Abstract
The detection of cutaneous phosphorylated alpha-synuclein (P-syn) in patients with Parkinson’s disease (PD) has ranged from 30% to 100% across different studies. We hypothesize that part of the variability in P-syn detection is due to methodological differences using sections of different tissue thickness. Three skin biopsies were obtained from 29 individuals with PD and 21 controls. Tissues were cut into 10-, 20-, and 50-µm-thick sections and double-stained with protein gene product (PGP) 9.5 and P-syn. We quantified the deposition of P-syn with and without PGP 9.5 in sweat glands, pilomotor muscle, and blood vessels using confocal digital images of autonomic structures. Overall, the P-syn-positive rates with PGP 9.5 colocalization in subjects with PD were 100% using 50 µm sections, 90% using 20 µm sections, and 73% using 10 µm sections with 100% specificity. (No P-syn was detected within control subjects.) Without PGP 9.5, colocalization of the P-syn-positive rates was 100% for all samples, but specificity dropped below 70%. In this study, double-immunostained 50 µm skin biopsy tissue sections are superior to 20 and 10 µm tissue sections at detecting P-syn in subjects with PD. The increased sensitivity is likely secondary to a combination of greater volume of tissue analyzed and improved visualization of nerve fiber architecture.
Keywords: autonomic nerve system, Parkinson’s disease, phosphorylated alpha-synuclein-s129, skin biopsy
Introduction
Phosphorylated alpha-synuclein S129 (P-syn) is an insoluble protein aggregate of the oligomeric form of α-synuclein and is one of the key proteins in the pathogenesis of neurodegenerative “synucleinopathies,” including Parkinson’s disease (PD), multiple system atrophy, Lewy body dementia, and pure autonomic failure.1–3 Alpha-synuclein pathology is closely associated with nerve degeneration.4,5 Synuclein pathology has traditionally been restricted to detection within the central nervous system, limiting the diagnostic utility of this protein in vivo. Recent dermatopathological advances have enabled detection of P-syn from within cutaneous autonomic nerve fibers obtained by simple skin punch biopsies.6–9 However, the rates of detecting P-syn from skin biopsies of patients with PD have ranged widely from 30% to 100% across different studies.8–14 The significant differences in detection of P-syn between publications highlight the potential impact of methodological variations on outcomes.
In the review of published studies, skin biopsies of various thicknesses ranging from 5 to 50 µm are included across different publications of P-syn.7,10,14–17 P-syn is frequently detected within autonomic substructures in the skin, such as sweat glands or piloerector muscles.18,19 The autonomic substructures are not always detected within skin tissue samples, and because of the increased likelihood of P-syn detection within these structures, we hypothesized that thicker tissue sections would increase the detection rate of P-syn within skin punch biopsies compared with thinner tissue sections.
The aim of this study was to compare P-syn detection rates across tissue sections of different thickness to successfully define the optimal method for analysis.
Materials and Methods
Subjects with a known diagnosis of PD confirmed by a movement disorder specialist were recruited from the neurology practices at Beth Israel Deaconess Medical Center. Healthy control subjects, with no known cardiac, neurodegenerative, or dermatological medical conditions, of similar age and gender distribution were recruited from our local referral patterns. All subjects signed an informed consent approved by the institutional review board. All study subjects underwent detailed history and neurological examination, including Movement Disorder Society Unified Parkinson Disease Rating Scale (MDS-UPDRS) scoring.20
Skin punch biopsies 3 mm in diameter and 3 to 4 mm in depth were obtained from the lateral distal leg, distal thigh, and proximal thigh after local anesthesia with 2% lidocaine. Skin biopsy specimens were fixed in Zamboni solution (2% paraformaldehyde-lysine-periodate) for 18 hr and cryoprotected overnight (20% glycerol and 20% 0.4 M Sorensen buffer). The skin biopsy was cut with a cryostat (Leica CM 3050 S; Leica Microsystem, Nußloch, Germany) at −25C into 10, 20, and 50 μm sections to include both epidermal and deeper dermal tissue. The 10- and 20-μm sections were collected and subsequently mounted on microscope slides (SuperFrost plus; Thermo Scientific, Waltham, MA) previously coated with an adhesive (DAKO No. S 2024). They were air-dried and subsequently stored at –80C. The 50-μm sections were subsequently stored in 96-well plates with antifreeze solution (30% glycerol, 30% ethylene glycol, 30% ddH20, and 10% PBS)21 at −20C. Five tissue sections of each thickness (10, 20, and 50 µm) from each skin biopsy were analyzed.
Immunohistochemistry Protocol
To improve antigen retrieval, we compared several methods for each tissue thickness to determine the optimal approach. Skin sections were treated in low concentrations of proteinase K (5, 10, or 20 µg/ml in TE buffer containing 50 mM Tris base, 1 mM EDTA, 0.5% Triton X-100, pH 8.0, and incubated at room temperature for 1, 5, and 10 min, respectively). Proteinase K treatment damaged the small nerve fibers within the skin at very low concentrations, so it was determined not to be a viable solution for antigen retrieval. Slide-mounted thin sections and free-floating thick sections were placed in citrate buffer (10 mM, pH 6.4) and microwaved at 800 W for 5 min (95–100C). The free-floating 50-µm-thick sections were severely damaged by microwave heating, but slide-mounted sections had excellent antigen retrieval on testing. Free-floating thick sections and slide-mounted sections were treated with formic acid (30%) as an alternative approach; this approach worked with free-floating sections but was not as effective for slide-mounted sections (compared with the microwave technique). All antigen retrieval methods were compared using full immunohistochemical staining protocols and compared with the same tissue thickness from the same patient samples. The optimal antigen retrieval method was selected based on quality review of dermal nerve fibers (stained by PGP9.5) within pilomotor, sudomotor, and vasomotor structures based on nerve fiber continuity, visibility, tissue disruption, and ease of quantification. The optimal antigen retrieval protocol for the 50-μm sections was treatment with formic acid (30% in ddH2O) for 30 min at room temperature and washing with TBS 3× for 10 min at room temperature. The optimal antigen retrieval method for the slide-mounted 10- and 20-μm sections was placement in citrate buffer (10 mM, pH 6.4), followed by microwave (800 W, 95–100C for 5 min), cooling, and washing with TBS (50 mM, pH 7.4, with 2% Triton X-100) 3× for 10 min at room temperature.
In an initial series of tests, we examined the following antibodies against P-syn: Abcam (rabbit, monoclonal [#51253] and polyclonal [#59264]; mouse monoclonal [#168381]), Wako (mouse, monoclonal [#015-25191]), Chemicon (rabbit, polyclonal [#AB9850]), Prothena (mouse, monoclonal [#11A5]), and Covance (mouse, monoclonal [#MMS-5091]). We determined that antibodies from Abcam, Chemicon, Prothena, Wako, and Covance worked, but that the Covance antibody provided the most reliable and repeatable data and the best visual contrast in the detection of P-syn (unpublished data).
After antigen retrieval, fluorescent immunostaining was performed using our previously published technique for covisualizing total nerve fibers.22 We used the pan-axonal marker PGP (PGP 9.5, rabbit polyclonal; RA95101, 1:10,000; UltraClone, Wellow, UK) and anti-P-syn (P-syn, mouse monoclonal; MMS-5091, 1:500; Covance, Princeton, NJ). Briefly, the slide-mounted thin sections of 10 and 20 μm were stained in an incubation chamber, and the free-floating 50-μm-thick sections were processed in 96-well plates on horizontal shaker at a speed of 50 cycles/min at room temperature. The tissues were incubated in block solution (5% bovine serum albumin, 5% normal goat serum, and 2% Triton X-100 in 50 mM TBS) for 2 hr at room temperature. Tissue sections were placed in primary antibody solution for 24 hr, at room temperature, followed by incubation in solution containing biotin-conjugated anti-mouse (715-065-151, 1:500; Jackson ImmunoResearch Lab, West Grove, PA) for 2 hr at room temperature and then visualized with streptavidin-conjugated fluorescence dye Cy3 (016-160-084, 1:500; Jackson ImmunoResearch Lab). After washing with TBS, the same tissue sections were incubated with rabbit anti-PGP in primary antibody solution for 12 hr at room temperature and then visualized with anti-rabbit conjugated fluorescence dye Cy2 (711-226-152, 1:200; Jackson ImmunoResearch Lab) for 2 hr at room temperature. All the secondary antibodies were incubated in TBS with 5% normal serum. All the slides and sections were washed with TBS 3× for 10 min after each incubation. Negative controls using no primary P-syn antibody to detect potential background and artifacts were included in the protocol. After staining, all floating stained 50 µm sections were mounted on slides and air-dried, and all slides were covered with an aqueous mounting medium (P36974; ThermoFisher Scientific, Waltham, MA).
Confocal Imaging
All stained sections were initially examined under a fluorescent microscope (Zeiss-Axioplan2; Carl Zeiss Microscopy, Jena, Germany), with areas of interest imaged by confocal microscopy (Zeiss LSM5 Pascal Exciter; Carl Zeiss). A series of images of optical sections was acquired at 2 μm intervals throughout the depth of the 50-μm section as a Z-stack (Lens Plan-Apochromat 20/0.8; Carl Zeiss). All regions of interest were identified and graded by two trained experts in a blinded manner.
Identification of Positive Fibers and Reporting of Results
Slides were de-identified and all investigators were blinded to study subject diagnosis. To ensure agreement, the following criteria were applied for interpretation of P-syn results:
P-syn-positive fibers must be completely colocalized within PGP 9.5–positive fibers (i.e., areas of P-syn that did not colocalize were defined as artifact).
P-syn-positive fibers must be clearly visible with adequate signal intensity.
P-syn fibers must track within the PGP 9.5–positive fibers in and out of the plane of focus.
The images for analysis must be taken by single-frame confocal scanned image to prevent the projection of artifacts onto PGP-positive fibers.
Any areas of tissue damage (including crush artifact during the biopsy procedure or staining artifact during immune processing) were excluded.
Grading of P-syn deposition for all tissue sections was developed using the following standard: 0, no visible P-syn staining; 1, faint P-syn staining, unable to convincingly distinguish from artifact; 2, bright P-syn deposits that colocalized with PGP 9.5–positive fibers; 3, very bright and clear P-syn fibers that colocalized with PGP 9.5–positive fibers. Only P-syn staining grades achieving level 2 or 3 were considered “positive.” The number of positive results was noted for each cutaneous dermal structure for each tissue section for each biopsy. For example, if one or more P-syn-positive fibers (grades 2–3) are found to innervate as single pilomotor muscle, the pilomotor muscle is defined as a “one” P-syn-positive structure. A second, separate pilomotor muscle with one or more P-syn-positive fibers would count as a second P-syn-positive structure. We report the number of P-syn-positive structures across sweat glands, pilomotor muscles, and blood vessels found in skin biopsies. Results are reported for each of the 10-, 20-, and 50-μm tissue sections. For each biopsy, five sections at each of the three thicknesses were analyzed for a total of 15 tissue sections.
Intraepidermal nerve fiber density (IENFD) was calculated using standard methodology with 50 μm florescent immunostained nerve fibers. A total of four tissue sections from each biopsy site were counted. Results are reported for PD and control subjects as nerve fibers/millimeter.23,24
All the samples from the PD and control subjects were reviewed in a blinded fashion at a later date using only immunofluorescent imaging for P-syn (without using PGP 9.5 colocalization) to determine rates of synuclein detection in PD and control subjects using single immunohistochemical imaging. These results are reported separately from the colocalized data.
Statistics
Results are presented as the mean ratio of P-syn-positive structure to total structures found and standard deviation for each parameter (the total number of P-syn-positive structures was normalized to the number of cutaneous substructures identified). The results were assessed for normal distribution by the Kolmogorov–Smirnov test. If normally distributed, the rate of positive structures to total structures obtained with sections of different thickness across different biopsy sites was compared by multivariate analysis of variance (MANOVA) with Bonferroni corrections for multiple pairwise tests. If not normally distributed, results were assessed through Kruskal–Wallis test. Unpaired t-tests were used to compare nerve fiber densities between PD and control subjects (only 50-µm-thick sections were used for nerve fiber density calculations). A p value <0.05 was considered to be statistically significant. Sensitivity, true positive/(true positives + false negatives), and specificity, true negatives/(true negatives + false positives), were calculated by biopsy site and by disease state.
Results
Demographics
A total of 29 individuals (16 males and 13 females) with PD were included in this study. The mean age was 66.3±7.7 years. The mean duration of PD was 5.5 ± 5.1 years. The Hoehn and Yahr score was 2.5 ± 0.8 (range, 1–4) with an MDS-UPDRS motor subscore (part 3) of 29.4 ± 11.2. A total of 21 control subjects (12 males and 9 females) were included with a mean age of 62.6 ± 10.7 years.
Detection of P-syn
The double fluorescent staining using P-syn and PGP 9.5 provided clear evidence of P-syn-labeled fibers in both the thick and thin tissue sections as can be seen in Fig. 1. In tissues of all thicknesses, the P-syn-positive fibers were detected within nerves of pilomotor muscles, blood vessels, and sweat glands. P-syn was not detected within epidermal nerve fibers.
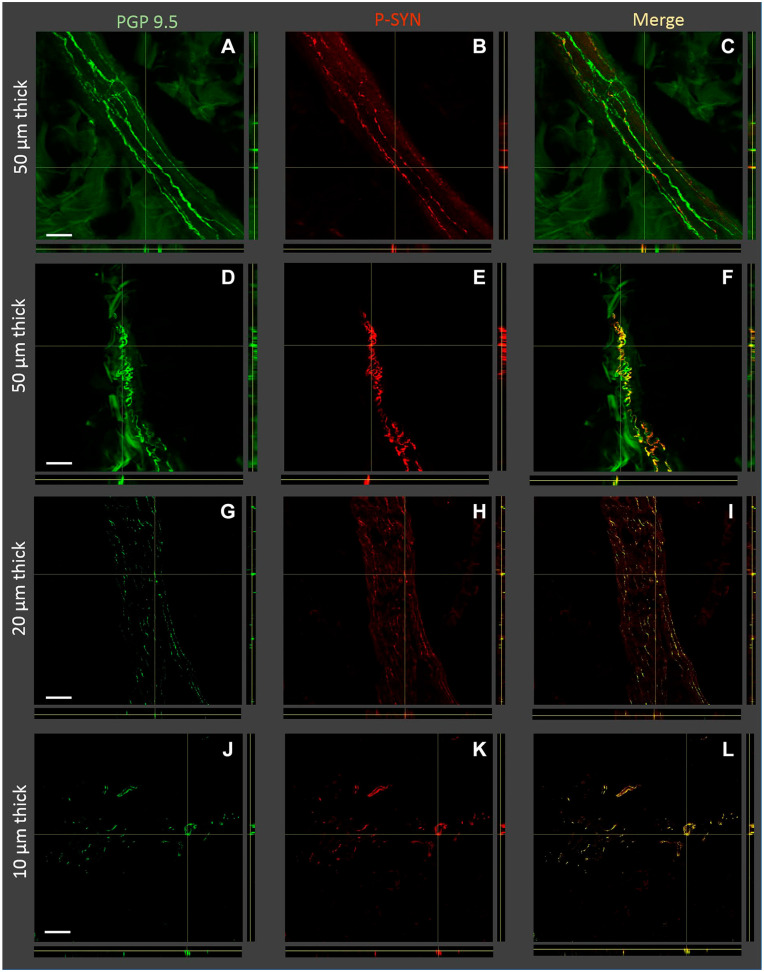
Figure 1.
Orthogonal Z-stack images of tissue sections with nerve fibers containing phosphorylated alpha-synuclein (P-syn) in individuals with Parkinson’s disease. Four examples of tissue sections are shown: two examples of 50 µm thickness, one example of 20 µm thickness, and one example of 10 µm thickness (displayed on the left Y-axis). The immunostains used for each image are shown on the top of the figure and included protein gene product 9.5 (PGP 9.5), P-syn, and the merged images. Sections A–C and G–I show pilomotor nerve fibers, and sections D–F are a nerve bundle. Sections J–L contain both pilomotor nerve fibers and nerve bundles. Thicker tissue sections tend to allow for easier identification of overlapping PGP 9.5 and P-syn. Scale bar, 100 μm.
The P-syn-positive autonomic fibers in PD subjects were identified most frequently in blood vessels, followed by pilomotor muscles and least often in sweat glands in both thick and thin sections. However, there were no differences in the rate of P-syn-positive structure (see section “Identification of Positive Fibers”) across the three biopsy locations (the distal leg, distal thigh, and proximal thigh). Subjects with PD had evidence of reduced nerve fiber densities compared with control subjects, particularly in distal biopsy sites (Table 1). Despite the distal denervation, P-syn-positive fibers were detected even in biopsies with severely reduced innervation. A summary of IENFD results is reported in Table 1, and a summary of P-syn results is reported in Table 2.
Table 1.
Demographic Information.
| PD | Control | |
|---|---|---|
| Number | 29 | 21 |
| Age | 66.3 ± 7.7 | 62 ± 10.7 |
| Gender | 16 F, 13 M | 11 F, 10 M |
| Duration of disease | 5.5 ± 5.1 years | N/A |
| MDS-UPDRS | 46 (22−69) | 0 |
| MDS-UPDRS part 3 | 29.4 ± 11.2 | 0 |
| IENFD distal leg | 4.5 ± 4.1 | 14.9 ± 3.8** |
| IENFD distal thigh | 12.4 ± 4.7 | 16.5 ± 5.7* |
| IENFD proximal thigh | 17.6 ± 6.1 | 21.3 ± 4.3* |
Abbreviations: MDS-UPDRS, Movement Disorder Society Unified Parkinson Disease Rating Scale; IENFD, intraepidermal nerve fiber density; PD, Parkinson’s disease.
p<0.05, **p<0.01.
Table 2.
The Results of P-syn in Skin Autonomic Structures in Thick and Thin Skin Sections From Subjects With PD.
| N=29 | Sweat Gland | Pili Muscle | Blood Vessel | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Thickness (µm) | 50 | 20 | 10 | 50 | 20 | 10 | 50 | 20 | 10 |
| Number of P-syn-positive PD subjects by structurea | 21 | 20 | 14 | 24 | 14 | 19 | 26 | 21 | 19 |
| Percentage of positive PD subjects by structure | 72 | 69 | 48 | 82 | 48 | 65 | 89 | 72 | 65 |
| Rate of P-synb | 0.53 ± 0.4 | 0.3 ± 0.28 | 0.25 ± 0.33 | 0.57 ± 0.38 | 0.16 ± 0.21 | 0.23 ± 0.25 | 0.68 ± 0.33 | 0.35 ± 0.32 | 0.31 ± 0.32 |
| p value (vs 50 µm) | <0.05 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |||
| Overall P-syn resultc | 100% in 50 µm, 90% in 20 µm, and 73% in 10 µm sections | ||||||||
Abbreviations: P-syn, phosphorylated alpha-synuclein; PD, Parkinson’s disease.
Number of P-syn-positive PD subjects by structure: The number of individuals with one or more positive autonomic structures (sweat gland, pili muscle, blood vessel) in any of the three biopsies.
Rate of P-syn: P-syn-positive structures divided by total structures in the three biopsies of each individual (mean and SD).
Overall P-syn result: The number of individuals who were found to have P-syn-positive autonomic structures SG, PM, or BV in any of the three skin biopsies.
The Impact of Tissue Thickness on P-syn Visualization
The quality of immunohistochemical double staining with PGP 9.5 and P-syn was similar in both thick and thin tissue sections. For those viewing slides under the microscope, P-syn-positive fibers were more easily detected in thicker sections, compared with thinner sections (Fig. 1). Thicker 50 µm tissue sections tended to preserve the continuity of P-syn within nerve fibers, allowing easier tracking of the nerve fiber through the tissue. In contrast, the P-syn within 10-µm-thick tissue sections would appear as segments of nerves or “dots” and not nerve fiber structures, thereby reducing the ease of visual identification and increasing the difficulty of distinguishing from artifact.
A summary of the overall results from the P-syn-positive fibers is shown in Table 2. The 50-µm tissue sections had greater number of P-syn-positive structures, greater number of positive biopsies, and a higher percentage of P-syn detection in patients with PD compared with 20- and 10-µm-thick sections (p<0.01). The percentage of P-syn-positive structures was significantly higher in 50-µm-thick sections than that in thin sections (p<0.01), but was similar between 20 and 10 µm sections. The overall P-syn-positive rates (the number of individuals in whom we detected P-syn-positive autonomic structures) were 100% of subjects with PD in 50 µm, 90% in 20 µm, and 73% in 10 µm sections.
P-syn Fibers and Staining Artifacts
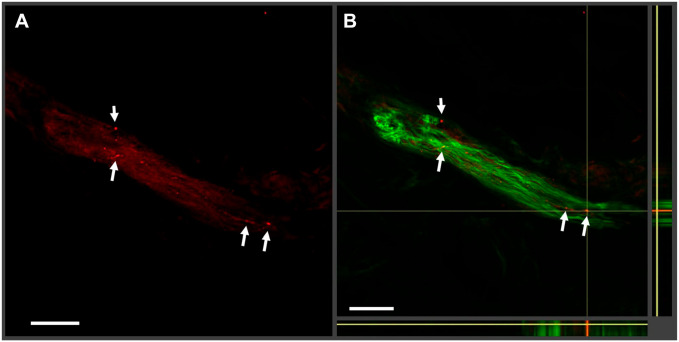
In most immunohistochemical studies, nonspecific background noise and staining artifacts are technically inescapable. In our study, low-intensity signals of short fiber-like or dot-like P-syn staining artifacts and background noise were identified in certain areas surrounding dermal autonomic substructures in sections that were not colocalized with PGP fibers. These artifacts could be misidentified as P-syn-positive fibers (Fig. 2).
Figure 2.
Examples of phosphorylated alpha-synuclein (P-syn) artifacts. In this 50-µm-thick tissue section, a nerve bundle is stained with P-syn (seen in red in (A)) and protein gene product 9.5 (PGP 9.5 in green) with the merged orthogonal image shown in (B). The white arrows indicate regions of possible P-syn deposition in red. In the merged image, the regions do not colocalize with PGP 9.5. Without colocalization, it is easy to misinterpret artifacts for actual alpha-synuclein staining.
P-syn and Colocalization
The comparison of 20 biopsies from individuals with PD and 20 biopsies from control subject using just P-syn without PGP 9.5 colocalization is provided in Table 3. The number of biopsies with P-syn-positive results increased in both the PD and control subjects. The specificity of P-syn testing without colocalization was 71% at the distal leg, 59% at the distal thigh, and 63% at the proximal thigh.
Table 3.
Detection of Phosphorylated Alpha-Synuclein Without Colocalization Using PGP 9.5.
| PD N=29 | Control N=21 | |||||
|---|---|---|---|---|---|---|
| Thickness | 50 | 20 | 10 | 50 | 20 | 10 |
| Distal leg (% P-syn positive) | 86% | 69% | 62% | 19% | 24% | 38% |
| Distal thigh (% P-syn positive) | 76% | 72% | 65% | 24% | 29% | 33% |
| Proximal thigh (% P-syn positive) | 86% | 65% | 72% | 19% | 29% | 38% |
Abbreviations: PD, Parkinson’s disease; PGP 9.5, protein gene product 9.5.
The sensitivity of detecting P-syn with results from three biopsies was 100% in the 50-, 20-, and 10-µm-thick tissue sections using PGP 9.5. The calculated specificity of detecting P-syn with results from three biopsies was 66% with 50 µm sections, 60% with 20 µm sections, and 57% with 10 µm sections without colocalizing with PGP 9.5.
Discussion
In this study, we report a detailed evaluation of tissue thickness as a critical variable in the detection of cutaneous P-syn. In this study, and in a review of prior work, it is clear that the number of P-syn-positive fibers is much lower than the density of nerve fibers imaged by PGP 9.5.9,14,25,26 Thus, the routine use of thin tissue sections would work well only if P-syn was distributed throughout the skin at a great enough frequency to be detected by low-volume tissue analysis. However, we have determined that P-syn-positive fibers are typically identified within dermal autonomic substructures (sweat glands, pilomotor muscles, and blood vessels) and that adequate tissue sampling is necessary to facilitate detection.
Our results have significant implications on the conduct of studies sampling cutaneous P-syn. In all measured outcomes, the 50-µm-thick tissue sections performed better than 20 or 10 µm tissue sections. These outcomes included number of positive tissue structures, number of positive biopsy, and number of subjects with PD who had P-syn detected.
There are a number of potential explanations for our findings. (1) It was only possible to visualize a P-syn-positive nerve fiber in the thicker tissue sections; the 10-µm sections were mostly visualized as P-syn-positive “dots.” The thinner tissue sections reduced the volume of nerve fiber evaluated, thereby diminishing the visually apparent fluorescent signal that was necessary to make a diagnosis. (2) The actual volume of the tissue sampled increased with greater section thickness when using the same number of sections. Thus, five 10 µm sections had only 50 µm of tissue sampled in total, whereas the five 20-µm sections had 100 µm of tissue evaluated and the five 50-µm sections had 250 µm of tissue evaluated. This significant increase in the total volume of tissue measured will increase the chances of P-syn detection. Increasing the number of 10 and 20 µm stained sections is likely to provide a similar result. (3) It is easier to identify autonomic substructures within thicker tissue sections, thereby allowing easier visual identification of potential areas of P-syn-positive fibers as seen in Figs. 1 and 2.
As noted in previous studies, the consistent penetration of antibodies can be achieved to a depth of 20- to 35-µm-thick tissue sections using single-sided conventional immunohistochemical staining.27,28 In this study, the use of free-floating tissue sections allowed for adequate penetration of the P-syn antibody in 50 μm sections after 24 hr as previously reported.9,25 Therefore, we prepared 50-μm-thick free-floating sections where penetration of the antibodies against P-syn and fluorescein labels was optimal, although some publications suggest that thinner sections are generally associated with improved morphology, but reduced chemical reactivity.27 The results show that P-syn-positive fibers in 50 µm sections were clear, bright, and visible and completely colocalized with PGP-positive fibers in autonomic structures. Free-floating staining cannot be performed using thinner tissue sections because the tissue is too fragile and will tear before mounting.
One of the challenges using thicker tissue sections is the need for confocal image analysis. Although automated image analysis systems are widely used for thinner paraffinized sections, several obstacles exist for automatic analysis of thicker sections. These include the need for thru-imaging of the entire tissue specimen (50-µm-thick tissue sections therefore require 25 complete images using 2 µm confocal “steps” to capture the complete section). The image resolution in the XY axis is high, and this imposes a significant memory burden for such a Z-stack image, typically resulting in files that exceed 2 terabytes per tissue section. Thus, for multiple biopsies per patient, each having multiple tissue sections, the average study would exceed 30 terabytes of memory and several hours of confocal imaging time. Although high-throughput high-resolution confocal Z-stack imaging systems exist, their costs are prohibitive and availability is limited. We do anticipate technological advances in imaging to continue to improve the potential for automation in the future.
In this study, we noted that low-intensity signal artifacts that did not colocalize with PGP 9.5 could be detected within the tissue sections of both subjects with PD and control subjects. These structures did not colocalize with PGP 9.5, but could be confused with P-syn-positive fibers in thin tissue sections, particularly if colocalization of PGP 9.5 was not required for confirmation of the result. As is reported in most immunohistochemical studies, some level of nonspecific background noise and staining artifacts are technically inescapable. We performed several attempts to reduce background noise and to enhance the signal for positive P-syn staining in tissue sections, including using different block solutions containing 5% to 10% normal goat serum, 5% to 10% normal donkey serum, 5% bovine serum albumin, or 5% non-fat dry milk; addition of Triton X-100; prolongation of washing during each incubation; modification of antibody concentrations; and using different methods for antigen retrievals; however, the artifacts still appeared. After optimization of technique, we compared the results with and without colocalization. Although both methods provided high sensitivity, the specificity decreased from 100% to <70% without colocalization of PGP 9.5–innervated nerve fibers. These results suggest that single immunohistochemical staining of P-syn cannot adequately differentiate between artifact and true positive results. Therefore, double staining of P-syn with a pan-axonal marker, such as PGP 9.5, is necessary to ensure adequate specificity.
In this study, as in many other recent reports, all of the patients with PD were positive for alpha-synuclein despite a range of disease duration.14,29 These results are consistent with the underlying pathology of PD where years of ongoing pathological progression occur before clinically apparent disease.30,31 More recently, the detection of phosphorylated alpha-synuclein from patients with idiopathic rapid eye movement sleep behavioral disorder suggests that early detection is an achievable goal.16,32,33 These findings suggest that the need to optimize methodological techniques to maximize rates of early detection will be a critically important technical advance.
There are several additional limitations to our study. We chose to use two different antigen optimization techniques for the free-floating and slide-mounted sections. Although microwaving worked well for slide-mounted sections, it caused the free-floating sections to fold and shrink, creating irreversible structural changes to the tissue. Conversely, the formic acid worked well for the free-floating sections, but was not as effective as microwaving for the slide-mounted sections. We attempted to compare the best antigen retrieval method possible for both approaches, but this does mean the methods are not identical. The analysis of slides is not performed in an automated fashion; thus, the results are to a certain extent subjective. This technique does require three skin biopsies for optimal specificity and sensitivity, an approach that is frequently performed within the field of neurology for evaluation of small fiber neuropathy.34
In summary, we demonstrate that double-immunostained 50 µm skin biopsy tissue sections (with P-syn and PGP 9.5) are superior to 20 and 10 µm tissue sections for the detection of P-syn in patients with PD. The increased rate of detection using thicker sections is likely secondary to a combination of greater volume of tissue analyzed and improved visualization of nerve fiber architecture.
Footnotes
Competing Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.H.G. has received research support from Grifols and serves as a scientific advisor for Cutaneous NeuroDiagnostics. C.H.G. has received personal compensation for his editorial activities (Associate Editor) with Autonomic Neuroscience—Basic and Clinical. R.F. has received personal compensation for serving on scientific advisory boards of Abbott, Astellas, Biogen, Cutaneous NeuroDiagnostics, Dong, Johnson and Johnson, Lundbeck, PamLab, Pfizer, Spinifex, and Zallicus. R.F. receives funding from the NIH NINDS and NHLBI. R.F. has received research support from Impeto and Pamlab. R.F. has received personal compensation for his editorial activities (Editor) from Autonomic Neuroscience—Basic and Clinical. N.W. and J.G. report no competing interests.
Author Contributions: NW was involved in study design, data collection, and writing the first draft of the manuscript. JG and CG was involved in study design, data collection, data analysis, and review of the manuscript. RF was involved in study design, data analysis, and review of the manuscript.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Ningshan Wang, Center for Autonomic and Peripheral Nerve Disorders, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Jennifer Garcia, Center for Autonomic and Peripheral Nerve Disorders, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Roy Freeman, Center for Autonomic and Peripheral Nerve Disorders, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Christopher H. Gibbons, Center for Autonomic and Peripheral Nerve Disorders, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA.
Literature Cited
- 1. Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117(6):613–634. doi: 10.1007/s00401-009-0538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES, Cairns NJ, Kotzbauer PT, Diamond MI. Parkinson’s disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J Biol Chem. 2019;294(3):1045–1058. doi: 10.1074/jbc.RA118.004471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85(5):404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caviness JN, Lue LF, Beach TG, Hentz JG, Adler CH, Sue L, Sadeghi R, Driver-Dunckley E, Evidente VG, Sabbagh MN, Shill HA, Walker DG. Parkinson’s disease, cortical dysfunction, and alpha-synuclein. Mov Disord. 2011;26:1436–1442. doi: 10.1002/mds.23697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doppler K, Ebert S, Uceyler N, Trenkwalder C, Ebentheuer J, Volkmann J, Sommer C. Cutaneous neuropathy in Parkinson’s disease: a window into brain pathology. Acta Neuropathol. 2014;128(1):99–109. doi: 10.1007/s00401-014-1284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doppler K, Weis J, Karl K, Ebert S, Ebentheuer J, Trenkwalder C, Klebe S, Volkmann J, Sommer C. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord. 2015;30:1688–1692. doi: 10.1002/mds.26293 [DOI] [PubMed] [Google Scholar]
- 8. Wang N, Gibbons CH. Skin biopsies in the assessment of the autonomic nervous system. Handb Clin Neurol. 2013;117:371–378. doi: 10.1016/B978-0-444-53491-0.00030-4 [DOI] [PubMed] [Google Scholar]
- 9. Gibbons CH, Garcia J, Wang N, Shih LC, Freeman R. The diagnostic discrimination of cutaneous alpha-synuclein deposition in Parkinson disease. Neurology. 2016;87(5):505–512. doi: 10.1212/WNL.0000000000002919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikemura M, Saito Y, Sengoku R, Sakiyama Y, Hatsuta H, Kanemaru K, Sawabe M, Arai T, Ito G, Iwatsubo T, Fukayama M, Murayama S. Lewy body pathology involves cutaneous nerves. J Neuropathol Exp Neurol. 2008;67(10):945–953. doi: 10.1097/NEN.0b013e318186de48 [DOI] [PubMed] [Google Scholar]
- 11. Shishido T, Ikemura M, Obi T, Yamazaki K, Terada T, Sugiura A, Saito Y, Murayama S, Mizoguchi K. alpha-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology. 2010;74(7):608–610. doi: 10.1212/WNL.0b013e3181cff6d5 [DOI] [PubMed] [Google Scholar]
- 12. Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116(3):277–288. doi: 10.1007/s00401-008-0409-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donadio V, Incensi A, Cortelli P, Giannoccaro MP, Jaber MA, Baruzzi A, Liguori R. Skin sympathetic fiber alpha-synuclein deposits: a potential biomarker for pure autonomic failure. Neurology. 2013;80(8):725–732. doi: 10.1212/WNL.0b013e3182825127 [DOI] [PubMed] [Google Scholar]
- 14. Donadio V, Incensi A, Leta V, Giannoccaro MP, Scaglione C, Martinelli P, Capellari S, Avoni P, Baruzzi A, Liguori R. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82(15):1362–1369. doi: 10.1212/WNL.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 15. Chahine LM, Beach TG, Seedorff N, Caspell-Garcia C, Coffey CS, Brumm M, Adler CH, Serrano GE, Linder C, Mosovsky S, Foroud T, Riss H, Ecklund D, Seibyl J, Jennings D, Arnedo V, Riley L, Dave KD, Mollenhauer B, Systemic Synuclein Sampling study. Feasibility and safety of multicenter tissue and biofluid sampling for alpha-synuclein in Parkinson’s disease: the systemic synuclein sampling study (S4). J Parkinson’s Dis. 2018;8(4):517–527. doi: 10.3233/JPD-181434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antelmi E, Donadio V, Incensi A, Plazzi G, Liguori R. Skin nerve phosphorylated alpha-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology. 2017;88(22):2128–2131. doi: 10.1212/WNL.0000000000003989 [DOI] [PubMed] [Google Scholar]
- 17. Haga R, Sugimoto K, Nishijima H, Miki Y, Suzuki C, Wakabayashi K, Baba M, Yagihashi S, Tomiyama M. Clinical utility of skin biopsy in differentiating between Parkinson’s disease and multiple system atrophy. Parkinson’s Disease. 2015;2015:167038. doi: 10.1155/2015/167038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JY, Illigens BM, McCormick MP, Wang N, Gibbons CH. Alpha-synuclein in skin nerve fibers as a biomarker for alpha-synucleinopathies. J Clin Neurol. 2019;15(2):135–142. doi: 10.3988/jcn.2019.15.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donadio V. Skin nerve alpha-synuclein deposits in Parkinson’s disease and other synucleinopathies: a review. Clin Auton Res. 2019;29:577–585. doi: 10.1007/s10286-018-0581-4 [DOI] [PubMed] [Google Scholar]
- 20. Goetz CG. Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): a new scale for the evaluation of Parkinson’s disease. Rev Neurol (Paris). 2010;166(1):1–4. doi: 10.1016/j.neurol.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 21. McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45(10):1848–1855. [DOI] [PubMed] [Google Scholar]
- 22. Wang N, Gibbons CH, Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. J Histochem Cytochem. 2011;59(4):382–390. doi: 10.1369/0022155410396931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333–338. doi: 10.1111/ene.12842 [DOI] [PubMed] [Google Scholar]
- 24. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Sole J. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a Joint Task Force of the European federation of neurological societies and the peripheral nerve society. Eur J Neurol. 2010;17(7):903–909. doi: 10.1111/j.1468-1331.2010.03023.x [DOI] [PubMed] [Google Scholar]
- 25. Wang N, Gibbons CH, Lafo J, Freeman R. alpha-synuclein in cutaneous autonomic nerves. Neurology. 2013;81(18):1604–1610. doi: 10.1212/WNL.0b013e3182a9f449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zange L, Noack C, Hahn K, Stenzel W, Lipp A. Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain. 2015;138(Pt 8):2310-2321. doi: 10.1093/brain/awv138 [DOI] [PubMed] [Google Scholar]
- 27. Melvin NR, Sutherland RJ. Quantitative caveats of standard immunohistochemical procedures: implications for optical disector-based designs. J Histochem Cytochem. 2010;58(7):577–584. doi: 10.1369/jhc.2009.954164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibbons CH, Wang N, Freeman R. Cutaneous alpha-synuclein from paraffin embedded autopsy specimens in Parkinson’s disease. J Parkinson’s Dis. 2017;7(3):503–509. doi: 10.3233/JPD-171088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parashos SA, Luo S, Biglan KM, Bodis-Wollner I, He B, Liang GS, Ross GW, Tilley BC, Shulman LM. Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurol. 2014;71(6):710–716. doi: 10.1001/jamaneurol.2014.391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Postuma RB, Gagnon JF, Montplaisir JY. REM sleep behavior disorder: from dreams to neurodegeneration. Neurobiol Dis. 2012;46(3):553–558. doi: 10.1016/j.nbd.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 32. Antelmi E, Pizza F, Donadio V, Filardi M, Sosero YL, Incensi A, Vandi S, Moresco M, Ferri R, Marelli S, Ferini-Strambi L, Liguori R, Plazzi G. Biomarkers for REM sleep behavior disorder in idiopathic and narcoleptic patients. Ann Clin Transl Neurol. 2019;6:1872–1876. doi: 10.1002/acn3.50833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doppler K, Jentschke HM, Schulmeyer L, Vadasz D, Janzen A, Luster M, Hoffken H, Mayer G, Brumberg J, Booij J, Musacchio T, Klebe S, Sittig-Wiegand E, Volkmann J, Sommer C, Oertel WH. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 2017;133(4):535–545. doi: 10.1007/s00401-017-1684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boruchow SA, Gibbons CH. Utility of skin biopsy in management of small fiber neuropathy. Muscle Nerve. 2013;48(6):877–882. doi: 10.1002/mus.23859 [DOI] [PubMed] [Google Scholar]