion of vasoactive substances, cellular handling of calcium and sodium and autonomous cardiovascular control.34 Therefore, from a mechanistic point of view, dyslipidemia can predispose to an increased sensitivity to hypertensive stimuli or even induce the clinical manifestation in individuals with a pathophysiological history favorable to the hypertension development. Other researchers and ourselves reported that lipid-lowering treatment with statins can improve blood pressure control.35,36 To what extent this effect stems from effects dependent or independent of LDL-C levels in the bloodstream we do not know. Thus, evaluation of 24-h AMBP change after evolocumab treatment was included as an exploratory endpoint to verify whether the association between BP and LDL-C also exists when lipid-lowering therapies other than statins are employed. The association between BP and PCSK9-i may also be related to adiposity. In fact, association has been reported between the excess weight and PCSK9 plasma concentration.37 A reciprocal interaction seems to exist since adiponectin inhibits the production of PCSK937 and PCSK9 is involved in visceral adipose tissue expansion.38 Hence, also the interaction between evolocumab treatment and the change in anthropometric and biological markers of adiposity will be explored.
The effect of monoclonal anti-PCSK9 antibodies on endothelial function is still unknown and, as such, a monotherapy study could directly clarify this knowledge gap. However, for the outcome of this clinical trial to become potentially clinically relevant, treatment with evolocumab must prove beneficial in patients treated with the best available therapy. As the effect of SGLT2-i on improving FMD was previously reported39 and it will be once again measured in the control arm, we believe that we will be able to estimate the magnitude of the additive effect of evolocumab on SGLT2-i therapy and in this way test its potential benefit in an optimal scenario of T2DM treatment.
The present protocol still bears some limitations. This will be a surrogate endpoint study, therefore could not be considered as an evidence for clinical practice change. The study will enroll a selected group of T2DM patients, thus the results cannot be extrapolated to a different subset of individuals. Nevertheless, the study will shed light on a clinically relevant and current issue, allowing new studies to be outlined more clearly.
In conclusion, the EXCEED-BHS3 trial is designed to verify whether an additive effect exists between PCSK9-i and SGLT2-i in mitigating endothelial dysfunction in T2DM patients.
Footnotes
Availability of data and material: The BHS group will hold intellectual propriety over data from this investigation. Data sets produced during the study will not be shared or left in the public domain. Based on a reasonable request addressed to the corresponding author, the availability could be considered.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Dissemination policy and consent for publication: Dissemination policy to participants and health professionals will occur through publications, defined exclusively by the BHS group without the interference from AMGEN. The authors will have full access to the data and take responsibility for its integrity. All authors have read and agree with the contents of this manuscript.
Ethics approval and consent to participate: This clinical trial was approved by the Ethical Committee from the University of Campinas, protocol CAAE 88800718.0.0000.5404. Every six months, a new report will be issued to this Committee, reporting the progress of the study. This trial was registered under the number NCT03932721 on 1 May 2019. It will be conducted according to the Declaration of Helsinki.40 All patients will receive a detailed explanation about AEs risk and confidentiality before signing the informed consent form.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was designed and proposed by the BHS group and it was funded as an Investigator Sponsored Studies (ISS) by AMGEN (grant number 20177605). As ISS, the BHS group has fully assumed the legal and regulatory responsibility for planning, conducting, managing and analyzing the research, as defined by applicable Brazilian regulations and laws. ACS was supported by a Research Career Awards grant from the Brazilian National Research Council (CNPq) (grant number 301465/2017-7).
ORCID iD: Andrei C. Sposito  https://orcid.org/0000-0001-7127-2052
https://orcid.org/0000-0001-7127-2052
Contributor Information
Ikaro Breder, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Jessica Cunha Breder, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Isabella Bonilha, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Daniel B. Munhoz, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil
Sheila T. Kimura Medorima, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Daniela C. Oliveira, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil
Helison R. do Carmo, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil
Camila Moreira, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Anatol Kontush, UMR-ICAN 1166, National Institute for Health and Medical Research (INSERM), Sorbonne University, Paris, France.
Francesca Zimetti, Department of Food and Drugs, University of Parma, Parma, Emilia-Romagna, Italy; BHS – Brazilian Heart Study Group, State University of Campinas, São Paulo, Brazil.
Ilaria Zanotti, Department of Food and Drugs, University of Parma, Parma, Emilia-Romagna, Italy; BHS – Brazilian Heart Study Group, State University of Campinas, São Paulo, Brazil.
Luiz Sergio F. Carvalho, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil
Wilson Nadruz, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Elza Muscelli, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Thiago Quinaglia, Atherosclerosis and Vascular Biology Laboratory, Cardiology Department, State University of Campinas, SP, Brazil.
Andrei C. Sposito, Brazilian Heart Study Group, Cardiology Division, State University of Campinas, Rua Tessália Vieira de Camargo, 126., Cidade Universitária Zeferino Vaz, Campinas, São Paulo, 13084-971, Brazil.
References
- 1. American Diabetes Association. Standards of medical care in diabetes-2018 abridged for primary care providers. Clin Diabetes 2018; 36: 14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walther G, Obert P, Dutheil F, et al. Metabolic syndrome individuals with and without type 2 diabetes mellitus present generalized vascular dysfunction: cross-sectional study. Arterioscler Thromb Vasc Biol 2015; 35: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 3. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5: 941–950. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Lachin JM, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2016; 374: 1092. [DOI] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. Epub ahead of print 10 November 2018. DOI: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 7. Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 2017; 9: 434–449. [DOI] [PubMed] [Google Scholar]
- 8. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 9. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: from research into clinical practice. Circulation 2012; 126: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broxterman RM, Witman MA, Trinity JD, et al. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 2019; 74: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009; 120: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thijssen DHJ, Bruno RM, van Mil A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019; 40: 2534–2547. [DOI] [PubMed] [Google Scholar]
- 13. Lunder M, Janic M, Japelj M, et al. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol 2018; 17: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawada T, Uzu K, Hashimoto N, et al. Empagliflozin’s ameliorating effect on plasma triglycerides: association with endothelial function recovery in diabetic patients with coronary artery disease. J Atheroscler Thromb 2020; 27: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irace C, Cutruzzola A, Parise M, et al. Effect of empagliflozin on brachial artery shear stress and endothelial function in subjects with type 2 diabetes: results from an exploratory study. Diab Vasc Dis Res 2020; 17: 1479164119883540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sezai A, Sekino H, Unosawa S, et al. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol 2019; 18: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tochiya M, Makino H, Tamanaha T, et al. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: a pilot study. J Diabetes Investig 2020; 11: 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun H, Krauss RM, Chang JT, et al. PCSK9 deficiency reduces atherosclerosis, apolipoprotein B secretion, and endothelial dysfunction. J Lipid Res 2018; 59: 207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013; 346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013; 310: 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zimetti F, Freitas WM, Campos AM, et al. Cholesterol efflux capacity does not associate with coronary calcium, plaque vulnerability, and telomere length in healthy octogenarians. J Lipid Res 2018; 59: 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bortnick AE, Rothblat GH, Stoudt G, et al. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J Biol Chem 2000; 275: 28634–28640. [DOI] [PubMed] [Google Scholar]
- 24. Chapman MJ, Goldstein S, Lagrange D, et al. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res 1981; 22: 339–358. [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostad MA, Eggeling S, Tschentscher P, et al. Flow-mediated dilation in patients with coronary artery disease is enhanced by high dose atorvastatin compared to combined low dose atorvastatin and ezetimibe: results of the CEZAR study. Atherosclerosis 2009; 205: 227–232. [DOI] [PubMed] [Google Scholar]
- 27. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 28. Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008; 371: 117–125. [DOI] [PubMed] [Google Scholar]
- 29. Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the firefighters and their endothelium (FATE) study. Circulation 2011; 123: 163–169. [DOI] [PubMed] [Google Scholar]
- 30. Miki T, Itoh T, Sunaga D, et al. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 2012; 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Q, He GW, Underwood MJ, et al. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: perspectives and implications for postischemic myocardial protection. Am J Transl Res 2016; 8: 765–777. [PMC free article] [PubMed] [Google Scholar]
- 32. Morgantini C, Natali A, Boldrini B, et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011; 60: 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rhainds D, Tardif JC. From HDL-cholesterol to HDL-function: cholesterol efflux capacity determinants. Curr Opin Lipidol 2019; 30: 101–107. [DOI] [PubMed] [Google Scholar]
- 34. Sposito AC. Emerging insights into hypertension and dyslipidaemia synergies. Eur Heart J Suppl 2004; 6: G8–G12. [Google Scholar]
- 35. Spósito AC, Mansur AP, Coelho OR, et al. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol 1999; 83: 1497–1499, a1498. [DOI] [PubMed] [Google Scholar]
- 36. Golomb BA, Dimsdale JE, White HL, et al. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med 2008; 168: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun L, Yang X, Li Q, et al. Activation of adiponectin receptor regulates proprotein convertase Subtilisin/Kexin type 9 expression and inhibits lesions in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2017; 37: 1290–1300. [DOI] [PubMed] [Google Scholar]
- 38. Roubtsova A, Munkonda MN, Awan Z, et al. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol 2011; 31: 785–791. [DOI] [PubMed] [Google Scholar]
- 39. Solini A, Seghieri M, Giannini L, et al. The effects of dapagliflozin on systemic and renal vascular function display an epigenetic signature. J Clin Endocrinol Metab 2019; 104: 4253–4263. [DOI] [PubMed] [Google Scholar]
- 40. World Medical Association. Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–926. [PubMed] [Google Scholar]