Abstract
Background:
Data regarding the real-life predictors of low disease activity (LDA) in rheumatoid arthritis (RA) patients are limited. Our aim was to evaluate the rate and predictors of LDA and treatment patterns in RA.
Methods:
This was a multicenter, prospective, RA cohort study where patients were evaluated in two different time points approximately 12 months apart. Statistical analysis was performed in order to identify predictors of LDA while patterns of disease-modifying anti-rheumatic drug [DMARDs; conventional synthetic (csDMARD) or biologic (bDMARD)] and glucocorticoid (GC) use were also recorded.
Results:
The total number of patients included was 1317 (79% females, mean age: 62.9 years, mean disease duration: 10.3 years). After 1 year, 57% had achieved LDA (DAS28ESR<3.2) while 43% did not (34%: moderate disease activity: DAS28ESR ⩾3.2 to <5.1, 9%: high disease activity, DAS28ESR ⩾5.1). By multivariate analysis, male sex was positively associated with LDA [odds ratio (OR) = 2.29 p < 0.001] whereas advanced age (OR = 0.98, p = 0.005), high Health Assessment Questionnaire (HAQ) score (OR = 0.57, p < 0.001), use of GCs (OR = 0.75, p = 0.037) or ⩾2 bDMARDs (OR = 0.61, p = 0.002), high co-morbidity index (OR = 0.86, p = 0.011) and obesity (OR = 0.62, p = 0.002) were negative predictors of LDA. During follow-up, among active patients (DAS28ESR >3.2), 21% initiated (among csDMARDs users) and 22% switched (among bDMARDs users) their bDMARDs.
Conclusion:
In a real-life RA cohort, during 1 year of follow-up, 43% of patients do not reach treatment targets while only ~20% of those with active RA started or switched their bDMARDs. Male sex, younger age, lower HAQ, body mass index and co-morbidity index were independent factors associated with LDA while use of GCs or ⩾2 bDMARDs were negative predictors.
Keywords: biologic therapy, co-morbidities, disease activity, rheumatoid arthritis
Introduction
The advances in the therapeutic landscape of rheumatoid arthritis (RA) have substantially improved the quality of life of patients living with the disease, as the introduction of biologic disease-modifying anti-rheumatic drugs (bDMARDs) has resulted in slower disease progression, less chronic irreversible damage, higher functional capacity, lower rates of arthroplasties and even lower risk for extra-articular complications, such as cardiovascular events.1 These favorable outcomes could be attributed to a number of factors,2 including earlier diagnosis, improved treatment strategies [implementation of the treat-to-target (T2T) approach],3 more aggressive management of co-morbidities4 and obviously the introduction of newer therapies such as the bDMARDs and oral targeted synthetic DMARDs (tsDMARDs).
However, despite all these advances, a substantial proportion of RA patients fail to achieve or to remain in remission/low disease activity (LDA).5 Real world data from registries have identified several factors associated with remission after treatment initiation in patients with early RA,6,7 albeit predictors of inadequate responses are ill-recognized in patients with longstanding disease.
Although the European League against Rheumatism (EULAR) Recommendations and the American College of Rheumatology (ACR) Guidelines for the management of RA suggest a T2T strategy consisting of adjusting therapy (initiating or switching DMARD therapies) if no improvement is observed in 3 months or target activity is not achieved in 6 months,3,8,9 the extent and success of the adoption of this approach in daily clinical practice has not been yet fully elucidated. Furthermore, for patients with moderate disease activity (MDA; DAS28ESR = 3.2–5.1), who currently constitute the most common RA patient subset in clinical practice, such real-life, longitudinal data are limited.10–12
In this study, we prospectively evaluated the rate and predictors of achievement of LDA/remission in a large, real-life, multicenter RA cohort with a special focus in the subgroup of patients with MDA.
Materials and methods
Patients and study design
We conducted a multicenter, prospective study under the auspices of the RA Study Group of the Greek Rheumatology Society as recently reported.13 Among the participating centers were academic and non-academic rheumatology departments, National Health System outpatient clinics and private offices. Inclusion criteria included age ⩾18 years and RA diagnosis according to the ACR/EULAR criteria.14 Ethical approval was provided by the Joint Rheumatology Program (Hippokration General Hospital as the co-ordinating center, 64/16-4-2015 and 7/23-3-2016) as well as by the local institutional boards of participating centers. Informed consent was provided by all patients at first evaluation.
According to the protocol, the study was divided into three successive phases. During phase I, an initial cross-section of RA patients was performed (first evaluation, June 2015–September 2016). Collected data were entered either through a printed case-reporting form or via a web-based form (www.rheumstudygrps.gr). The results of phase I have been published recently.13
During phase I, the following data were collected:
- Patient and disease characteristics: age, sex, weight, height, disease duration, working status, educational status, smoking and alcohol habits, disease activity assessed by the DAS28 (Disease Activity Score using 28 joints) erythrocyte sedimentation rate (ESR) score, functional status assessed by the Health Assessment Questionnaire (HAQ), serological status [presence or absence of rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP)], presence of erosions in plain joint X-rays (physician reported), history of joint arthroplasties and RA-related interstitial lung disease. According to their disease duration, patients were further classified to those with “early” (⩽2 years) or “longstanding” (>2 years) disease.
- Treatment patterns: for each patient, past and current use of anti-rheumatic medications (including current dose and reasons for previous discontinuation) was also recorded. Anti-rheumatic medications included non-steroidal anti-inflammatory drugs, analgesics, glucocorticoids (GCs) and DMARDs, either conventional synthetic (cs)DMARDs or bDMARDs.
- Comorbidities: hyperlipidemia (use of lipid-lowering therapy), coronary artery disease (treatment and/or history of stable angina, acute coronary syndrome or angioplasty/coronary artery bypass surgery), cerebrovascular disease (treatment and/or history of thrombotic or hemorrhagic stroke), peripheral vascular disease (specific treatment and/or history of revascularization), diabetes mellitus (use of antidiabetic agents and/or insulin), chronic obstructive pulmonary disease (with or without use of oxygen therapy at home), arterial hypertension (use of anti-hypertensive medications), depression (use of anti-depressives), osteoporosis (use of anti-osteoporotic therapies and/or history of osteoporotic fractures), current or past hepatitis B virus (HBV) infection (documented by the specific serology including HBsAg, anti-HBc and anti-HBs antibodies), current or past hepatitis C virus (HCV) infection (documented by anti-HCV antibodies and HCV-RNA testing), history of tuberculosis (TB) or latent TB infection, documented by a positive tuberculin skin test or an interferon-gamma release assay, history of herpes zoster, current or past history of neoplastic diseases and history of vaccination against influenza (in the last year or in the past), Streptococcus pneumoniae and HBV, history of hospitalization during the last 12 months and history of serious infection. Rheumatic disease co-morbidity index (RDCI) was used as a composite co-morbidity score.15
During phase II (June 2016–September 2017), patients from the initial cohort were prospectively re-evaluated approximately 12 months after their first evaluation and a new set of data was collected with the same methods as phase I (printed or web-based forms).
These included:
- Disease characteristics (DAS28ESR, HAQ);
- Serious events between the two evaluations (serious infections requiring hospitalization, arthroplasties, cardiovascular events, hospitalization for any reason, osteoporotic fractures, neoplasias);
- Treatment changes (discontinuation and current treatment).
In the current study, we included RA patients who had available DAS28ESR at both time points (first evaluation visit during phase I and 2nd evaluation during phase II approximately 1 year later). Patients’ disease activity was categorized as low (LDA, <3.2), medium (MDA, ⩾3.2 to <5.1) and high (HDA, ⩾5.1) according to the DAS28ESR score. Furthermore, patients with MDA (n = 493, DAS28ESR = 3.2–5.1) were further categorized into those with “lower” (DAS28ESR: 3.2–4.1, n = 199, 40.4%) or “higher” (DAS28ESR: 4.2–5.1 n = 294, 59.6%) disease activity at first evaluation.
Statistical analysis
All statistical analyses were performed with SPSS (IBM SPSS Statistics for Windows, v. 20.0., IBM Corp., Armonk, NY, USA) and STATA 13 (StataCorp) software. Demographic and descriptive continuous variables with normal distribution are expressed as mean (SD), whereas non-normally distributed data are presented as median values (interquartile range). Categorical variables are expressed as percentages. Chi square or Fisher’s exact test was used for comparison of dichotomous and Mann–Whitney, t-test or one-way analysis of variance for continuous variables. Related samples were compared with Wilcoxon and McNemar’s tests. Threshold of statistical significance was defined as a p-value < 0.05. Among patients with MDA or HDA, univariate and multivariate logistic regression analysis was implemented in order to identify factors associated with changes in disease activity and initiation or switch in treatment type. Variables with p-value < 0.2 were included in the multivariate model. Variables with p-value < 0.1, as well as those of biological significance (sex and age), were retained until the final stage of multivariate analysis (backward selection). Outcomes of logistic regression analysis are displayed as odds ratios (ORs) and their respective 95% confidence intervals (CIs).
Results
Patient characteristics at first evaluation
Among 2491 patients evaluated initially as reported in details in our previous publication,13 1549 (62.2%) were available for a second evaluation after a median period of 13 months. With the exception of the more frequent use of bDMARDs (45% versus 35%) and dyslipidemia (35% versus 30%) and less frequent use of GCs (37% versus 45%), no other significant differences were noted between those with both evaluations (n = 1549) and those with only the first evaluation available, respectively (n = 942; see Supplemental Material Table 1 online). A total of 1317 patients (85%) with available complete DAS28ESR data were included in the current study. With the exception of lower HAQ and higher csDMARD use at first evaluation, there were no other statistically significant differences between those included (n = 1317) and not included (n = 232), respectively in the analysis (Supplemental Table 2).
The characteristics of the 1317 patients are shown in Table 1; most patients were women (79%) with a mean age of 62.9 years and long disease duration (mean: 10.3 years). Almost half (54%) were RF and/or anti-CCP+, 44% had erosive disease while 9% had had an arthroplasty in the past. Regarding disease activity, the mean DAS28ESR was 3.36 and the median HAQ 0.48. Co-morbidities were common (63%; 825/1317) including hypertension (43%), dyslipidemia (34%), osteoporosis (28%) and diabetes mellitus (13%). Approximately one-third of patients (34%) had ⩾2 co-morbidities (see Supplemental Figure 1) while one out four patients was obese [body mass index (BMI) >30 kg/m2; 24.6%; Table 1].
Table 1.
Patient and disease characteristics at first evaluation.
| Patient characteristics | |
|---|---|
| n | 1317 |
| Female, n (%) | 1012 (79%) |
| Age, years, mean (SD) | 62.9 ± 12.6 |
| Disease characteristics | |
| Disease duration, years, mean (SD) | 10.3 ± 9.3 |
| Early RA, duration <2 years | 125 (10.3%) |
| Seropositivity, RF and/or anti-CCP, n (%) | 696 (54%) |
| Erosions, n (%) | 477 (44%) |
| DAS28ESR, mean (SD) | 3.36 ± 1.29 |
| HAQ, median (IQR) | 0.48 (0.8) |
| History of arthroplasties, n (%) | 119 (9%) |
| Treatment patterns | |
| No treatment, n (%) | 55 (4%) |
| csDMARDs, n (%) | 1112 (85%) |
| Monotherapy | 652 (50%) |
| bDMARDs, n (%) | 610 (46%) |
| Monotherapy | 150 (11%) |
| Combination of cs- and bDMARDs, n (%) | 460 (35%) |
| bDMARDs, n (%) | 610 (46%) |
| 1st agent | 309 (51%) |
| 2nd agent | 158 (26%) |
| ⩾3rd agent | 143 (23%) |
| Glucocorticoids, n (%) | 480 (36%) |
| Prednisolone daily dose, mg, mean (SD) | 4.7 ± 3.4 |
| Co-morbidities | |
| RDCI, median (IQR) | 1 (2) |
| Current smokers | 230 (18.2%) |
| Obesity, BMI >30 kg/m2 | 283 (24.6%) |
| Hypertension | 563 (43%) |
| Dyslipidemia | 443 (34%) |
| Osteoporosis | 367 (28%) |
| Diabetes | 178 (13%) |
| Depression | 165 (12%) |
| Coronary artery disease | 75 (6%) |
| COPD | 80 (6%) |
| Cancer, current/past | 66 (5%) |
| Stroke | 41 (3%) |
anti-CCP, anti-cyclic citrullinated peptide antibodies; bDMARD, biologic DMARD; COPD, chronic obstructive pulmonary disease; csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; DAS28, Disease Activity Score using 28 joints; DMARD, disease-modifying anti-rheumatic drug; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; IQR, interquartile range; RA, rheumatoid arthritis; RDCI, Rheumatic Disease Comorbidity Index; RF, rheumatoid factor; SD, standard deviation.
In terms of treatment patterns (Table 1), 85% were on csDMARDs (50% as monotherapy and 35% in combination with bDMARDs) and 46% on bDMARDs (11% as monotherapy and 35% in combination with csDMARDs). Among bDMARD-treated patients, almost half had been already exposed to >1 bDMARD (49%). Approximately one-third of patients (36%) were on GCs (mean daily prednisolone dose: 4.7 mg).
Attainment of LDA during follow-up
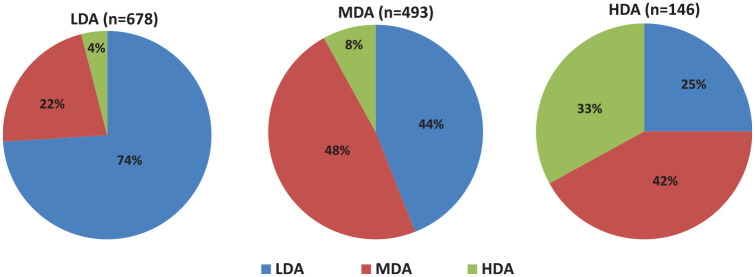
At first evaluation 52% of patients were on LDA, 37% on MDA and 11% on HDA; at re-evaluation 1 year later, the respective rates were 57% (+5%), 34% (–3%) and 9% (–2%, p < 0.001; Figure 1). During the same period, 74% of patients with LDA (n = 678) remained at LDA, while 44% and 25% of patients with MDA (n = 493) or HDA (n = 146) achieved the same target, respectively (Figure 2).
Figure 1.
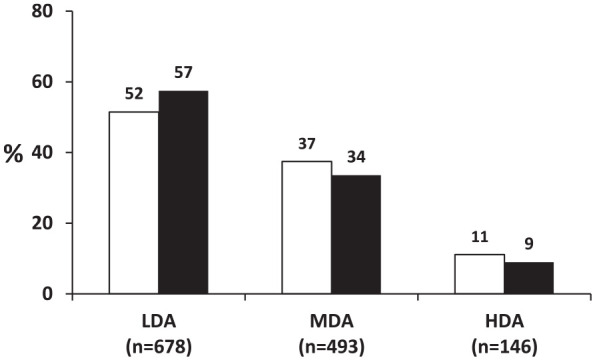
Disease activity at the first and second evaluation of the whole rheumatoid arthritis cohort (n = 1317).
The percentages (%) of patients with low (LDA; DAS28ESR < 3.2), moderate (MDA; DAS28ESR ⩾3.2 to <5.1) and high (HDA; DAS28ESR ⩾5.1) disease activity at first evaluation (white bars) and at the end of the 1 year follow-up (dark bars) are shown.
DAS28, Disease Activity Score using 28 joints; ESR, erythrocyte sedimentation rate
Figure 2.
Disease activity outcomes according to the initial disease activity status.
The percentages (%) of patients who achieved the specific outcome [low disease activity (LDA): DAS28ESR <3.2; moderate disease activity (MDA): DAS28ESR ⩾3.2 to <5.1; high disease activity (HAD): DAS28ESR ⩾5.1] in each patient group during the 1 year follow-up period is depicted.
DAS28, Disease Activity Score using 28 joints; ESR, erythrocyte sedimentation rate
The characteristics of patients who attained LDA versus those who retained active disease are shown in Supplemental Table 3. By multivariate analysis, male sex (OR = 2.29, 95% CI: 1.62–3.23, p < 0.001) was a positive predictor of LDA while a high HAQ (OR = 0.57, 95% CI: 0.45–0.72, p < 0.001) and co-morbidity index (RDCI, OR = 0.86, 95% CI: 0.76–0.96, p = 0.011) as well as obesity (BMI >30 kg/m2, OR = 0.62, 95% CI: 0.46–0.84, p = 0.002) and GC use (OR = 0.75, 95% CI: 0.57–0.98, p = 0.037) were negative predictors (Table 2). Regarding bDMARDs, patients who had been on their ⩾2nd bDMARD were less likely to achieve LDA (OR = 0.61, 95% CI: 0.44–0.84, p = 0.002; Table 2). Similar results were obtained when patients with early disease were excluded (data not shown).
Table 2.
Univariate and multivariate analysis of factors associated with achievement of low disease activity (DAS28ESR <3.2) for the whole RA cohort (n = 1317).
| Variable | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | ||||||
| n | OR | Lower | Upper | OR | Lower | Upper | |||
| Sex, male | 1317 | 2.48 | 1.84 | 3.33 | <0.001 | 2.29 | 1.62 | 3.23 | <0.001 |
| Age | 1317 | 0.98 | 0.97 | 0.99 | <0.001 | 0.98 | 0.97 | 0.99 | 0.005 |
| Disease duration | 1219 | 0.98 | 0.97 | 0.99 | 0.035 | ||||
| Seropositivity, RF/anti-CCP | 1280 | 0.62 | 0.49 | 0.77 | <0.001 | ||||
| GC use | 1317 | 0.62 | 0.49 | 0.77 | <0.001 | 0.75 | 0.57 | 0.98 | 0.037 |
| bDMARD use | 1317 | ||||||||
| 1st line versus no bDMARD | 0.92 | 0.7 | 1.2 | 0.53 | 0.84 | 0.62 | 1.16 | 0.3 | |
| ⩾2nd line versus no bDMARD | 0.59 | 0.45 | 0.78 | <0.001 | 0.61 | 0.44 | 0.84 | 0.002 | |
| HAQ score at first evaluation | 1195 | 0.46 | 0.37 | 0.57 | <0.001 | 0.57 | 0.45 | 0.72 | <0.001 |
| Co-morbidity index (RDCI) | 1317 | 0.79 | 0.72 | 0.86 | <0.001 | 0.86 | 0.76 | 0.96 | 0.011 |
| Active smoking | 1265 | 1.26 | 0.94 | 1.69 | 0.117 | ||||
| Obesity, BMI >30 kg/m 2 | 1149 | 0.51 | 0.39 | 0.67 | <0.001 | 0.62 | 0.46 | 0.84 | 0.002 |
| Erosions | 1317 | 0.66 | 0.51 | 0.84 | <0.001 | ||||
Number of patients included in the final model n = 1092, Hosmer–Lemeshow test = 8.88 (p = 0.35), the model predicted correctly 67.3% of cases. Variables with statistically significant differences (p < 0.05) between groups by multivariate analysis are shown in bold.
anti-CCP, anti-cyclic citrullinated peptide antibodies; bDMARD, biologic disease-modifying anti-rheumatic drug; BMI, body mass index; CI, confidence interval; DAS28, Disease Activity Score using 28 joints; ESR, erythrocyte sedimentation rate; GC, glucocorticoid; HAQ, Health Assessment Questionnaire; OR, odds ratio; RDCI, Rheumatic Disease Comorbidity Index; RF, rheumatoid factor.
Clinical outcome and predictors of LDA among patients with MDA
Among patients with MDA (n = 493, DAS28ESR = 3.2–5.1), 44% reached LDA (Figure 2). By multivariate analysis, male sex (OR = 2.13, 95% CI: 1.3–3.99, p = 0.004) and a lower co-morbidity index (RDCI, OR = 0.81, 95% CI: 0.68–0.97, p = 0.024) were independently associated with LDA, as was the case for the entire RA cohort (Table 3). Additionally, for this subgroup of patients, a “lower” (DAS28ESR: 3.2–4.1) MDA status (OR = 1.93, 95% CI: 1.27–2.94, p = 0.002) at first evaluation was a positive predictor of LDA (Table 3). Similar results were obtained when patients with early disease were excluded (data not shown).
Table 3.
Univariate and multivariate analysis of factors associated with achievement of low disease activity (DAS28ESR <3.2) among patients with moderate disease activity (n = 493).
| Variable | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | p | 95% CI | p | ||||||
| n | OR | Lower | Upper | OR | Lower | Upper | |||
| Sex, male | 493 | 2.15 | 1.32 | 3.51 | 0.002 | 2.13 | 1.30 | 3.99 | 0.004 |
| Age | 493 | 0.99 | 0.98 | 1.01 | 0.98 | 1.002 | 0.98 | 1.02 | 0.81 |
| Working status, <67 years | 444 | 1.59 | 1.02 | 2.48 | 0.041 | ||||
| bDMARD use | |||||||||
| 1st line versus no bDMARD | 493 | 0.86 | 0.55 | 1.34 | 0.50 | ||||
| ⩾2nd line versus no bDMARD | 0.60 | 0.38 | 0.93 | 0.22 | |||||
| HAQ score at first evaluation | 433 | 0.7 | 0.49 | 1.001 | 0.051 | ||||
| Co-morbidity index, RDCI | 493 | 0.87 | 0.75 | 1.009 | 0.065 | 0.81 | 0.68 | 0.97 | 0.024 |
| History of serious infection | 493 | 0.49 | 0.27 | 0.89 | 0.018 | ||||
| “Lower” versus “Higher” MDA status | 493 | 2.03 | 1.40 | 2.95 | <0.001 | 1.93 | 1.27 | 2.94 | 0.002 |
Number of patients included in the final model = 407. Variables with statistically significant differences (p < 0.05) between groups by multivariate analysis are shown in bold. “Higher” moderate disease activity (MDA) status = DAS28ESR, 4.2–5.1; “Lower” MDA status = DAS28ESR: 3.2–4.1.
bDMARD, biologic disease-modifying anti-rheumatic drug; CI, confidence interval; DAS28, Disease Activity Score using 28 joints; ESR, erythrocyte sedimentation rate; HAQ, Health Assessment Questionnaire; OR, odds ratio; RDCI, Rheumatic Disease Comorbidity Index.
Overall, “lower” MDA patients had a higher chance of achieving LDA (51%) compared with the “higher” MDA ones (34%, p = 0.0002; Figure 3) while the rates of worsening to HDA were similar between the two groups (8% and 10%, respectively, p = 0.51).
Figure 3.
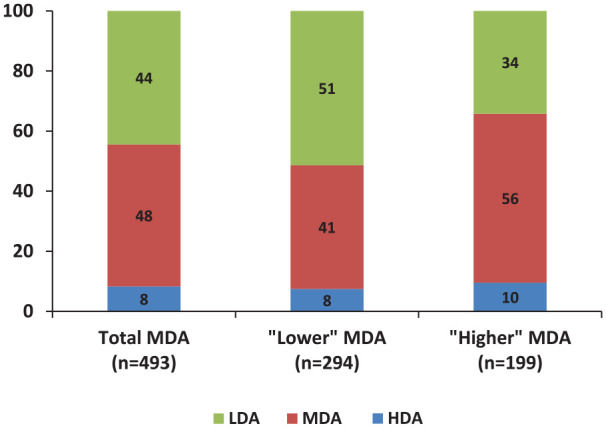
Outcomes among patients with moderate disease activity (MDA; n = 493).
The clinical outcomes of patients with MDA (DAS28ESR: ⩾3.2 to <5.1) as a total group (n = 493) and those with “lower” (DAS28ESR: 3.2–4.1, n = 294) or “higher” (DAS28ESR: 4.2–5.1, n = 199) MDA status during the 1 year follow-up are shown.
DAS28, Disease Activity Score using 28 joints; ESR, erythrocyte sedimentation rate
bDMARD pattern use
Current treatment Guidelines and Recommendations support bDMARD initiation (among csDMARD users) or switching (in bDMARD users) in RA patients who do not achieve their treatment targets.3,8,9 Overall, the rate of bDMARD initiation or switching among csDMARD and bDMARD users was 14% (91/652) and 16% (98/610), respectively (Figure 4). More specifically among patients with active disease (DAS28ESR >3.2), the respective rates were 21% (62/292) and 22% (68/315). These rates were higher among HDA patients compared with those with MDA (bDMARD initiation: 39% versus 17%, p = 0.0005, bDMARD switching: 36% versus 17%, p = 0.0008).
Figure 4.
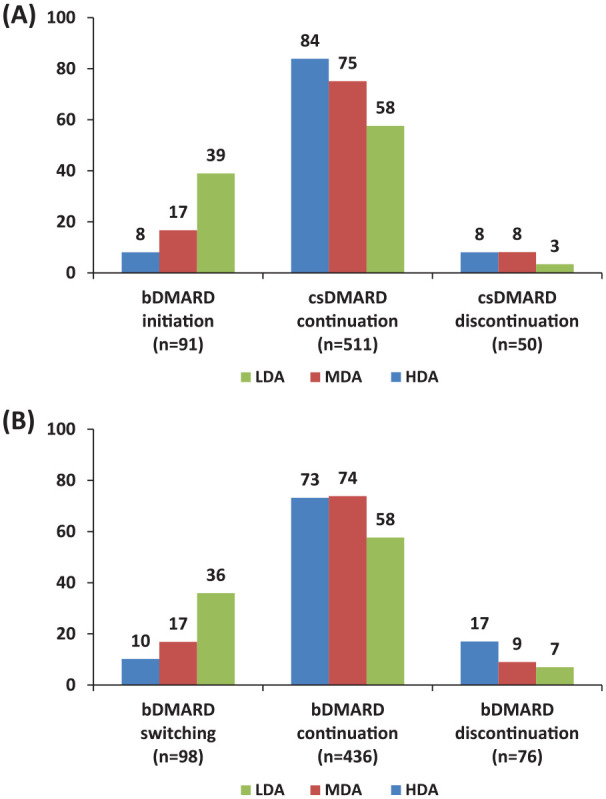
(A) Changes in DMARD use among csDMARD (n = 652) or (B) bDMARD users (n = 610).
LDA, low disease activity: DAS28ESR <3.2; MDA, moderate disease activity: DAS28ESR ⩾3.2 to <5.1; HDA, high disease activity: DAS28ESR ⩾5.1.
We finally studied the bDMARD pattern use according to disease duration. Among patients who were on csDMARDs at first evaluation, we did not notice any difference in the rates of bDMARD initiation between those with and those without early disease (defined as ⩽2 years), neither in the total cohort of csDMARD users (12.5% versus 12.4%, p = 0.98) nor in those csDMARD users not in remission (11.1% versus 9.6%, p = 0.3). In general, those with active disease who started bDMARDs were younger compared with those who did not (61.7 ± 10.7 versus 64.9 ± 12 years, p = 0.055).
When we performed the same analysis in bDMARD users, a statistically significant difference in the respective rates of bDMARD switching was found both in the total bDMARD cohort (34.8% versus 17.4%, p = 0.03) and those not in remission (43.8% versus 23.4%, p = 0.049).
Discussion
The primary goals of our prospective RA cohort study were two-fold: first to assess the rate of achievement of current treatment targets (LDA or remission) and second to track changes in treatment patterns (mainly initiation or switching of a bDMARD) in real-life settings. In contrast to previous inception or early RA cohorts, most of our patients had long standing (mean duration: ~10 years) disease with frequent presence of co-morbidities, treated both with csDMARDs and bDMARDs (46%). This context is important, since older studies have described not only gene expression differences in peripheral blood mononuclear cells and synovial tissue between early and longstanding RA,16,17 but also attenuated responses to anti-rheumatic therapies.18
Regarding our first goal, at our initial cross-sectional evaluation more than half of our patients (52%) had already achieved the treatment targets of LDA/remission. Although this is an important achievement, it should be noted that in a large cohort of real-life RA patients (mainly from referral centers) where bDMARDs were used in 46% of cases, the rate of residual active disease (48%) remains high. This rate is somewhat lower from what has been recently reported from other RA registries worldwide (range: 58–76%).19 For example, in the US CORRONA registry among 24,176 RA patients (with 38% bDMARD use), 58% of patients had still active disease (DAS28ESR ⩾3.2).20
One year later, a small but statistically significant increase in the proportion of patients who achieved LDA was noted (57%; +5%). During this period, the overall rate of bDMARD initiation (among csDMARD users) or switching (among bDMARD users) was rather low at 14% and 16%, respectively. However, these low annual rates of bDMARD initiation or switching in real life prevalent RA cohorts are close to the ones reported recently from US insurance databases (11% and 17%, respectively).21 Even among patients with active disease (DAS28ESR ⩾3.2), only one out of five RA patients started or switched their bDMARD. Although it is expected that in prevalent patients with long-standing RA, such therapy changes may be less frequent than in newly diagnosed incident cases, nevertheless our findings emphasize that a significant proportion of RA patients (~43%) in real life fail to achieve the recommended treatment target and thus a more aggressive treatment approach is needed.
A number of reasons for not complying with T2T strategies have been proposed. For example, rheumatologists in the CORRONA registry were more reluctant to initiate bDMARDs in older patients22 as was the case in our study. Other factors, such as patients’ preferences, concerns for potential treatment-related complications or patient–physician discordances in disease activity assessment23 could also play a role.
In the entire RA cohort, we identified, by multivariate analysis, male sex as a positive and older age, obesity (BMI >30 kg/m2), high HAQ and co-morbidity burden as well as use of GCs or ⩾2 bDMARDs as negative predictors of low DAS28 1 year later.
Whether or not gender is an important factor in determining treatment responses in RA is currently unclear.24–26 Recently, data from the British Society for Rheumatology Biologics Register–RA, have shown that female gender was a negative predictor of sustained remission and LDA in anti-tumor necrosis factor (TNF) treated RA patients.27 Couderc et al. showed higher rates of remission after rituximab therapy in men but only in the setting of previous anti-TNF failure,24 whereas in the Orencia and Rheumatoid Arthritis registry, there was no difference in response to abatacept between men and women.25 However, DAS28, tender joint count and patient global assessment were consistently lower in men during follow-up.25 In a study from Italy that included RA patients treated with 1st line anti-TNFs, male gender was associated with higher odds of remission/LDA after 2 years,28 while similar findings were reported in a recent meta-analysis.29 Although previous studies had attributed these discrepancies to subjective rather than objective components of disease activity metrology and not to RA itself,26 our data suggest that gender may have an impact in treatment responses in RA patients.
In our RA cohort, we found also that obesity, which was a common co-morbidity (24.6%), represented a negative predictor of LDA at 1 year of follow-up (OR = 0.6). These findings are in accordance with two previous meta-analyses30,31 and recent UK studies,27,32 showing that obese RA patients are less likely to achieve remission compared with non-obese or normal weight patients. It remains though unclear at the moment whether there is true association between obesity and inadequate treatment responses or whether obesity could bias disease activity measurements such as DAS28.33 In a recent post-hoc analysis of two randomized controlled trials (RCTs) of RA patients treated with golimumab, George et al. showed that although obese RA patients had a lower chance of achieving DAS28 remission compared with non-obese, their likelihood to reach low synovitis and inflammation scores, as measured subjectively by MRI, was similar between the two groups.34 Certainly more prospective long-term data with objective and subjective measurements of disease activity are needed before a final conclusion can be made.
Co-morbidities are common in RA patients worldwide15,35–37 and impact significantly upon treatment decisions and disease outcomes in real-life settings,28,38–41 since in RCTs such patients are usually excluded. In our RA cohort, co-morbidities were also very common (63%) and their presence decreased the chance of reaching LDA. This finding emphasizes the difficulties that physicians are facing in making treatment decisions and achieving treatment targets in daily clinical practice.
As for the role of treatment on disease activity control, we found that GC users were less likely to achieve LDA. Indeed, several studies have shown that although GCs may contribute to more rapid disease control at therapy initiation,42 their efficacy in maintaining remission is questionable.43 We also showed that patients being treated with second or higher line of biologics were also less likely to achieve LDA. This could be due to either physicians’ reluctance to switch bDMARDs in patients that have already failed at least one bDMARD or the presence of resistant, long-standing disease.
A novel finding of our study has to do with the analysis of predictors of response in patients with MDA (DAS28ESR = 3.2–5.1). This patient subgroup represents probably the most common RA subgroup in real-life settings with a recent review of five RA registries worldwide estimating its frequency at ~40% (range: 25–53%).19 Previous studies in patients with early RA have shown that persistent MDA is associated with worse clinical, functional and radiological outcomes compared to patients with LDA,10–12 while a recent RCT of MDA patients who had not responded to methotrexate showed that combination therapy of a csDMARD (methotrexate) with a bDMARD (etanercept) was more efficacious in inducing and maintaining LDA compared with csDMARD (methotrexate) monotherapy.44 These data indicate that aggressive therapy is efficacious for this group of patients.
Nevertheless, real-life, longitudinal data for MDA patients with established RA are limited. In our cohort, 37% of patients had MDA and 1 year later, 44% of them had achieved LDA/remission. By multivariate analysis, as for the whole RA cohort, male sex and a lower co-morbidity burden were independently associated with a good clinical outcome at 1 year.
In addition, for this group of patients a “lower” MDA status (DAS28ESR = 3.2–4.1) at first evaluation was another independent factor associated with transition to LDA. A recent analysis of 1274 csDMARD-treated RA patients with MDA from the British Registry, showed that seven different trajectory groups according to disability score (HAQ) could be distinguished which, during a 3 year follow-up period, remained rather stable.45 Our data, in accordance to these findings, may indicate the existence of distinct patient subgroups with a different clinical outcome within the MDA group. Whether or not this “lower” disease activity subgroup has a more benign course, responding better to DMARDs, is currently uncertain.
On the other hand, it should be mentioned that half of patients with “lower” MDA status do not achieve LDA after 1 year of follow-up. This could be due to the fact that physicians choose a more conservative approach and are reluctant to intensify therapy by initiating or switching bDMARDs (17% overall rate) in these patients. Well-designed prospective studies are needed in order to define the natural course and response to therapy of these MDA subgroups, before any change in existing treatment recommendations is made.
Our study has certain strengths and limitations. Its strengths include the high number of included patients, its prospective and multicenter nature, the inclusion of patients with established disease who were being treated with cs- and/or bDMARDs in a real-life setting and the specific focus on the MDA patient subgroup.
Regarding its limitations, loss to follow-up and missing data are inarguable concerns in real-life prospective patient cohorts. In our case, follow-up evaluation was not available in 38% of patients. Although undesirable, these loss to follow-up rates are not infrequent in real-life observational studies of patients not only with rheumatic diseases (24–35%),46 but also in other settings, such as post-operatively after spine surgery (59%).47 Moreover, 15% of patients were excluded from final analysis due to missing disease activity data, a percentage similar to or even lower from other RA registries.36,48 Their characteristics, though, did not differ significantly from those who were analyzed. Another issue was the absence of a specific therapeutic protocol followed by all centers with the potential of heterogeneity in treatment decisions. This is the issue, though, in most RA registries worldwide, while it should be noted that since 2009 the Greek Rheumatology Society has been regularly issuing updated recommendations for RA management and its therapeutic algorithm has been implemented in the obligatory electronic prescription of anti-rheumatic therapies over the last 6 years. Last, since most of our patients had been recruited from hospital referral centers, this could have created a “referral bias” with inclusion of the most severe, difficult to treat cases with more frequent use of bDMARDs compared with the general RA patient population.
In conclusion, our prospective, real-life RA study clearly shows that 20 years after the introduction and widespread implementation of biologics in clinical practice, a significant proportion of RA patients with established disease (~40%) do not achieve the predefined treatment targets of LDA or remission. In contrast to early, incident RA cohorts, the annual rates of bDMARD initiation or switching among RA patients with active disease were rather low (~20%), emphasizing the need for better compliance with existing treatment Recommendations and Guidelines. Furthermore, we observed that approximately one-third of patients remain in moderately active disease despite therapy with cs- and/or bDMARDs, while within this group, a subgroup of patients at the lower end of disease activity (DAS28ESR = 3.2–4.1) had a higher likelihood of reaching low DAS28.
Overall, our findings demonstrate that there are still a number of unmet therapeutic needs in real-life settings while at the same time they indicate that MDA subgroups according to their disease activity level deserve to be studied further.
Supplemental Material
Supplemental material, TAB937132_Supplemental_Material_CLN for Treatment patterns and achievement of the treat-to-target goals in a real-life rheumatoid arthritis patient cohort: data from 1317 patients by Konstantinos Thomas, Argiro Lazarini, Evripidis Kaltsonoudis, Alexandros Drosos, Ioannis Papalopoulos, Prodromos Sidiropoulos, Panagiota Tsatsani, Sousana Gazi, Lina Pantazi, Kyriaki A. Boki, Pelagia Katsimbri, Dimitrios Boumpas, Kalliopi Fragkiadaki, Maria Tektonidou, Petros P. Sfikakis, Konstantina Karagianni, Lazaros I. Sakkas, Eleftheria P. Grika, Panagiotis G. Vlachoyiannopoulos, Gerasimos Evangelatos, Alexios Iliopoulos, Theodoros Dimitroulas, Alexandros Garyfallos, Konstantinos Melissaropoulos, Panagiotis Georgiou, Maria Areti, Constantinos Georganas, Periklis Vounotrypidis, George D. Kitas and Dimitrios Vassilopoulos in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We would like to thank Prof. Katerina Chatzidionysiou for her constructive comments and suggestions.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Greek Rheumatology Society and the Professional Association of Greek Rheumatologists and by the Special Account for Research Grants (S.A.R.G.), National and Kapodistrian University of Athens, Athens, Greece (DV, Grants #12085, 12881).
ORCID iDs: Konstantinos Thomas  https://orcid.org/0000-0002-7489-7776
https://orcid.org/0000-0002-7489-7776
Maria Tektonidou  https://orcid.org/0000-0003-2238-0975
https://orcid.org/0000-0003-2238-0975
Dimitrios Vassilopoulos  https://orcid.org/0000-0003-2288-3863
https://orcid.org/0000-0003-2288-3863
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Konstantinos Thomas, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Argiro Lazarini, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Evripidis Kaltsonoudis, Rheumatology Clinic, University of Ioannina, Ioannina, Greece.
Alexandros Drosos, Rheumatology Clinic, University of Ioannina, Ioannina, Greece.
Ioannis Papalopoulos, Clinical Immunology and Allergy Department, University of Crete, Heraklion, Greece.
Prodromos Sidiropoulos, Clinical Immunology and Allergy Department, University of Crete, Heraklion, Greece.
Panagiota Tsatsani, Rheumatology Unit, KAT Hospital, Athens, Greece.
Sousana Gazi, Rheumatology Unit, KAT Hospital, Athens, Greece.
Lina Pantazi, Rheumatology Unit, Sismanoglio Hospital, Athens, Greece.
Kyriaki A. Boki, Rheumatology Unit, Sismanoglio Hospital, Athens, Greece
Pelagia Katsimbri, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Dimitrios Boumpas, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Kalliopi Fragkiadaki, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Maria Tektonidou, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece.
Petros P. Sfikakis, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Konstantina Karagianni, Department of Rheumatology, University of Thessaly, Larissa, Greece.
Lazaros I. Sakkas, Department of Rheumatology, University of Thessaly, Larissa, Greece
Eleftheria P. Grika, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Panagiotis G. Vlachoyiannopoulos, Joint Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Athens, Greece
Gerasimos Evangelatos, Rheumatology Unit, NIMTS Hospital, Athens, Greece.
Alexios Iliopoulos, Rheumatology Unit, NIMTS Hospital, Athens, Greece.
Theodoros Dimitroulas, 4th Department of Medicine, Aristotle University, Thessaloniki, Greece.
Alexandros Garyfallos, 4th Department of Medicine, Aristotle University, Thessaloniki, Greece.
Konstantinos Melissaropoulos, Rheumatology Unit, Agios Andreas Hospital, Patras, Greece.
Panagiotis Georgiou, Rheumatology Unit, Agios Andreas Hospital, Patras, Greece.
Maria Areti, Private Practice, Greece.
Constantinos Georganas, Private Practice, Greece.
Periklis Vounotrypidis, Private Practice, Greece.
George D. Kitas, Hygeia Hospital, Athens, Greece
Dimitrios Vassilopoulos, Joint Rheumatology Program, Clinical Immunology-Rheumatology Unit, 2nd Department of Medicine and Laboratory, National and Kapodistrian University of Athens, School of Medicine, Hippokration General Hospital, 114 Vass. Sophias Avenue, Athens, 115 27, Greece.
References
- 1. Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28</=3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis 2017; 76: 1693–1699. [DOI] [PubMed] [Google Scholar]
- 2. Conigliaro P, Triggianese P, De ME, et al. Challenges in the treatment of rheumatoid arthritis. Autoimmun Rev 2019; 18: 706–713. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biggioggero M, Mesina F, Favalli EG. The use of rheumatic disease comorbidity index for predicting clinical response and retention rate in a cohort of rheumatoid arthritis patients receiving tumor necrosis factor alpha inhibitors. Biomed Res Int 2019; 2019: 6107217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince FH, Bykerk VP, Shadick NA, et al. Sustained rheumatoid arthritis remission is uncommon in clinical practice. Arthritis Res Ther 2012; 14: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayakumar K, Norton S, Dixey J, et al. Sustained clinical remission in rheumatoid arthritis: prevalence and prognostic factors in an inception cohort of patients treated with conventional DMARDS. Rheumatology (Oxford) 2012; 51: 169–175. [DOI] [PubMed] [Google Scholar]
- 7. van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum 2009; 60: 2262–2271. [DOI] [PubMed] [Google Scholar]
- 8. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 9. Singh JA, Saag KG, Bridges SL, Jr, et al. 2015. American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016; 68: 1–26. [DOI] [PubMed] [Google Scholar]
- 10. Combe B, Logeart I, Belkacemi MC, et al. Comparison of the long-term outcome for patients with rheumatoid arthritis with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann Rheum Dis 2015; 74: 724–729. [DOI] [PubMed] [Google Scholar]
- 11. Kiely P, Walsh D, Williams R, et al. Outcome in rheumatoid arthritis patients with continued conventional therapy for moderate disease activity—the early RA network (ERAN). Rheumatology (Oxford) 2011; 50: 926–931. [DOI] [PubMed] [Google Scholar]
- 12. Conaghan PG, Hensor EM, Keenan AM, et al. Persistently moderate DAS-28 is not benign: loss of function occurs in early RA despite step-up DMARD therapy. Rheumatology (Oxford) 2010; 49: 1894–1899. [DOI] [PubMed] [Google Scholar]
- 13. Thomas K, Lazarini A, Kaltsonoudis E, et al. Multicenter cross-sectional study of patients with rheumatoid arthritis in Greece: results from a cohort of 2.491 patients. Mediterr J Rheumatol 2018; 29: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aletaha D, Neogi T, Silman AJ, et al. 2010. rheumatoid arthritis classification criteria: an American college of rheumatology/european league against rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 15. Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007; 21: 885–906. [DOI] [PubMed] [Google Scholar]
- 16. Olsen N, Sokka T, Seehorn CL, et al. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Ann Rheum Dis 2004; 63: 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lequerre T, Bansard C, Vittecoq O, et al. Early and long-standing rheumatoid arthritis: distinct molecular signatures identified by gene-expression profiling in synovia. Arthritis Res Ther 2009; 11: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Symmons DP, Silman AJ. Aspects of early arthritis. What determines the evolution of early undifferentiated arthritis and rheumatoid arthritis? An update from the Norfolk arthritis register. Arthritis Res Ther 2006; 8: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamanaka H, Askling J, Berglind N, et al. Infection rates in patients from five rheumatoid arthritis (RA) registries: contextualising an RA clinical trial programme. RMD Open 2017; 3: e000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michaud K, Berglind N, Franzen S, et al. Can rheumatoid arthritis (RA) registries provide contextual safety data for modern RA clinical trials? The case for mortality and cardiovascular disease. Ann Rheum Dis 2016; 75: 1797–1805. [DOI] [PubMed] [Google Scholar]
- 21. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm 2017; 23: 809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ranganath VK, Maranian P, Elashoff DA, et al. Comorbidities are associated with poorer outcomes in community patients with rheumatoid arthritis. Rheumatology (Oxford) 2013; 52: 1809–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Challa DN, Kvrgic Z, Cheville AL, et al. Patient-provider discordance between global assessments of disease activity in rheumatoid arthritis: a comprehensive clinical evaluation. Arthritis Res Ther 2017; 19: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Couderc M, Gottenberg JE, Mariette X, et al. Influence of gender on response to rituximab in patients with rheumatoid arthritis: results from the autoimmunity and rituximab registry. Rheumatology (Oxford) 2014; 53: 1788–1793. [DOI] [PubMed] [Google Scholar]
- 25. Nourisson C, Soubrier M, Mulliez A, et al. Impact of gender on the response and tolerance to abatacept in patients with rheumatoid arthritis: results from the ‘ORA’ registry. RMD Open 2017; 3: e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 2009; 11: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamann PDH, Pauling JD, McHugh N, et al. Predictors, demographics and frequency of sustained remission and low disease activity in anti-tumour necrosis factor-treated rheumatoid arthritis patients. Rheumatology (Oxford) 2019; 58: 2162–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conigliaro P, Triggianese P, Chimenti MS, et al. Factors Predicting 2 Years of Remission and Low Disease Activity in Rheumatoid Arthritis Patients Treated with TNF-inhibitors. Isr Med Assoc J 2017; 19: 467–472. [PubMed] [Google Scholar]
- 29. Yu C, Jin S, Wang Y, et al. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol 2019; 38: 727–738. [DOI] [PubMed] [Google Scholar]
- 30. Lupoli R, Pizzicato P, Scalera A, et al. Impact of body weight on the achievement of minimal disease activity in patients with rheumatic diseases: a systematic review and meta-analysis. Arthritis Res Ther 2016; 18: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Hazlewood GS, Kaplan GG, et al. Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2017; 69: 157–165. [DOI] [PubMed] [Google Scholar]
- 32. Nikiphorou E, Norton S, Young A, et al. The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: data from the early rheumatoid arthritis study/early rheumatoid arthritis network UK prospective cohorts. Rheumatology (Oxford) 2018; 57: 1194–1202. [DOI] [PubMed] [Google Scholar]
- 33. Finckh A, Turesson C. The impact of obesity on the development and progression of rheumatoid arthritis. Ann Rheum Dis 2014; 73: 1911–1913. [DOI] [PubMed] [Google Scholar]
- 34. George MD, Ostergaard M, Conaghan PG, et al. Obesity and rates of clinical remission and low MRI inflammation in rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1743–1746. [DOI] [PubMed] [Google Scholar]
- 35. Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014; 73: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radner H, Dixon W, Hyrich K, et al. Consistency and utility of data items across European rheumatoid arthritis clinical cohorts and registers. Arthritis Care Res (Hoboken) 2015; 67: 1219–1229. [DOI] [PubMed] [Google Scholar]
- 37. Dougados M. Comorbidities in rheumatoid arthritis. Curr Opin Rheumatol 2016; 28: 282–288. [DOI] [PubMed] [Google Scholar]
- 38. Radner H, Yoshida K, Smolen JS, et al. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol 2014; 10: 252–256. [DOI] [PubMed] [Google Scholar]
- 39. Radner H, Yoshida K, Frits M, et al. The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2015; 54: 2076–2084. [DOI] [PubMed] [Google Scholar]
- 40. Conti F, Atzeni F, Massaro L, et al. The influence of comorbidities on the efficacy of tumour necrosis factor inhibitors, and the effect of tumour necrosis factor inhibitors on comorbidities in rheumatoid arthritis: report from a National Consensus Conference. Rheumatology (Oxford) 2018; 57: vii11–vii22. [DOI] [PubMed] [Google Scholar]
- 41. Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 2016; 75: 965–973. [DOI] [PubMed] [Google Scholar]
- 42. Hua C, Buttgereit F, Combe B. Glucocorticoids in rheumatoid arthritis: current status and future studies. RMD Open 2020; 6: e000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haraoui B, Jovaisas A, Bensen WG, et al. Use of corticosteroids in patients with rheumatoid arthritis treated with infliximab: treatment implications based on a real-world Canadian population. RMD Open 2015; 1: e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013; 381: 918–929. [DOI] [PubMed] [Google Scholar]
- 45. Pan Y, Norton S, Gwinnutt JM, et al. Not all moderate disease is the same - Identification of disability trajectories among patients with rheumatoid arthritis and moderate disease activity. PLoS One 2019; 14: e0215999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tien YC, Chiu YM, Liu MP. Frequency of lost to follow-up and associated factors for patients with rheumatic diseases. PLoS One 2016; 11: e0150816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schroder ML, de Wispelaere MP, Staartjes VE. Predictors of loss of follow-up in a prospective registry: which patients drop out 12 months after lumbar spine surgery? Spine J 2019; 19: 1672–1679. [DOI] [PubMed] [Google Scholar]
- 48. Verstappen SM, Askling J, Berglind N, et al. Methodological challenges when comparing demographic and clinical characteristics of international observational registries. Arthritis Care Res (Hoboken) 2015; 67: 1637–1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAB937132_Supplemental_Material_CLN for Treatment patterns and achievement of the treat-to-target goals in a real-life rheumatoid arthritis patient cohort: data from 1317 patients by Konstantinos Thomas, Argiro Lazarini, Evripidis Kaltsonoudis, Alexandros Drosos, Ioannis Papalopoulos, Prodromos Sidiropoulos, Panagiota Tsatsani, Sousana Gazi, Lina Pantazi, Kyriaki A. Boki, Pelagia Katsimbri, Dimitrios Boumpas, Kalliopi Fragkiadaki, Maria Tektonidou, Petros P. Sfikakis, Konstantina Karagianni, Lazaros I. Sakkas, Eleftheria P. Grika, Panagiotis G. Vlachoyiannopoulos, Gerasimos Evangelatos, Alexios Iliopoulos, Theodoros Dimitroulas, Alexandros Garyfallos, Konstantinos Melissaropoulos, Panagiotis Georgiou, Maria Areti, Constantinos Georganas, Periklis Vounotrypidis, George D. Kitas and Dimitrios Vassilopoulos in Therapeutic Advances in Musculoskeletal Disease